Abstract
AIM
To review published clinical studies examining the effect of natamycin in the treatment of fungal keratitis.
METHODS
We selected the publications in CENTRAL, MEDLINE, EMBASE, CNKI, and CBM. This study systematically reviewed published randomized controlled trials (RCTs) that compared natamycin to other antifungal agents, and conducted feasible Meta-analysis of efficacy results using Revman 5.2 software.
RESULTS
We included seven trials which were mainly carried out in developing countries of Asia, with five trials conducted in India, one each in China and Bangladesh. A total of 804 participants were randomized to following comparisons: 2% econazole versus 5% natamycin showed little difference in the effects of treatment of fungal keratitis [RR=0.99, 95% confidence interval (CI), 0.8 to 1.21]; chlorhexidine gluconate versus 5% natamycin indicated that the results on healing of the ulcer at 21d was less conclusive (RR=0.77, 95% CI, 0.55 to 1.08; I2=0%); 1% voriconazole versus 5% natamycin suggested that natamycin treatment appeared to be significantly better outcomes than voriconazole (regression coefficient =-0.18 logMAR; 95% CI, -0.30 to -0.05; P=0.006), especially in Fusarium cases (regression coefficient=-0.41 logMAR; 95% CI, -0.61 to -0.20; P<0.001); natamycin versus fluconazole showed a significant difference in cure rate (χ2=5.048, P<0.05) and natamycin group was more effective than fluconazole in average period of therapy (t=7.94, P<0.01).
CONCLUSION
Natamycin was a preferable choice in the treatment of fungal keratitis, especially in the early period of Fusarium cases.
Keywords: eye infection, fungal, natamycin, Meta-analysis
INTRODUCTION
Fungal keratitis is a leading cause of blindness in corneal diseases, which is relatively common in warm climates and developing countries[1]–[3]. Recent reports suggest the prevalence for fungal ketatitis was increasing[4],[5]. A study reported that fungal ulcers as a serious public health problem in north China, in which the dominating pathogen was genus Fusarium (77.6%), and the second common pathogen was genus Aspergillus (10.8%)[6]. Moreover, both of Fusarium and Aspergillus were mostly sensitive to natamycin. Fungal keratitis results in severe visual impairment, and the treatment is more difficult than other corneal infections[7],[8]. The gold standard for the treatment of fungal keratitis has not been identified[9],[10], and the main management is antifungal agents involving topic antifungal drops such as natamycin and topical amphotericin B. For acute corneal perforation and visual rehabilitation, therapeutic penetrating keratoplasty is needed.
The antifungal agents used for treatment of fungal keratitis include three classes: polyenes, triazoles, and echinocandins. Natamycin is a tetraene polyene which has been regarded as the most important agent in the management of fungal keratitis. It acts by binding with ergosterol, which is an essential component in fungal cell wall, and blocks fungal growth. Natamycin is the only antifungal medication approved by U.S. Food and Drug Administration[11]. There were previous studies reporting the efficacy of natamycin and comparing it with other agents in management of fungal keratitis, but the results were not completely consistent. FlorCruz et al[9] reported that there is no evidence to date that any particular drug, or combination of drugs, is more effective in the treatment of fungal keratitis. Therefore, a systematic review of available reports will conduce to the evidence base, and we performed this Meta-analysis to assess the efficacy of natamycin in the treatment of fungal keratitis.
MATERIALS AND METHODS
Search Strategy
We searched the publications in CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (The Cochrane Library 2014, Issue 1), MEDLINE, EMBASE, CNKI (China National Knowledge Infrastructure), CBM (Chinese Biological Medicine Database), following the Cochrane's highly sensitive search strategy and used relevant keywords and medical subject heading (MeSH) terms, including “natamycin or pimaricin” and “eye infections, fungal” “antifungal agents”. We also handsearched the reference lists of identified trial reports and case reports for to find relevant articles. There were on language restrictions in the search for trials.
Trial Selection
Two reviewers (Qiu S and Wang X) independently scanned the titles and abstracts to exclude the trials which were obviously not conform to the inclusion criteria. Full text reports of the studies that definitely or possibly met the inclusion criteria were examined for further assessment. They cross checked into the results, and determined that whether the paper should be excluded or included by discussion or the third reviewer. We also contacted with the authors to perfect our data.
