Abstract
AIM
To compare the effectiveness of topical cyclosporine A emulsion with that of oral doxycycline for rosacea associated ocular changes and dry eye complaints.
METHODS
One hundred and ten patients with rosacea were screened. Thirty-eight patients having rosacea associated eyelid and ocular surface changes and dry eye complaints were included in the study. Patients were randomly divided into two groups: nineteen patients were given topical cyclosporine twice daily and nineteen patients were given oral doxycycline 100 mg twice daily for the first month and once daily for the following two months. Symptom and sign scores, ocular surface disease index questionnarie and tear function tests were evaluated at baseline and monthly for 3mo. Three months after results were compared with that of baseline.
RESULTS
Mean values of symptom, eyelid sign and corneal/conjunctival sign scores of each treatment group at baseline and 3mo after treatments were compared and both drugs were found to be effective on rosacea associated ocular changes (P<0.001). Cyclosporine was more effective in symptomatic relief and in the treatment of eyelid signs (P=0.01). There was statistically significant increase in the mean Schirmer score with anesthesia and tear break up time scores in the cyclosporine treatment group compared to the doxycycline treatment group (P<0.05).
CONCLUSION
Cyclosporine as a topical drug can be used in the treatment of rosacea associated ocular complications because it is more effective than doxycycline. In addition ocular rosacea as a chronic disease requires long term treatment and doxycycline has various side effects limiting its long term usage.
Keywords: cyclosporine A, doxycycline, rosacea
INTRODUCTION
Rosacea is a chronic skin disorder affecting the sebaceous glands especially in the central area of the face. It is characterised by facial flushing, erythema and telangiectasia, inflammatory papules, occasionally connective tissue hypertrophy and ocular involvement[1]. The disease typically affects patients between the ages of 40 and 50, but ocular rosacea is more common between the ages of 50 and 60[1],[2]. The incidence of ocular involvement varies between 6%-72% in different studies[2],[3]. Ocular manifestations may accompany primary skin findings but could appear independently[4].
Ocular signs of rosacea usually involve following: periorbital lymphedema; eyelid margin erythema and telangiectasia; blepharitis and inspissated meibomian gland orifices; hordeola and chalasia; dry eye; bacterial colonization; corneal erosion, vascularisation and thinning; episcleritis, scleritis[5]. Ocular symptoms of rosacea may be out of proportion to the signs and include burning, foreign body sensation, itching, redness, watering, photophobia, pain and lid swelling[5]–[7]. Although the etiopathogenetic mechanism of rosacea remains still unclear, the current knowledge is that it is an inflammatory disorder. The primary defect in ocular rosacea is the meibomian gland inflammation with dilation and plugging of the gland orifices leading to dysfunction of the glands. Chronic inflammation can lead to hyperkeratinization of the ductal epithelium and loss of secretion[1]. These result in blepharitis, meibomitis, chalasia, telangiectasias and eyelid irregularities. The abnormal lipid secretions from the meibomian glands can lead to qualitative abnormalities of the tear film and rapid evaporation[8].
Moreover more than one third of the ocular rosace patients have decreased tear production. Both qualitative and quantitative abnormalities of the tear film can lead to dry eye complaints. The instability of the tear film results in hyperosmolarity of the ocular surface, stimulation of imflammatory cell cytokine release and activation of matrix metalloproteinase leading to damage of the ocular surface[9]–[11]. Conjunctival hyperemia is common in ocular rosacea, usually found in the interpalpebral zone and worsened by tear film abnormalities. Punctate keratopathy and superficial vascularization of peripheral cornea are also common findings[1],[2].
There is currently no standard and satisfactory treatment for ocular manifestations of rosacea. Traditional treatments include lid hygiene, oral tetracyclines, steroids and artificial tears. Measures to protect the ocular surface from irritation such as lid hygiene and artificial tears are seldom sufficient. Oral doxycycline has been the mainstay of tretament for ocular rosacea but it seems to provide moderate benefit and to have many side effects limiting its usage[5],[12]–[16]. Systemic cyclosporine has been used as an immune supressant and inhibits T-lymphocyte activation, proliferation and migration[11],[12]. It also inhibits cell apoptosis which is a useful therapeutic effect for the management of ocular surface disorders[17],[18]. In ophthalmology topical cyclosporine has been used in various inflammatory ocular surface disorders including ocular rosacea since early 1980s[19],[20].
As our knowledge there is no study comparing the efficiencies of topical cyclosporine and oral doxycycline on ocular rosacea. This study is the first to compare the efficiencies of both drugs on rosacea associated ocular surface changes and dry eye complaints.
