Abstract
Neuronal signals related to visual attention are found in widespread brain regions, and these signals are generally assumed to participate in a common mechanism of attention. However, the behavioral effects of attention in detection can be separated into two distinct components: spatially selective shifts in either the criterion or sensitivity of the subject. Here we show that a paradigm used by many single-neuron studies of attention conflates behavioral changes in the subject’s criterion and sensitivity. Then, using a task designed to dissociate these two components, we found that multiple aspects of attention-related neuronal modulations in area V4 of monkey visual cortex corresponded to behavioral shifts in sensitivity but not criterion. This result suggests that separate components of attention are associated with signals in different brain regions, and that attention is not a unitary process in the brain but instead consists of distinct neurobiological mechanisms.
INTRODUCTION
Attending to a location in a visual scene enhances behavioral performance there even when gaze is directed elsewhere (Posner et al., 1980; Carrasco, 2011). At the attended location, subjects detect target stimuli more readily and respond with shorter delays. These improvements in detection could depend on either of two components: a more lenient criterion for detecting targets, or higher sensitivity at discriminating targets from nontargets. Lowering the criterion for the visual location where a target is expected results in more targets being detected at that location. Enhancing the sensitivity of discrimination between targets and nontargets at a location also increases the frequency of target detection at that location.
Many psychophysical studies have used signal detection theory (Green and Swets, 1966), a statistical model of perceptual decisions, to measure how a subject’s criterion and sensitivity differ between the attended and unattended locations (Bashinski and Bacharach, 1980; Müller and Findlay, 1987; Downing, 1988; Hawkins et al., 1990; Müller and Humphreys, 1991; Kinchla, 1992; Wyart et al. 2012). These studies found that subjects can shift either their criterion or sensitivity at the attended location relative to the unattended location. When it is adaptive to do so, subjects often modulate both to improve their performance. Thus, spatially selective changes in both criterion and sensitivity contribute to the behavioral enhancement in detection associated with attention. Moreover, like sensitivity shifts, criterion changes could also depend on perceptual mechanisms (White et al., 2012; Ferrera et al., 2009). Thus, here we refer to spatially specific shifts in criterion and sensitivity as components of attention.
Neuronal signals related to visual attention have been found in many brain regions, including the cerebral cortex, thalamus, and brainstem (Desimone and Duncan, 1995). These widespread signals are generally thought to participate in a unitary mechanism of attention. However, attention is associated with distinguishable perceptual and behavioral phenomena (Carrasco, 2011), and it has not been investigated whether the attention-related signals in any of these brain structures reflect the same or distinct components of attention. In particular, it is unknown how behavioral changes in criterion and sensitivity are related to neuronal signals associated with attention.
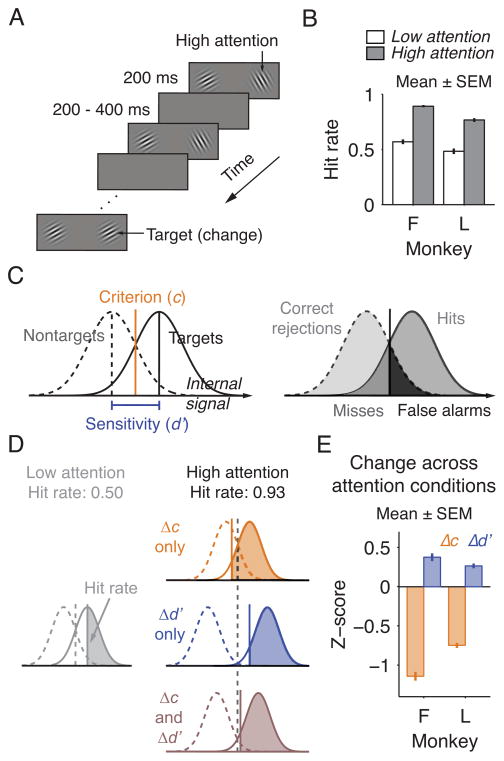
Many single-neuron studies of attention use a paradigm introduced by Posner and colleagues (Posner et al., 1980). Variants of this paradigm have been used to investigate attention in visual cortex (Reynolds et al., 2000; Cohen and Maunsell, 2009), parietal cortex (Herrington and Assad, 2001), prefrontal cortex (Armstrong et al., 2009), superior colliculus (Robinson and Kertzman, 1995), and thalamus (Petersen et al., 1987), as well as the relationship between the attention-related signals in different structures (Gregoriou et al., 2009; Zénon and Krauzlis, 2012). In this paradigm, the subject has to detect a target that appears at one of two locations (Figure 1A). More attention is directed to the location where the target appears more frequently or is rewarded more highly. Appropriate allocation of attention is often ascertained by a higher target detection rate (hit rate) at the attended location (Figure 1B). However, any improvements in hit rate could depend on a change in only criterion, only sensitivity, or both. This ambiguity is apparent when behavior is analyzed using signal detection theory (Figure 1C–D; criterion and sensitivity are indexed as criterion location (c) and d′, respectively). Because of the ambiguity in the behavior, the neuronal modulations attributed to attention in these studies could reflect shifts in the subject’s criterion or sensitivity. Thus, it is uncertain whether the neuronal signals associated with attention in any brain area correspond to changes in one or both components of attention.