Inclusion Criteria
The inclusion criteria included: 1) type of studies: randomized controlled trials (RCTs) that compared efficacy of natamycin with control or other antifungal eye drop; 2) type of participants: all age patients with fungal keratitis diagnosed clinically or microbiologically, and we excluded the patients infected by mixed bacteria and fungi; 3) type of interventions: we considered studies using different concentrations of natamycin in the treatment of fungal keratitis. This included placebo controlled trials or trials comparing natamycin to other antifungal agents; 4) type of outcome measures: a) primary outcomes: best spectacle-corrected visual acuity (BSCVA) at 3mo; b) secondary outcomes: the time to be defined as a healed or healing ulcer; the safety of medication; complication including scar size, perforations; assessment and presence or absence of toxicity after treatment.
Assessment of Risk of Bias
The risk of bias in the included studies was assessed in accordance with Cochrane handbook. Two authors (Qiu S and Wang X) independently assessed the risk bias of studies and resolved the disagreement by discussion. Each bias domain listed in the Cochrane risk of bias tool was assessed and graded as “low risk of bias”, “high risk of bias” and “unclear”. We need to contact the authors for illustration of any parameter graded as unclear.
Data Extraction and Analysis
Two reviewers (Qiu S and Wang X) independently implemented the data extraction that met the inclusion criteria. The full texts of selected trials were read to determine whether they contained useful information. Any disagreement was resolved by discussion to reach a consensus among the investigators. The following data were collected from each study: 1) publication data: the first author's last name, year of publication, country of origin; 2) characteristics of the participants: the setting, sample size, gender, age; 3) interventions: natamycin, other antifungal agents, dose of medication, and administration route; 4) follow-up time; 5) outcome measurement: the number of healed or healing ulcers treated with natamycin or other agents, the number of other outcomes and the complications.
We used Review Manager 5.2 for Meta-analysis. We calculated a relative risk ratio for dichotomous data and the weighted mean difference for continuous data. We calculated the point estimate and confidence intervals (CIs) with a 95% CI for each result. We evaluated the statistical heterogeneity by Cochrane χ2 tests and qualified it by calculating the I2 statistic. If significant heterogeneity was observed between studies (I2>50%), a random-effects model was used to pool the data; otherwise, a fixed-effects model was used.
We considered to conducting a sensitivity analysis by excluding studies which were at high risk of bias in the protocol, but the current study does not include many more Meta-analysis so it was not done. If possible we will do further sensitivity analysis, so that we can judge the importance of review results to crucial decisions and assumptions that we have made during the review. Data analysis will be repeated with the following methods: exclusion of trials at high risk of bias; exclusion of unpublished studies; changing inclusion criteria of the studies, participants, interventions or outcome measures; reanalyzing the data using another statistical approach, such as using a random-effects model instead of a fixed-effects model.
RESULTS
Studies Selection
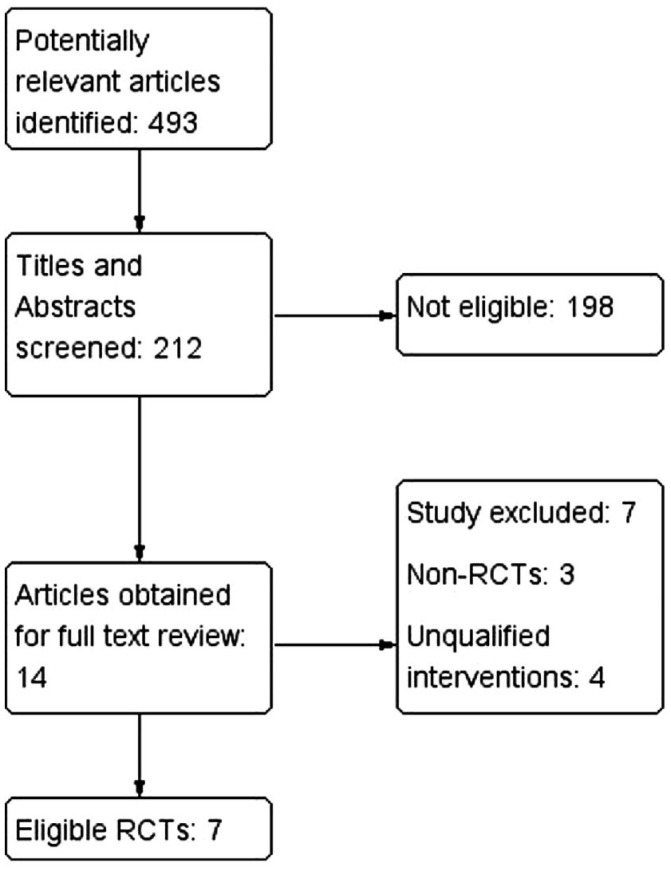
Figure 1 showed the selection of eligible studies. We identified 493 articles through primary literature search. Two hundred and twelve articles were selected to screen the abstract and titles. After that, 198 articles were excluded and 14 potential relevant articles were obtained for full text review. Finally, 7 eligible studies were included for the systematic review and Meta-analysis[11]–[17].