SUBJECTS AND METHODS
Subjects
This study was approved by the local ethics comittee of the hospital and conducted in accordance with the Declaration of Helsinki. All participants were informed about the study and signed informed written consents were obtained. This study was conducted during a one-year period and included 110 patients with rosacea who attended Dermatology and Ophthalmology Outpatient Clinics of Ankara Atatürk Training and Research Hospital. Inclusion criteria were written informed consent provided by the patients and diagnosis of rosacea based on the clinical criteria stated by the Expert National Rosacea Society Comittee[4]. Patients' age, sex and systemic ilnesses were recorded. Clinical stage of rosacea was determined according to the standard classification system reported by the Expert National Rosacea Society Comittee[4].
Methods
All patients diagnosed as rosacea were consulted to ophthalmology clinic and screened for the signs and symptoms of ocular rosacea, completed ocular surface disease index (OSDI) questionnarie, underwent full ophthalmologic examination including tear function tests. Patients having rosacea associated eyelid and ocular surface changes were randomly divided into two treatment groups. Pregnant women, nursing mothers, patients with eyelid defects, lagophthalmos, active ocular infections and allergies, patients with a history of hypersensitivity to cyclosporine and doxycycline, patients who have undergone ocular surgery within the past 6mo were excluded from the study. The first group is given topical cyclosporine emulsion twice daily for 3mo and the second group is given oral doxycycline 100 mg twice daily for the first month and once daily for the following two months. All patients were instructed about lid hygiene and given artificial eye drops four times daily.
Patients underwent a panel of rosacea associated symtoms and signs at baseline and monthly thereafter. Symptoms and signs are recorded as present or absent (1/0) at each visit. Symptom panel included burning, stinging or foreign body sensation, photophobia, itching, redness, blurring, watering, pain and lid swelling. The sum of scores were calculated at each visit. Maximum symptom score a patient could get was 9.
Eyelid sign panel included blepharitis, meibomian gland inspissation, erythema and telangiectasia, chalasia and lid margin irregularity. The sum of scores were calculated at each visit. Maximum sum of eyelid sign scores a patient could get was 5.
Corneal and conjunctival sign panel included conjunctival hyperemia, episcleritis or scleritis, punctate epithelial keratopathy, corneal infiltration, corneal vascularisation and corneal thinning or perforation. The sum of scores were calculated at each visit. Maximum corneal and conjunctival sign score a patient could get was 6.
Tear funtions were evaluated by Schirmer test with anesthesia and tear break up time (TBUT). For Schirmer test with anesthesia a drop of topical anesthetic agent (0.5% proparacaine hydrochloride) was instilled in both eyes and excess drop on the eyelid margins was dried with a cotton tip applicator. After 5min sterile test strips (SNO strips Laboratory Chauvin) were placed in the inferotemporal conjunctival fornices, avoiding contact with the cornea. After 5min strips were removed and the size of the wet area was measured in milimeters.
For TBUT measurement fluorescein strips (Fluorescein Strips Chauvin, Laboratory Chauvin) were moistened with sterile saline and placed in the inferior palpebral conjunctival fornices of both eyes. Patients were asked to blink few times to stabilise the tear film. After removal of the strips, with the help of a broad spectrum cobalt blue light, time taken from the last blink until the appearance of the first randomly distributed dark spot or streak within the fluorescein enhanced tear film was measured.
Statistical Analysis
Mean changes within each treatment group were analyzed by paired sample t-tests and between-group differences were analyzed by two-sample t-tests. SPSS version 16 was used for statistical analysis.
RESULTS
One hundred and ten patients dignosed as rosacea by the Dermatology outpatient clinic were consulted to Ophthalmology clinic. Thirty-eight patients were symptomatic and demonstrated rosacea associated ocular changes (30%). Patients were enrolled sequentially as they were diagnosed as ocular rosacea into two treatment groups. Each group included nineteen patients. The mean age of patients in the cyclosporine treatment group was 49.58±11.77 and in the doxycycline treatment group was 53.84±12.57. Patient demographics were given in Table 1.