Figure 1. Behavioral Improvement in a Typical Attention Task Conflates Changes in Criterion and Sensitivity.
(A) Standard attention task. The subject has to detect a target (orientation change) that occurred at either of two stimulus locations. In alternating blocks of trials, the subject directed more attention to one of two locations. (B) Monkeys detected targets more frequently at the high attention location. (C) In the signal detection model, each stimulus evokes a noisy internal signal. If the signal is stronger than the criterion (c), the stimulus is reported as a target. The distributions of signals evoked by the target and by the nontarget overlap, and the separation between these two distributions is indexed as sensitivity (d′). The response to each stimulus is categorized as a hit, miss, false alarm, or correct rejection, and these responses are used to calculate c and d′. (D) Any improvement in hit rate could be due to changes in only criterion (Δc), only sensitivity (Δd′), or both (Δc and Δd′). (E) Monkeys changed both criterion and sensitivity between attention conditions. (B, E) Monkey F, n = 65 sessions; monkey L, n = 50.
The presence of attention-related signals in widespread brain structures and the heterogeneity of the behavioral changes associated with attention suggest that each of these brain structures mediates a distinct component of attention. Investigating this possibility would provide insight into whether attention is a monolithic brain process or depends on distinguishable neurobiological mechanisms. Here we examine whether the neuronal mechanisms of attention in visual cortex are associated with behavioral changes in criterion or sensitivity. We focused on area V4, a region with reliable attention-related signals (e.g. Cohen and Maunsell, 2009), as well as modulation by visual target selection (Chelazzi et al., 2001) and contextual modulation unrelated to the neuron’s sensory selectivity (Ferrera et al., 1994). The extrasensory signals in V4 suggest that the previously observed attention-related modulation may be related to behavioral shifts in either criterion or sensitivity, or both.
RESULTS
In a preliminary experiment, we examined how two monkeys (F and L) changed their criterion and sensitivity in a task of the sort commonly used in neurophysiological studies of attention (“standard attention task”; Figure 1A). Both monkeys performed with a lower criterion and higher sensitivity at the attended location relative to the unattended location (Figure 1E). Criterion and sensitivity both changed regardless of whether attention was directed using higher target probability, larger reward size, or both (Figure S1). Criterion changes accounted for most of the behavioral improvement (Figure S2). These results indicate that although attention-related modulations in neuronal activity in visual cortex are frequently described as related to behavioral changes in sensitivity (e.g. Reynolds et al., 2000; Cohen and Maunsell, 2009), the omission to examine for shifts in criterion or sensitivity means that the neuronal modulations might have reflected either. This uncertainty exists not only for tasks like the one used here, where targets occur at the unattended location (e.g. Cohen and Maunsell, 2009), but also for tasks where animals are trained never to respond to targets at the unattended location (e.g., Reynolds et al., 2000; Zénon and Krauzlis, 2012).