Figure 1. Flow diagram of selection process of articles for this Meta-analysis.
Study Characteristics
Table 1 summarizes the characteristic of the 7 included studies. A total of 804 patients with 804 eyes in 7 included trials were enrolled in this review. The baseline characteristics are summarized as follows. The countries of participants were mainly developing countries in Asia (5 in India[11]–[13],[15],[17], 1 each in China[14] and Bangladesh[16]). Sample size was range from 30-323 eyes. The mean age of participants was 43.49y, and 64% were male. The follow-up time was range from 21d to 3mo. All the trials tested the efficacy of natamycin by comparing with other antifungal drugs. Only 1 study tested 2.5% natamycin[16]. Natamycin regimen and duration were almostly once for 2h or 3h, only 1 trial using natamycin 7 times per day[14]. All the durations were up to the follow-up time (Tables 1, 2).
Table 1. Basic characteristics of included trials.
| Study (a) | Country | n | Mean age± SD (a) |
M/F |
||||
| NAT | Control | Overall | NAT | Control | Overall | |||
| Prajna et al[12] (2013) | India | 323 | NS | NS | NS | NS | NS | NS |
| Arora et al[11] (2011) | India | 30 | 37.93±15.14 | 48.47±3.53 | NS | 10/5 | 11/4 | 21/9 |
| Prajna et al[13] (2010) | India | 120 | 49.8±11.9 | 47.0±14.5 | NS | 42/18 | 37/23 | 79/41 |
| Wang et al[14] (2010) | China | 84 | 45.4±15.38 (20-66) | 46.7±15.56 (5-72) | NS | 23/19 | 22/20 | NS |
| Prajna et al[15] (2003) | India | 116 | NS | NS | 37.0±13.8 (7-84) | NS | NS | 72/44 |
| Rahman et al[16] (1998) | Bangla-desh | 71 | NS | NS | NS | 27/9 | 25/10 | 52/19 |
| Rahman et al[17] (1997) | India | 60 | 44.3±17.3 | 42.6±16.2 | NS | NS | NS | NS |
NS: Not specified in RCT; NAT: Natamycin.
Table 2. Administration of natamycin in included studies.
| Study (a) | Follow-up | Concentration of NAT(%) | NAT regimen and duration | Completion of follow-up (No. of patients) |
||
| NAT | Control | Overall | ||||
| Prajna et al[12] (2013) | 3mo | 5 | once/2h for 3mo | 141/162 | 143/161 | 284/323 |
| Arora et al[11] (2011) | 2mo | 5 | once/h for 2mo | 15/15 | 15/15 | 30/30 |
| Prajna et al[13] (2010) | 3mo | 5 | once/2h for 3mo | 56/60 | 53/60 | 109/120 |
| Wang et al[14] (2010) | 35d | 5 | 7 times/d for 35d | 42/42 | 42/42 | 84/84 |
| Prajna et al[15] (2003) | 1mo | 5 | once/2h for 1mo | 59/61 | 52/55 | 111/116 |
| Rahman et al[16] (1998) | 21d | 2.5 | once/3h for 21d | 27/36 | 26/35 | 53/71 |
| Rahman et al[17] (1997) | 21d | 5 | once/3h for 21d | 16/18 | 42/42 | 58/60 |
Quantitative Data Synthesis
Risk of bias in included randomized controlled trials
Figures 2 and 3 show the risk of bias assessment on included trials. For selection bias, 4 trials[12],[13],[16],[17] of 7 RCTs reported adequate methods of sequence generation and allocation concealment. It was always difficult for masking of participants. For performance and detection biases, only 2 studies[12],[13] reported adequate masking of participants, personnel and outcome assessment. For attrition bias, 5 trials[11]–[14],[17] of 7 had reasonably complete data and were judged as low risk. In the other studies, attrition bias was considered to be possible. In the included trials, reporting bias was not considered to be a major problem but it was always difficult to evaluate it sufficiently.