Table 1. Patient demographics of cyclosporine and doxycycline treatment groups.
| Patients demographics | Cyclosporine (n=19) | Doxycycline (n=19) | P |
| Mean age (a) | 49.58±11.77 | 53.84±12.57 | 0.30 |
| Gender | 0.74 | ||
| M | 8 (42.1) | 7 (36.8) | |
| F | 11 (57.9) | 12 (63.2) | |
| Systemic diseases | |||
| Diabetes mellitus | 1 (5.2) | 2 (10.5) | |
| Hypertension | 6 (31.5) | 6 (31.5) | |
| Others | 1 (5.2) | 3 (15.8) |
n (%)
Ocular sign and symptom scores of patients in each treatment group before and 3mo after treatments are given in Table 2. Burning and stinging were present in all patients before treatment and the most common eyelid sign was meibomian gland inspissation (100%) followed by blepharitis (94.7%) in the cyclosporine treatment group. The most common eyelid signs were meibomian gland inspissation (100%) and blepharitis (100%) in the doxycycline treatment group. The most common corneal/conjunctival sign was conjunctival hyperemia (100%) in both groups followed by punctate keratopathy (94.7%) in the cyclosporine treatment group and episcleritis (94.7%) in the doxycycline treatment group.
Table 2. Symptom, eyelid sign and corneal/conjunctival sign panel of patients in cyclosporine and doxycycline treatment groups at baseline and 3mo after treatments.
| Patients' signs and symptoms | Cyclosporine (n=19) |
Doxycycline (n=19) |
||
| Baseline | 3mo after treatment | Baseline | 3mo after treatment | |
| Symptoms | ||||
| Burning | 19 (100.0) | 4 (21.1) | 19 (100.0) | 14 (73.7) |
| Stinging | 19 (100.0) | 9 (47.3) | 19 (100.0) | 14 (73.7) |
| Itching | 11 (57.9) | 4 (21.1) | 16 (84.2) | 8 (42.1) |
| Photophobia | 19 (100.0) | 2 (10.5) | 18 (94.7) | 4 (21.1) |
| Redness | 18 (94.7) | 4 (21.1) | 19 (100.0) | 9 (47.3) |
| Blurred vision | 8 (42.1) | 1 (5.3) | 6 (31.6) | 1 (5.3) |
| Watering | 17 (89.5) | 6 (31.6) | 17 (89.5) | 6 (31.6) |
| Pain | 11 (57.9) | 1 (5.3) | 7 (36.8) | 4 (21.1) |
| Lid swelling | 10 (52.6) | 0 (0.0) | 6 (31.6) | 2 (10.5) |
| Eyelid Signs | ||||
| Meibomian gland inspissation | 19 (100.0) | 8 (42.1) | 19 (100.0) | 14 (73.7) |
| Blepharitis | 18 (94.7) | 3 (15.8) | 19 (100.0) | 10 (52.6) |
| Lid margin telangiectasia | 14 (73.7) | 3 (15.8) | 17 (89.5) | 11 (57.9) |
| Hordeola/chalazia | 4 (21.1) | 0 (0.0) | 5 (26.3) | 0 (0.0) |
| Lid margin irregularity | 12 (63.2) | 3 (15.8) | 11 (57.9) | 5 (26.3) |
| Corneal /Conjonctival Signs | ||||
| Conjuctival hyperemia | 19 (100.0) | 5 (26.3) | 19 (100.0) | 12 (63.2) |
| Punctate keratopathy | 18 (94.7) | 0 (0.0) | 15 (78.9) | 0 (0.0) |
| Corneal vascularization | 3 (15.8) | 3 (15.8) | 2 (10.5) | 2 (10.5) |
| Episcleritis/scleritis | 17 (89.5) | 2 (10.5) | 18 (94.7) | 3 (15.8) |
| Corneal infiltrates | 3 (15.8) | 1 (5.3) | 2 (10.5) | 1 (5.3) |
| Corneal ulceration/perforation | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
n (%)
Mean values of symptom, eyelid sign and corneal/conjunctival sign scores of each treatment group at baseline and 3mo after treatments were given in Table 3. Comparison of baseline and post treatment scores demonstrated improvement of rosacea associated ocular signs and symptoms for each treatment group (P<0.01).
Table 3. Mean symptom, eyelid sign and corneal/conjuctival sign scores of cyclosporine and doxycycline treatment groups baseline and 3mo after treatments.
| Symptom and sign score comparison | Symptom score |
Eyelid sign score |
Corneal/conjuctival |
|||
| Baseline | After treatment | Baseline | After treatment | Baseline | After treatment | |
| Cyclosporine | 7.16±1.21 | 1.79±0.98a | 3.89±0.74 | 1.21±0.86a | 3.16±0.77 | 0.58±0.77a |
| Doxycycline | 6.79±1.08 | 3.32±1.41a | 3.79±0.79 | 2.21±0.92a | 3.05±0.78 | 1.00±0.67a |
aP<0.01.