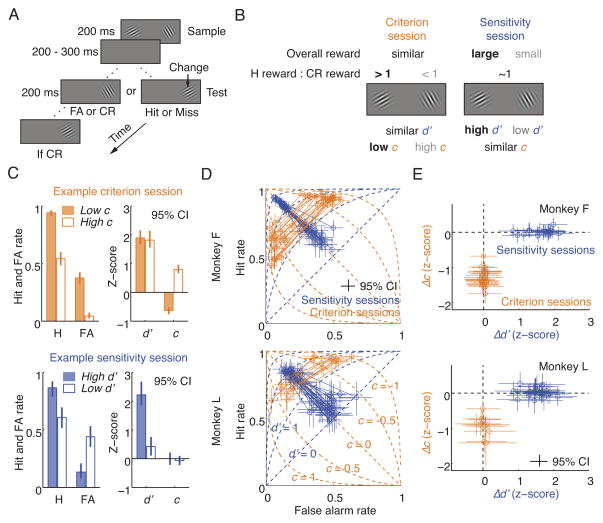
To more accurately characterize the neuronal signals associated with attention, we designed a task to dissociate changes in criterion and sensitivity (“dissociation task”; Figure 2A). In each trial, two stimuli (“samples”) appeared concurrently for a brief time. After a short delay, a single stimulus (“test”) appeared at one of the two sample locations, selected at random. The monkey had to saccade to the test if it differed in orientation from the sample at the same location. If not, the monkey had to wait and saccade to a second test stimulus that always differed from the sample. The response to the first test stimulus in each trial was categorized as a hit, miss, false alarm, or correct rejection, and these responses were used to compute c and d′.
Figure 2. Dissociation task.
(A) Monkeys detected a target (orientation change) that occurred on either the first or the second test stimulus. Behavioral responses to the first test stimulus were categorized as hits (H), misses (M), correct rejections (CR), or false alarms (FA). (B) Reward manipulations to isolate spatially selective changes in criterion (c) and sensitivity (d′). (C) A criterion session of monkey F and a sensitivity session of monkey L. (D) All sessions. Each circle is the behavior in one task condition from one daily session, and a solid line connects the two conditions of each session. Dashed lines are isocriterion and isosensitivity lines. (E) Differences in criterion and sensitivity between the two task conditions of each session. Same data as panel D. (C–E) Error bars represent 95% confidence intervals. (D–E) Monkey F: 22 criterion and 22 sensitivity sessions. Monkey L: 10 criterion and 25 sensitivity sessions.
As in other neurophysiological experiments, we controlled attention by manipulating reward contingencies, but here with additional refinements to control the subject’s criterion and sensitivity (Figure 2B; Figure S3A; Experimental Procedures). The relative reward between hits and correct rejections was manipulated independently at each stimulus location to control criterion for that location. The relative overall reward between the two locations was used to control the difference in sensitivity between locations. These reward parameters were varied between two task conditions of each daily session to isolate a change in either criterion (in “criterion sessions”) or sensitivity (in “sensitivity sessions”) (Figure 2C). These isolated behavioral changes were spatially selective and unrelated to the global changes due to arousal.
We trained the same two monkeys on this task and achieved precise behavioral dissociation in more than 90% of sessions (Figure 2D–E, Figure S3B–C). To our knowledge, this is the first demonstration of consistent, precise separation of spatially specific changes in criterion and sensitivity. We then implanted an array of microelectrodes in each animal’s area V4 and measured how neuronal responses are modulated as the animal shifted either its criterion or sensitivity.
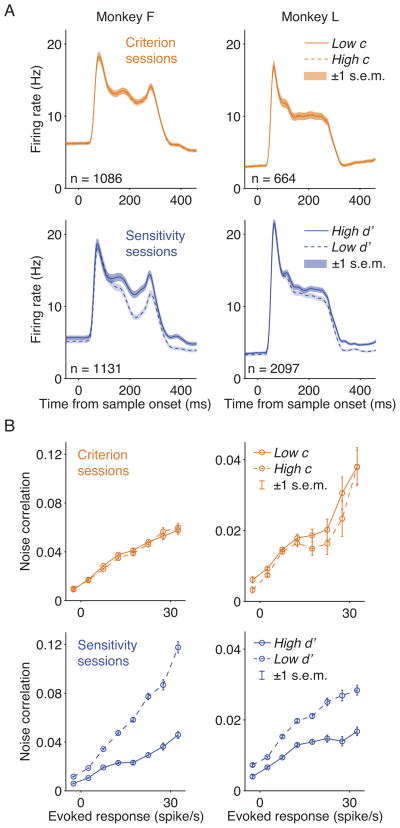
Because criterion changes accounted for most of the behavioral improvements in the standard attention task (Figure S2), we expected attention-related modulations in V4 to be primarily associated with shifts in criterion. But when we isolated changes in criterion and sensitivity, we found that attention-related changes corresponded to changes in sensitivity and not criterion (Figure 3; Figure S4). In sensitivity sessions, neuronal responses were stronger in the high d′ condition than in the low d′ condition, but in criterion sessions, responses were similar between low c and high c conditions despite large behavioral changes in criterion. To quantify the difference in neuronal responses between the two task conditions of each session, we calculated a modulation index using responses to the sample stimulus (firing rates 60 ms to 260 ms after sample onset; Supplemental Experimental Procedures). Modulation indices differed significantly from zero in sensitivity sessions but not in criterion sessions, and modulation indices from sensitivity sessions were significantly larger than indices from criterion sessions (Table 1).