Figure 2. Risk of bias.
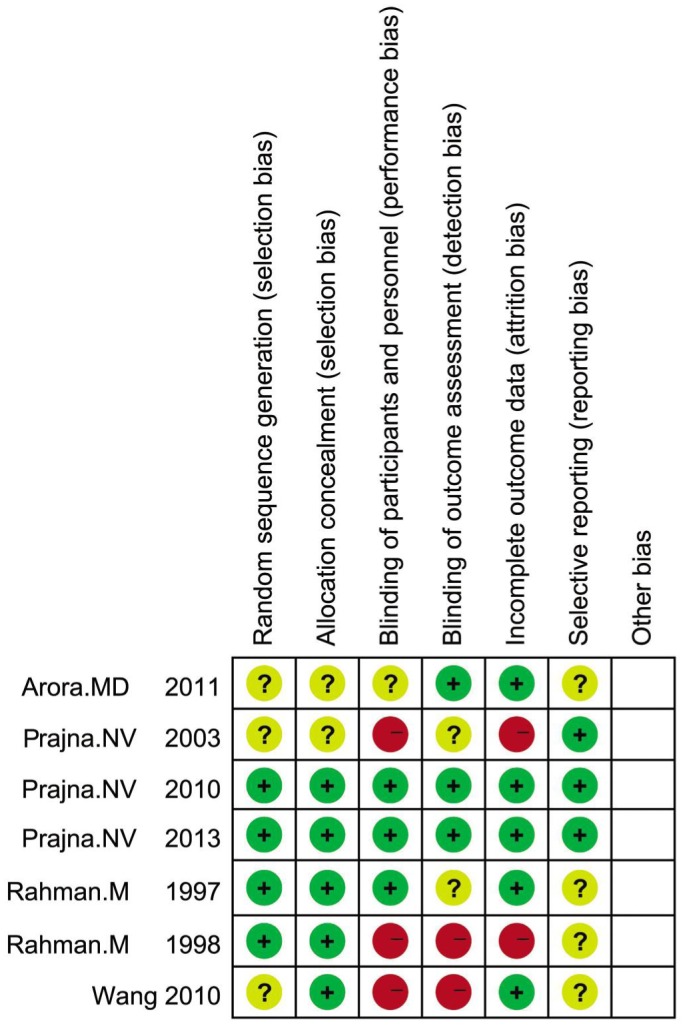
Each risk of bias item for each selected study.
Figure 3. Risk of bias.
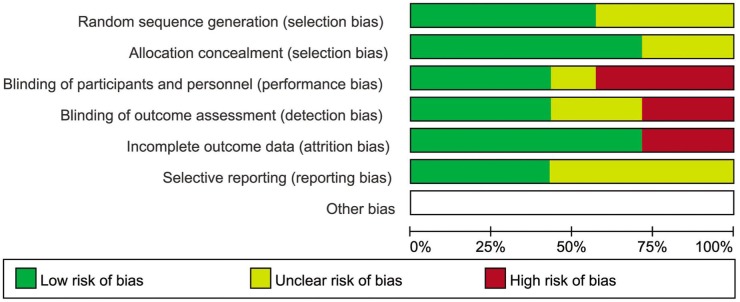
Each risk of bias event presented as percentages across all selected trials.