The reduction of symptom and sign scores of each patient was determined by substracting the 3mo after treatment score from the baseline score. Mean symptom and sign score reductions of cyclosporine and doxycycline treatment groups were given in Table 4. Cyclosporine was found to be more effective in symptomatic relief and in the treatment of eyelid signs (P=0.01). There was not statistically significant difference in the mean corneal/conjunctival sign score reductions between the drugs (P=0.08).
Table 4. Comparison of symptom, eyelid sign and corneal/conjunctival sign score reductions of cyclosporine and doxycycline treatment groups.
| Symptom and sign score reduction | Cyclosporine | Doxycycline | P |
| Symptom score reduction | 5.32±1.25 | 3.47±1.12 | 0.01 |
| Eyelid sign score reduction | 2.68±0.82 | 1.58±0.96 | 0.01 |
| Corneal/conjonctival sign score reduction | 2.58±0.69 | 2.05±0.52 | 0.08 |
OSDI questionnarie at baseline 34.76±7.70 for cyclosporine treatment group and 29.64±10.30 for the doxycycline treatment group also indicated dry eye complaints. There was no significant difference between groups in the mean OSDI scores before treatment (P=0.06). There was statistically significant decrease in OSDI scores of cyclosporine (-20.04±8.06) compared to doxycycline (-11.22±9.20) (P< 0.05; Figure 1).
Figure 1. Change in OSDI scores between baseline and 3mo after treatment.
In our study the mean Schirmer scores with anesthesia at baseline were 4.21±2.69 mm and 4.05±2.40 mm, the mean TBUT scores were 3.68±1.63 and 4.00±1.63 for cyclosporine and doxycycline treatment groups respectively
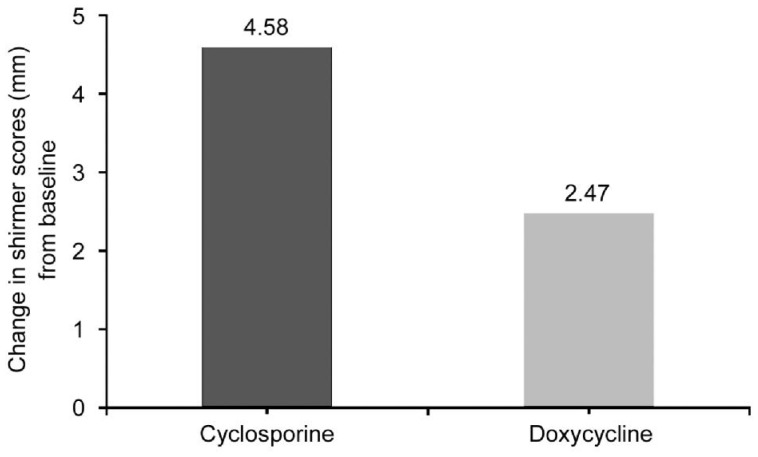
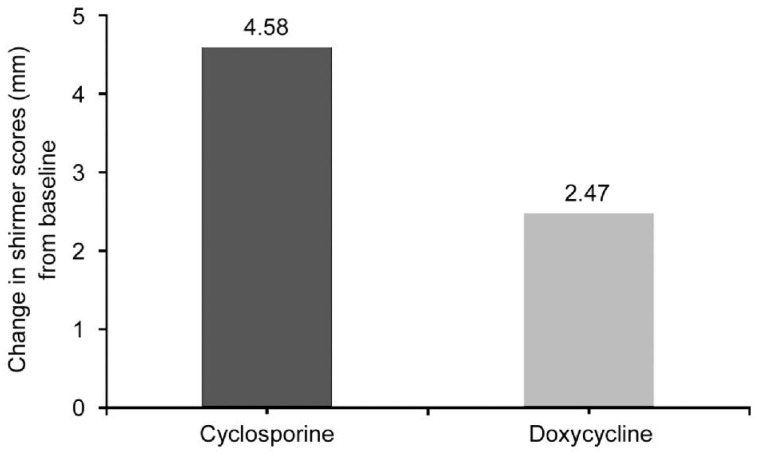
There was no significant between group differences in the mean Schirmer scores with anesthesia at baseline (P=0.85). After 3mo there was statistically significant increase in the mean.
Schirmer score with anesthesia in the cyclosporine treatment group (4.58±2.46 mm) compared to the doxycycline treatment group (2.47±1.47 mm) (P<0.05; Figure 2).