Figure 3. Neuronal Modulations in V4 Correspond to Changes in Sensitivity but not Criterion.
Data are from the same sessions in Figure 3D–E and Table 1. (A) Peristimulus histograms showing the population response to the sample stimuli. Histograms used 1-ms bins and were smoothed with a Gaussian filter (σ = 5 ms). Responses were modulated by changes in sensitivity but not in criterion. (B) Noise correlations between pairs of simultaneously recorded neurons binned by the geometric mean of their evoked responses. Noise correlations were reduced when behavioral sensitivity increased, but were unaffected by shifts in criterion. The y-axis scaling differs for monkeys F and L.
Table 1.
Modulation Indices of Attention-Related Neuronal Changes.
| Modulation Index | Criterion sessions (Δc)
|
Sensitivity sessions (Δd′)
|
Criterion sessions vs. sensitivity sessions
|
|||
|---|---|---|---|---|---|---|
| Monkey F (n = 22) | Monkey L (n = 10) | Monkey F (n = 22) | Monkey L (n = 25) | Monkey F | Monkey L | |
| Firing rate (Sample stimulus) | 0.006 ± 0.005 p < 0.31 |
0.002 ± 0.007 p < 0.78 |
0.060 ± 0.004 p < 10−11 |
0.028 ± 0.003 p < 10−8 |
p < 10−8 | p < 10−3 |
| Firing rate (Delay period) | 0.009 ± 0.005 p < 0.09 |
0.004 ± 0.012 p < 0.75 |
0.109 ± 0.006 p < 10−13 |
0.078 ± 0.005 p < 10−13 |
p < 10−14 | p < 10−7 |
| Noise correlation | 0.040 ± 0.026 p < 0.13 |
0.057 ± 0.056 p < 0.34 |
−0.295 ± 0.020 p < 10−11 |
−0.198 ± 0.030 p < 10−6 |
p < 10−12 | p < 10−3 |
| Fano factor | 0.002 ± 0.004 p < 0.68 |
0.007 ± 0.014 p < 0.62 |
−0.043 ± 0.010 p < 10−3 |
−0.019 ± 0.004 p < 10−4 |
p < 10−3 | p < 0.02 |
Each of the four columns to the left reports the mean ± SEM aross sessions and the probability that the indices have a mean 0 (t-test). The remaining two columns indicate the probability that the modulation indices from the two types of sessions have the same mean (paired t-test). A single modulation index was computed for each session. A positive index for a sensitivity session indicates a higher measure (e.g. firing rates) in the high d′ task condition, and a positive index for a criterion session reflects a higher measure in the low c condition. Indices were computed using both correct and error trials, but results are highly similar if only correct trials were used.
We also analyzed the firing rates during the delay period between the sample and the first test stimulus (60 ms to 260 ms after sample offset). Similar to responses to the sample stimulus, firing rates during the delay were stronger in conditions of higher d′, and there was no detectable modulation by criterion changes (Table 1). We also found that the modulation by sensitivity was stronger during the delay than during the sample stimulus period (91% and 290% larger and p < 10−6 and p < 10−10, t-test, for monkeys F and L, respectively). Despite the stronger firing rate modulation associated with sensitivity changes during the delay epoch, there was no detectable modulation associated with criterion changes.