Outcome Measures
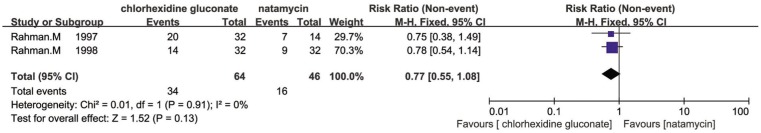
Seven trials reported the comparison of natamycin to different antifungal agents. Prajna et al[15] showed that there were little difference in the effects of econazole and natamycin which was published in 2003 (RR=0.99, 95% CI, 0.8 to 1.21). Two of seven trials carried out by the same investigator indicated that there was some evidence for a favourable effect of chlorhexidine gluconate compared to natamycin in response at five days (RR=0.45, 95% CI, 0.13 to 1.56; I2 =80%; Figure 4), the results on healing of the ulcer at 21d was less conclusive (RR=0.77, 95% CI, 0.55 to 1.08; I2 = 0%; Figure 5) [16],[17]. In studies of Arora et al[11] and Prajna et al [12],[13] there were no evidence for any difference between natamycin and voriconazole. However, the study of Arora et al[11] was rather small and it was impossible to combine the data of these studies because of differences in outcomes presented. In 2010, Prajna et al[13] found that people treated with voriconazole had a 1 line better best correct visual acuity compared to people treated with natamycin at three months, however, this difference was not statistically significant (P=0.29). Otherwise, Prajna et al [12] conducted another trial to compare natamycin with voriconazole in 2013, suggesting that natamycin treatment appeared to be significantly better clinical and microbiological outcomes than voriconazole in management of fungal keratitis (regression coefficient =-0.18 logMAR; 95% CI, -0.30 to -0.05; P=0.006), and much of the variance due to elevated results in Fusarium cases (regression coefficient =-0.41 logMAR; 95% CI, -0.61 to -0.20; P<0.001). Wang et al[14] found that natamycin was more effective than fluconazole in the management of fungal keratitis, and there was a significant difference between natamycin and fluconazole in cure rate (χ2 =5.048, P<0.05). Additionally, the natamycin group was more effective than fluconazole in average period of therapy (t=7.94, P<0.01).
Figure 4. Comparison of the response of chlorhexidine gluconate and natamycin in management of fungal keratitis at five days.
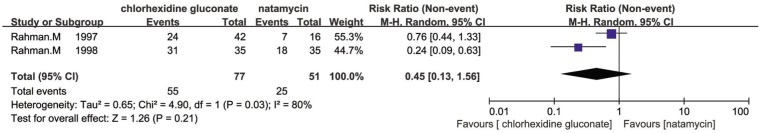
Figure 5. Comparison of the effect of chlorhexidine gluconate and natamycin in management of fungal keratitis on corneal ulcer healed at 21d.
Prajna et al[12] concluded that natamycin-treated cases had less likely to have perforation or require therapeutic penetrating keratoplasty than voriconazole-treated cases in the study which was published in 2013 (OR=0.42; 95% CI, 0.22 to 0.80; P=0.009),but did not describe the adverse reaction of drugs in details, so as to his two other trials[13],[15]. No adverse reactions to study antifungal agents were noted in Arora et al's[11] trial. There was no descriptions of significant systemic or ocular adverse reactions from natamycin and chlorhexidine gluconate groups, but a case of temporary punctate epitheliopathy was reported in one patient receiving chlorhexidine gluconate due to frequent application of the drops[16],[17]. No early cataract formation for participants treated with chlorhexidine gluconate and natamycin was recorded at six months to one year after treatment. There were no systematic mild side effects observed in the trial of Wang et al[14] which compared natamycin to fluconazole.
DISCUSSION
Meta-analysis attempts to analyze and combine the results of previous reports[18].This systematic review provided a critical overview of previous clinical reports and combined effect measures of natamycin in multiple small clinical trials to increase statistical power. It included seven trials comparing natamycin to different antifungal drugs for the treatment of fungal keratitis. All trials were implemented in developing countries because of the higher incidence than developed countries. There are still no large multicentre randomised trials to assess the efficacy of natamycin on the treatment of fungal keratitis.
Four antifungal agents, namely, voriconazole, econazole, fluconazole and chlorhexidine gluconate were comparing to natamycin. Former three drugs are conventional agents for fungal infection. They are triazoles and act by inhibiting the biosynthesis of ergosterol, which natamycin binds with. Natamycin has been considered to be mainstay for treatment of filamentous fungal keratitis[19]. Natamycin is the only commercial antifungal drug in ophthalmic form and is expensive[9],[20],[21]. Since fungal keratitis often occurs in developing countries, natamycin performs limited availability although natamycin is offered as a service drug[22]. There were a few clinical trials have been done on natamycin to evaluate the efficacy by comparing to other agents.