Figure 2. Change in schirmer scores with anesthesia between baseline and 3mo after treatment.
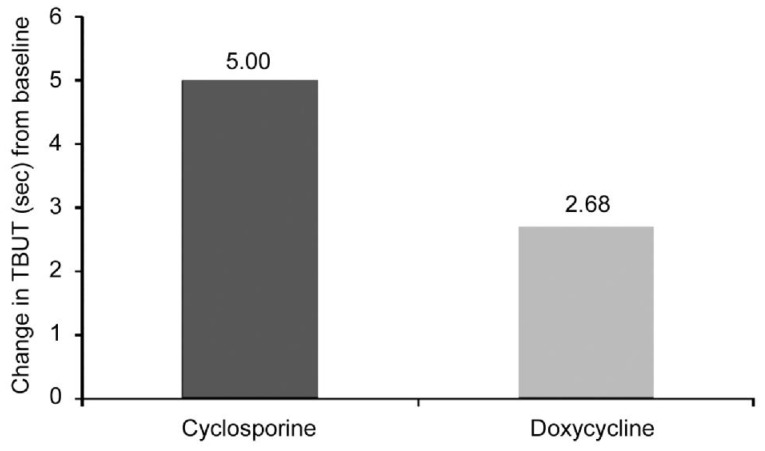
Although there was no statistically significant between group difference in TBUT scores before treatment (P=0.56); cyclosporine improved the mean TBUT score (5.00±2.69) more than oral doxycycline (2.68±2.00) (P<0.05; Figure 3).
Figure 3. Change in TBUT scores between baseline and 3mo after treatment.
DISCUSSION
Rosacea is a chronic cutaneous disorder affecting sebaceous glands of the face, meibomian glands of the eyes and found in up to 10% of the adult population[21],[22]. The disease typically affects patients between the ages of 40-50y and has no serious adverse effect on vital functions[23]. To date there is no reliable dignostic test for cutaneous and ocular rosacea[24],[25]. There are studies on tear and saliva glycomics of rosacea patients as a biomarker to facilitate early and accurate diagnosis but further studies are needed[10],[25]. Today the diagnosis of ocular rosacea is clinical and relies on colloborative study of both ophthalmologists and dermatologists and careful observation and interpretation of skin and ocular manifestations. Yet other ophthalmic and cutaneous disorders may present with similar findings further chalenging the diagnosis.
Because patients with mild rosacea may not seek medical help, patients who need medical treatment may be unaware of their ocular findings, ocular rosacea is often underdiagnosed. Studies estimate that 6%-72% of rosacea patients have ocular involvement[3],[24]. In our study 30% of the rosacea patients had ocular findings. A recent study by Bakar et al[24] showed much higher ophthalmic involvement in rosacea patients (72%).
Primary defect in ocular rosacea is chronic inflammation and dysfunction of the meibomian glands leading to eyelid changes including blepharitis, inspissation of gland orifices, hordeola and chalasia, eyelid margin telangiectasia and irregularities. In the study of Bakar et al[24] the most common eyelid finding was blepharitis (94.4%) followed by meibomian gland inspissation (88.8%). In our study, all patients diagnosed as ocular rosacea had meibomian gland inspissation and the second most common eyelid sign was blepharitis (97%). Conjunctival hyperemia is a common finding usually affecting interpalpebral zone and worsened by tear film abnormalities. Corneal findings are punctate keratopathy, peripheral corneal vascularization, subepithelial infiltrates and rarely ulceration and perforation. Bakar et al[24] reported conjonctival hyperemia (98%) and punctate epithelial keratopathy (66%). All patients in our study had conjunctival hyperemia and 87% of them had punctate keratopathy. Ghanem et al[26] reported that one third of rosacea patients had potentially sight threatening corneal findings. Although Bakar et al[24] have not detected serious corneal complications, 5 patients (12%) in our study had peripheral corneal vascularization and infiltration.
Inflammation of the meibomian glands leads to capping and plugging of the gland orifices with dilation of the glands and production of excessive and abnormal lipid secretions leading to tear film instability and rapid evaporation of tears. This results in reduced TBUT scores. Additionally more than one third of rosacea patients have decreased tear production[1],[8]. Both increased evaporation and decreased production cause quantitative abnormality of tears and reduced Schirmer scores with anesthesia. Abnormalities of the tear function tests have been reported in different studies[26],[27]. In our study, mean Schirmer scores with anesthesia and mean TBUT results were consistent with tear film abnormality and dry eye. Mean OSDI scores were consistent with increased complaints of dry eye. There was not statistically significant difference in the baseline mean tear function test results between the treatment groups. Follow up examinations showed increase of mean test results for both drugs. Moreover, cyclosporine was found to increase Schirmer and TBUT scores and decrease OSDI scores more efficiently than doxycycline.