There is no significant evidence suggesting that natamycin is more effective than other antifungal agents for the treatment of fungal keratitis in terms of the seven trials included in this review. The evidence supporting natamycin as a gold standard for the treatment of fungal keratitis seemed to be weak. The reasons were that other effective antifungal agents such as amphotericin B[7],[23],[24], itraconazole[25], miconazole and sliver sulphadiazine[26]–[28] have not yet been compared in a large scale RCTs, and the trials compared natamycin to different antifungal agents with different outcome measures. So it was inadvisable to put the data together, and we did not pool all the results since there was no large scale trial for natamycin comparing to particular agent. However, natamycin has been regarded as the first line in the management of fungal keratitis[29]. A few researches had reported that natamycin was significantly more effective than voriconazole or fluconazole in management of fungal keratitis. Participants exposed to natamycin had preferable 3mo BSCVA or higher cure rate, particularly in Fusarium cases.
In addition to conventional route of administration, such as local eye drops, oral, intravenous injection, eye ointment, there has been subconjunctival injection and injection in corneal stroma for treating fungal keratitis. The main treatment of fungal keratitis is using antifungal eye drops. Systematic use of agents possesses serious side effects. Natamycin poorly penetrates into the aqueous and is unable to achieve therapeutic levels by intravenous injection and subconjunctival injection. Culture and sensitivity results play a vital role for conducting medical therapy for infectious diseases, but fungi grow slower than other pathogens[30]. Therefore, we need to apply antifungal drugs as soon as discovering fungal elements on the examination such as smear, confocal microscopy and so on[31]–[33]. So we need to find a more effective agent to be administered in the early period of fungal keratitis. However, the drugs used in the current RCTs were different, and the evidence that verified which agent was more effective for fungal keratitis was weak due to the small sample size. In our review, we found that natamycin was useful than some other antifungal agents like voriconazole and fluconazole. So we recommend that natamycin is more favorable applying in the early period of fungal keratitis.
In summary, natamycin was a preferable choice in the treatment of fungal keratitis, especially in the Fusarium cases. Importantly, further RCTs with large sample size are needed and the search for more effective and cheaper interventions for fungal keratitis would be necessary.
Acknowledgments
Foundations: Supported by National Natural Science Foundation of China (No.81170825; No.81470609); Shandong Province Natural Science Foundation (No. ZR2013HQ007; No. ZR2012HZ001); the Specialized Research Fund for the Doctoral Program of Higher Education, 2012 (No.20123706110003).
Conflicts of Interest: Qiu S, None; Zhao GQ, None; Lin J, None; Wang X, None; Hu LT, None; Du ZD, None; Wang Q, None; Zhu CC, None.
REFERENCES
- 1.Hu LT, Du ZD, Zhao GQ, Jiang N, Lin J, Wang Q, Xu Q, Cong L, Qiu S. Role of TREM-1 in response to Aspergillus fumigatus infection in corneal epithelial cells. Int Immunopharmacol. 2014;23(1):288–293. doi: 10.1016/j.intimp.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Shi WY, Wang T, Xie LX, Li SX, Gao H, Liu JC, Li HP. Risk Factors, clinical features, and outcomes of recurrent fungal keratitis after corneal transplantation. Ophthalmology. 2010;117:890–896. doi: 10.1016/j.ophtha.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Day S, Lalitha P, Haug S, Fothergill AW, Cevallos V, Vijayakumar R, Prajna NV, Acharya NR, McLeod SD, Lietman TM. Activity of antibiotics against Fusarium and Aspergillus. Br J Ophthalmol. 2009;93(1):116–119. doi: 10.1136/bjo.2008.142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alfonso EC, Cantu-Dibildox J, Munir WM, Miller D, O'Brien TP, Karp CL, Yoo SH, Forster RK, Culbertson WW, Donaldson K, Rodila J, Lee Y. Insurgence of Fusarium keratitis associated with contact lens wear. Arch Ophthalmol. 2006;124(7):941–947. doi: 10.1001/archopht.124.7.ecs60039. [DOI] [PubMed] [Google Scholar]