The etiology of rosacea and its ocular manifestations are still unknown. The instability of tear film and reduced production result in hyperosmolarity of the ocular surface and stimulation of inflammatory cells, cytokine release, activation of matrixmetalloproteinases leading to damage of the ocular surface[9],[11]. Several studies have documented the presence of elevated levels of interleukin-1α (IL-1α), matrix metalloproteinase-9 (MMP-9) and tumor necrosis factor-α (TNF-α) in the tears and ocular surface of patients with ocular rosacea supporting its inflammatory origin[9],[22],[28]. A recent study stated that cutaneous biopsies taken from the eyelid of rosacea patients demonstrated vascular abnormalities representing activated inflamed vessels but not increase in the total number of vessels[29]. Oral doxycycline with its anti-inflammatory and anti-angiogenic properties has long been used for ocular rosacea. Topical cyclosporine has been used in ocular rosacea and posterior blepharitis because of its anti-inflammatory properties and its effect on aquous tear production.
Cyclosporine is a cyclic polipeptide and an immunomodulating agent that inhibits the activation of T cells and induction of inflammatory cytokines[17]. The anti-inflammatory effect of cyclosporine on ocular surface may be due to the reduction of activated lymphocytes within the conjunctiva. This effect leads to decreased ocular surface inflammation and increased tear production and improvements in patients signs and symptoms. Turner et al[30] demonstrated that after 6mo use of topical cyclosporine, inflammmatory cytokine levels of conjunctival epithelium has decreased supporting the inflammatory origin of rosacea.
In a prospective study of 30 patients with posterior blepharitis cyclosporine was found to increase TBUT and Schirmer scores and improve meibomian gland secretions significantly greater than topical dexamethasone/tobramycine combination. In another prospective double masked study, Perry et al[31] found that cyclosporine improved meibomian gland inflammation but had no effect on mean ocular symptom scores and tear function tests and conjunctival staining. A recent double masked randomized clinical trial comparing the efficacy of cyclosporine and artificial tears on ocular rosacea demonstrated significant improvements in TBUT, Schirmer test, corneal staining and OSDI scores with cyclosporine treatment[32]. In our study cyclosporine had significant improvements on symptom scores, eyelid sign scores, OSDI scores and tear function tests compared to oral doxycycline.
Tetracycline derivates have been the mainstay of treatment, but they seem to provide moderate benefit. The clinical effect of doxycycline is presumably by its non-antibiotic anti-inflammatory properties. Doxycycline has inhibitory effect on MMPs and it has anti-angiogenic properties[12],[13],[33]. It regulates cytokines and diminishes neutrophil chemotaxis[13],[14]. It may be prescribed 100 mg once or twice daily for 6 to 12wk. Many patients experience flareups after discontinuation of the drug and require long term maintenance therapy. Yet there are drawbacks to long term use of the drug including side effects such as gastrointestinal upset, increased sensitivity to UV light, decreased compliance with long tern use. Slow release forms (30 mg immediately releasded/10 mg delayed release) have been approved by the Food and Drug Administration to overcome these problems[34],[35]. But improvement of the sign and symptoms is delayed with slow release forms[15].
In this prospective comperative study, both drugs were found to be effective on the treatment of rosacea associated eyelid and ocular surface changes. Cyclosporine were more effective in the symptomatic relief, in the treatment of eyelid signs. Additionally cyclosporine provided more significant improvements in tear function tests. Given that ocular rosacea is a chronic disease, requires long term treatment, we conclude that cyclosporine as a topical drug can be used for ocular rosacea, because it is more effective than oral doxycycline which has side effects limiting its long term usage.
Acknowledgments
Conflicts of Interest: Arman A, None; Demirseren DD, None; Takmaz T, None.