- 5.Xie L, Zhong W, Shi W, Sun S. Spectrum of fungal keratitis in north China. Ophthalmology. 2006;113(11):1943–1948. doi: 10.1016/j.ophtha.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 6.Xie L, Zhai H, Zhao J, Sun S, Shi W, Dong X. Antifungal susceptibility for common pathogens of fungal keratitis in Shandong Province, China. Am J Ophthalmol. 2008;146(2):260–265. doi: 10.1016/j.ajo.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 7.Mellado F, Rojas T, Cumsille C. Fungal keratitis: review of diagnosis and treatment. Arq Bras Oftalmol. 2013;76(1):52–56. doi: 10.1590/s0004-27492013000100016. [DOI] [PubMed] [Google Scholar]
- 8.Srinivasan M. Fungal keratitis. Curr Opin Ophthalmol. 2004;15(4):321–327. doi: 10.1097/00055735-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 9.FlorCruz NV, Peczon IV, Evans JR. Medical interventions for fungal keratitis. Cochrane Database Syst Rev. 2012;2(1):CD004241. doi: 10.1002/14651858.CD004241.pub3. [DOI] [PubMed] [Google Scholar]
- 10.Florcruz NV, Peczon I., Jr Medical interventions for fungal keratitis. Cochrane Database Syst Rev. 2008;(1):CD004241. doi: 10.1002/14651858.CD004241.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Arora R, Gupta D, Goyal J, Kaur R. Voriconazole versus natamycin as primary treatment in fungal corneal ulcers. Clin Experiment Ophthalmol. 2011;39(5):434–440. doi: 10.1111/j.1442-9071.2010.02473.x. [DOI] [PubMed] [Google Scholar]
- 12.Prajna NV, Krishnan T, Mascarenhas J, Rajaraman R, Prajna L, Srinivasan M, Raghavan A, Oldenburg CE, Ray KJ, Zegans ME, McLeod SD, Porco TC, Acharya NR, Lietman TM, Mycotic Ulcer Treatment Trial G The mycotic ulcer treatment trial: a randomized trial comparing natamycin vs voriconazole. JAMA Ophthalmol. 2013;131(4):422–429. doi: 10.1001/jamaophthalmol.2013.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prajna NV, Mascarenhas J, Krishnan T, Reddy PR, Prajna L, Srinivasan M, Vaitilingam CM, Hong KC, Lee SM, McLeod SD, Zegans ME, Porco TC, Lietman TM, Acharya NR. Comparison of natamycin and voriconazole for the treatment of fungal keratitis. Arch Ophthalmol. 2010;128(6):672–678. doi: 10.1001/archophthalmol.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang DM, Chen GS, Huang MH. Observation of therapeutical effect of natamyc in fungal corneal ulcer. Guoji Yanke Zazhi ( Int Eye Sci) 2010;10(4):744–745. [Google Scholar]
- 15.Prajna NV, John RK, Nirmalan PK, Lalitha P, Srinivasan M. A randomised clinical trial comparing 2% econazole and 5% natamycin for the treatment of fungal keratitis. Br J Ophthalmol. 2003;87(10):1235–1237. doi: 10.1136/bjo.87.10.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rahman MR, Johnson GJ, Husain R, Howlader SA, Minassian DC. Randomised trial of 0.2% chlorhexidine gluconate and 2.5% natamycin for fungal keratitis in Bangladesh. Br J Ophthalmol. 1998;82(8):919–925. doi: 10.1136/bjo.82.8.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahman MR, Minassian DC, Srinivasan M, Martin MJ, Johnson GJ. Trial of chlorhexidine gluconate for fungal corneal ulcers. Ophthalmic Epidemiol. 1997;4(3):141–149. doi: 10.3109/09286589709115721. [DOI] [PubMed] [Google Scholar]