REFERENCES
- 1.Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology:a review of recent findings. J Am Acad Dermatol. 2013;69:15–26. doi: 10.1016/j.jaad.2013.04.045. [DOI] [PubMed] [Google Scholar]
- 2.Vieira AC, Höfling-Lima AL, Mannis MJ. Ocular rosacea-a review. Arq Bras Oftalmol. 2012;75(5):363–369. doi: 10.1590/s0004-27492012000500016. [DOI] [PubMed] [Google Scholar]
- 3.Michel JL, Cabibel F. Frequency, severity and treatment of ocular rosacea during cutaneous rosacea. Ann Dermatol Venereol. 2003;130(1):20–24. [PubMed] [Google Scholar]
- 4.Wilkin J, Dahl M, Detmar M, Drake L, Liang MH, Odom R, Powell F, National Rosacea Society Expert Committee Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2004;50(6):907–912. doi: 10.1016/j.jaad.2004.01.048. [DOI] [PubMed] [Google Scholar]
- 5.Eiseman AS. The ocular manifestations of atopic dermatitis and rosacea. Curr Allergy Asthma Rep. 2006;6(4):292–298. doi: 10.1007/s11882-006-0062-z. [DOI] [PubMed] [Google Scholar]
- 6.Oltz M, Check J. Rosacea and its ocular manifestations. Optometry. 2011;82(2):92–103. doi: 10.1016/j.optm.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Dahl MV. Rosacea subtypes: a treatment algorithm. Cutis. 2004;7421-27(3 Suppl):32–34. [PubMed] [Google Scholar]
- 8.Suzuki T. Meibomitis-related keratoconjunctivitis: implications and clinicalsignificance of meibomian gland inflammation. Cornea. 2012;31(Suppl 1):S41–44. doi: 10.1097/ICO.0b013e31826a04dd. [DOI] [PubMed] [Google Scholar]
- 9.Solomon A, Dursun D, Liu Z, Xie Y, Macri A, Pflugfelder SC. Pro-and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001;42(10):2283–2292. [PubMed] [Google Scholar]
- 10.Vieira AC, Mannis MJ. Ocular rosacea: common and commonly missed. J Am Acad Dermatol. 2013;69(6 Suppl 1):S36–41. doi: 10.1016/j.jaad.2013.04.042. [DOI] [PubMed] [Google Scholar]
- 11.Määttä M, Kari O, Tervahartiala T, Peltonen S, Kari M, Saari M, Sorsa T. Tear fluid levels of MMP-8 are elevated in ocular rosacea-treatment effect of oral doxycycline. Graefes Arch Clin Exp Ophthalmol. 2006;244(8):957–962. doi: 10.1007/s00417-005-0212-3. [DOI] [PubMed] [Google Scholar]
- 12.Gupta AK, Chaudhry MM. Rosacea and its management: an overview. J Eur AcadDermatol Venereol. 2005;19(3):273–285. doi: 10.1111/j.1468-3083.2005.01216.x. [DOI] [PubMed] [Google Scholar]
- 13.Iovieno A, Lambiase A, Micera A, Stampachiacchiere B, Sgrulletta R, Bonini S. In vivo characterization of doxycycline effects on tear metalloproteinases in patients with chronic blepharitis. Eur J Ophthalmol. 2009;19(5):708–716. doi: 10.1177/112067210901900504. [DOI] [PubMed] [Google Scholar]
- 14.Monk E, Shalita A, Siegel DM. Clinical applications of non-antimicrobial tetracyclines in dermatology. Pharmacol Res. 2011;63(2):130–145. doi: 10.1016/j.phrs.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Pfeffer I, Borelli C, Zierhut M, Schaller M. Treatment of ocular rosacea with 40 mg doxycycline in a slow release form. J Dtsch Dermatol Ges. 2011;9(11):904–907. doi: 10.1111/j.1610-0387.2011.07723.x. [DOI] [PubMed] [Google Scholar]
- 16.Bikowski JB. Subantimicrobial dose doxycycline for acne and rosacea. Skinmed. 2003;2(4):234–245. doi: 10.1111/j.1540-9740.2003.03014.x. [DOI] [PubMed] [Google Scholar]
- 17.Donnenfeld E, Pflugfelder SC. Topical ophthalmic cyclosporine: pharmacology and clinical uses. Surv Ophthalmol. 2009;54(3):321–338. doi: 10.1016/j.survophthal.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Durrani K, Zakka FR, Ahmed M, Memon M, Siddique SS, Foster CS. Systemic therapy with conventional and novel immunomodulatory agents for ocular inflammatory disease. Surv Ophthalmol. 2011;56(6):474–510. doi: 10.1016/j.survophthal.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Shimazaki J, Den S, Omoto M, Satake Y, Shimmura S, Tsubota K. Prospective, randomized study of the efficacy of systemic cyclosporine in high-risk corneal transplantation. Am J Ophthalmol. 2011;152(1):33–39. doi: 10.1016/j.ajo.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 20.Utine CA, Stern M, Akpek EK. Clinical review: topical ophthalmic use of cyclosporin A. Ocul Immunol Inflamm. 2010;18(5):352–361. doi: 10.3109/09273948.2010.498657. [DOI] [PubMed] [Google Scholar]