- 18.Sacks HS, Berrier J, Reitman D, Ancona-Berk VA, Chalmers TC. Meta-analyses of randomized controlled trials. N Engl J Med. 1987;316(8):450–455. doi: 10.1056/NEJM198702193160806. [DOI] [PubMed] [Google Scholar]
- 19.Lalitha P, Vijaykumar R, Prajna NV, Fothergill AW. In vitro natamycin susceptibility of ocular isolates of Fusarium and Aspergillus species: comparison of commercially formulated natamycin eye drops to pharmaceutical-grade powder. J Clin Microbiol. 2008;46(10):3477–3478. doi: 10.1128/JCM.00610-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neoh CF, Daniell M, Chen SC, Stewart K, Kong DC. Clinical utility of caspofungin eye drops in fungal keratitis. Int J Antimicrob Agents. 2014;44(2):96–104. doi: 10.1016/j.ijantimicag.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 21.El-Mofty HM, Abdelhakim MA, El-Miligi MF, El-Nabarawi MA, Khalil IA. A new combination formula for treatment of fungal keratitis: an experimental study. J Ophthalmol. 2014;2014(3):173298. doi: 10.1155/2014/173298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mimouni M, Tam G, Paitan Y, Kidron D, Segev F. Safety and efficacy of intrastromal injection of 5% natamycin in experimental Fusarium keratitis. J Ocul Pharmacol Ther. 2014;30(7):543–547. doi: 10.1089/jop.2014.0004. [DOI] [PubMed] [Google Scholar]
- 23.Lai J, Pandya V, McDonald R, Sutton G. Management of Fusarium keratitis and its associated fungal iris nodule with intracameral voriconazole and amphotericin B. Clin Exp Optom. 2014;97(2):181–183. doi: 10.1111/cxo.12091. [DOI] [PubMed] [Google Scholar]
- 24.Mahdy RA, Nada WM, Wageh MM, Kader MA, Saleh MM, Alswad MM. Assessment safety and efficacy of a combination therapy of topical amphotericin B and subconjunctival fluconazole for the treatment of fungal keratitis. Cutan Ocul Toxicol. 2010;29(3):193–197. doi: 10.3109/15569521003801454. [DOI] [PubMed] [Google Scholar]
- 25.Agarwal PK, Roy P, Das A, Banerjee A, Maity PK, Banerjee AR. Efficacy of topical and systemic itraconazole as a broad-spectrum antifungal agent in mycotic corneal ulcer. A preliminary study. Indian J Ophthalmol. 2001;49(3):173–176. [PubMed] [Google Scholar]
- 26.Mohan M, Gupta SK, Kalra VK, Vajpayee RB, Sachdev MS. Silver sulphadiazine in the treatment of mycotic keratitis. Indian J Med Res. 1987;85(1):572–575. [PubMed] [Google Scholar]
- 27.Mohan M, Gupta SK, Kalra VK, Vajpayee RB, Sachdev MS. Silver sulphadiazine-a new drug for keratomycosis. Indian J Ophthalmol. 1987;35(5–6):83–87. [PubMed] [Google Scholar]
- 28.Mohan M, Gupta SK, Kalra VK, Vajpayee RB, Sachdev MS. Topical silver sulphadiazine-a new drug for ocular keratomycosis. Br J Ophthalmol. 1988;72(3):192–195. doi: 10.1136/bjo.72.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rasool BK, Salmo HM. Development and clinical evaluation of clotrimazole-beta-cyclodextrin eyedrops for the treatment of fungal keratitis. AAPS PharmSciTech. 2012;13(3):883–889. doi: 10.1208/s12249-012-9813-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylan Sekeroglu H, Erdem E, Yagmur M, Gumral R, Ersoz R, Ilkit M, Harbiyeli II. Successful medical management of recalcitrant Fusarium solani keratitis: molecular identification and susceptibility patterns. Mycopathologia. 2012;174(3):233–237. doi: 10.1007/s11046-012-9542-y. [DOI] [PubMed] [Google Scholar]
- 31.Liang QF, Sun XG, Labbe A. Role of in vivo confocal microscopy in the management of infectious keratitis. Zhonghua Yan Ke Za Zhi. 2013;49(10):951–955. [PubMed] [Google Scholar]
- 32.Thomas PA, Kaliamurthy J. Mycotic keratitis: epidemiology, diagnosis and management. Clin Microbiol Infect. 2013;19(3):210–220. doi: 10.1111/1469-0691.12126. [DOI] [PubMed] [Google Scholar]
- 33.Garg P. Fungal, Mycobacterial, and Nocardia infections and the eye: an update. Eye (Lond) 2012;26(2):245–51. doi: 10.1038/eye.2011.332. [DOI] [PMC free article] [PubMed] [Google Scholar]