- 21.Bikowski JB. Rosacea: a tiered approach to therapy. Cutis. 2000;66(4 Suppl):3–6. [PubMed] [Google Scholar]
- 22.Crawford GH, Pelle MT, James WD. Rosacea: I. Etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51(3):327–341. doi: 10.1016/j.jaad.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 23.Lazaridou E, Fotiadou C, Ziakas NG, Giannopoulou C, Apalla Z, Ioannides D. Clinical and laboratory study of ocular rosacea in northern Greece. J Eur Acad Dermatol Venereol. 2011;25(12):1428–1431. doi: 10.1111/j.1468-3083.2011.03995.x. [DOI] [PubMed] [Google Scholar]
- 24.Bakar O, Demircay Z, Toker E, Cakir S. Ocular signs, symptoms and tear function tests of papulopustular rosacea patients receiving azithromycin. J EurAcad Dermatol Venereol. 2009;23(5):544–549. doi: 10.1111/j.1468-3083.2009.03132.x. [DOI] [PubMed] [Google Scholar]
- 25.Vieira AC, An HJ, Ozcan S, Kim JH, Lebrilla CB, Mannis MJ. Glycomic analysis of tear and saliva in ocular rosacea patients: the search for a biomarker. Ocul Surf. 2012;10(3):184–192. doi: 10.1016/j.jtos.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghanem VC, Mehra N, Wong S, Mannis MJ. The prevalence of ocular signs in acne rosacea: comparing patients from ophthalmology and dermatology clinics. Cornea. 2003;22(3):230–233. doi: 10.1097/00003226-200304000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Yaylali V, Ozyurt C. Comparison of tear function tests and impression cytology with the ocular findings in acne rosacea. Eur J Ophthalmol. 2002;12(1):11–17. doi: 10.1177/112067210201200103. [DOI] [PubMed] [Google Scholar]
- 28.Baudouin C. A new approach for better comprehension of diseases of the ocularsurface. J Fr Ophtalmol. 2007;30(3):239–246. doi: 10.1016/s0181-5512(07)89584-2. [DOI] [PubMed] [Google Scholar]
- 29.Wladis EJ, Carlson JA, Wang MS, Bhoiwala DP, Adam AP. Toll-like receptors and vascular markers in ocular rosacea. Ophthal Plast Reconstr Surg. 2013;29(4):290–293. doi: 10.1097/IOP.0b013e318293764c. [DOI] [PubMed] [Google Scholar]
- 30.Turner K, Pflugfelder SC, Ji Z, Feuer WJ, Stern M, Reis BL. Interleukin-6 levels in the conjunctival epithelium of patients with dry eye disease treated with cyclosporine ophthalmic emulsion. Cornea. 2000;19(4):492–496. doi: 10.1097/00003226-200007000-00018. [DOI] [PubMed] [Google Scholar]
- 31.Perry HD, Doshi-Carnevale S, Donnenfeld ED, Solomon R, Biser SA, Bloom AH. Efficacy of commercially available topical cyclosporine A 0.05% in the treatment of meibomian gland dysfunction. Cornea. 2006;25(2):171–175. doi: 10.1097/01.ico.0000176611.88579.0a. [DOI] [PubMed] [Google Scholar]
- 32.Schechter BA, Katz RS, Friedman LS. Efficacy of topical cyclosporine for the treatment of ocular rosacea. Adv Ther. 2009;26(6):651–659. doi: 10.1007/s12325-009-0037-2. [DOI] [PubMed] [Google Scholar]
- 33.Fallen RS, Gooderham M. Rosacea: update on management and emerging therapies. Skin Therapy Lett. 2012;17(10):1–4. [PubMed] [Google Scholar]
- 34.Valentín S, Morales A, Sánchez JL, Rivera A. Safety and efficacy of doxycycline in the treatment of rosacea. Clin Cosmet Investig Dermatol. 2009;2:129–140. doi: 10.2147/ccid.s4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Korting HC, Schöllmann C. Tetracycline actions relevant to rosacea treatment. Skin Pharmacol Physiol. 2009;22(6):287–294. doi: 10.1159/000235550. [DOI] [PubMed] [Google Scholar]