We next examined two other neuronal correlates of attention in visual cortex. Attention is associated with a modest decrease in the trial-to-trial variability in the responses of individual neurons, measured as the Fano factor (Mitchell et al., 2007), and a large reduction in the correlated variability in pairs of neurons, measured as noise correlation (Cohen and Maunsell, 2009; Mitchell et al., 2009). Fano factor and noise correlation were calculated using the sample period. Reduction in both Fano factor and noise correlation both corresponded to enhancement in sensitivity but not shifts in criterion (Figure 3B and Table 1). Taken together with the observations on firing rates, these results indicate that multiple aspects of attention-related modulation of V4 neuronal activity all correspond to shifts in sensitivity but not criterion.
DISCUSSION
Accurate detection of a signal requires proper spatial distribution of criterion and sensitivity. For example, a radar operator needs to adjust his or her criterion for where a signal is expected and where a successful detection is more important than a correct rejection. Sensitivity needs to be focused to where successful detections and rejections have the greatest overall importance. Failure to optimize either criterion or sensitivity undermines performance.
Here we show that these two distinct components of attention are conflated in a paradigm used by many single-neuron studies of attention. Using a task designed to dissociate these two components, we found that the neuronal mechanisms of attention in area V4 of visual cortex corresponded to shifts in sensitivity but not criterion. This result shows that spatially selective criterion changes must be mediated by brain structures separate from V4, and perhaps outside of visual cortex. Furthermore, this result indicates that separate brain regions support distinct components of attention, and suggests that attention depends on multiple neurobiological mechanisms.
Task Difficulty
Because the magnitude of attention-related modulation of firing rates in V4 is larger for tasks of greater difficulty (Boudreau et al., 2006), the modulations related to sensitivity shifts would likely be larger in a more difficult task. A more challenging task might also reveal modulation associated with criterion changes, which we did not detect here. But even if criterion-related modulation were found in a more difficult task, it is likely to be much smaller than the sensitivity-related modulation in the same task, and hence, V4 modulations would still be dominated by behavioral changes in sensitivity and not criterion. In the task used here, the firing rate modulation related to criterion changes was ten-fold smaller than the modulation related to sensitivity changes (Table 1). Even if V4 modulation related to criterion shifts were revealed in a more difficult task, it is unlikely that V4 contributes substantially to the animal’s changes in criterion.
Neural mechanisms of criterion and sensitivity
While criterion is generally formulated as a post-perceptual process in signal detection theory (Green and Swets, 1966; Macmillan and Creelman, 2004), a subject’s criterion can depend on perceptual as well as decisional and motor processes. For example, neuronal signals related to whether a visual stimulus is a target or nontarget are observed in V4 and other areas of the ventral visual pathway (Chelazzi et al., 2001; Pagan et al., 2013). A simple perceptual mechanism of criterion shifts could be to selectively control the gain of these signals for different spatial locations. However, the results here suggest that such signals in V4 are unlikely to support behavioral shifts in criterion.
Spatial shifts of sensitivity are likely to be mediated by sensory regions of cerebral cortex, but the structures mediating criterion changes are less clear. It is possible that criterion shifts are associated with subcortical structures, such as superior colliculus. If so, this dichotomy would explain a puzzling result from pharmacological inhibition of superior colliculus (Zénon and Krauzlis, 2013). During collicular inactivation, monkeys showed behavioral deficits in attention, but neuronal modulations related to attention were intact in visual cortex. This result was unexpected because the behavioral deficits from collicular inactivation were thought to arise from perturbation of cortical modulations. But this result would be expected if cortex and colliculus contribute to distinct components of attention. In that case, the behavioral impairment due to collicular inhibition could be explained by a perturbation of the animal’s criterion. A different study has shown that inactivation of colliculus within a given attention condition changed monkeys’ criterion but not sensitivity (McPeek and Keller, 2004). These observations make it possible that shifts in criterion are associated with neuronal modulations in the colliculus.
Attention as an aggregate process
Attention is associated with a broad range of perceptual and behavioral phenomena. These include increased perceived contrast and spatial resolution, even when these effects are irrelevant or impair behavioral performance (Carrasco et al., 2004; Yeshurun and Carrasco, 1998). Psychophysical studies show that sensitivity enhancement can be separated further into multiple component mechanisms (Lu and Dosher, 2000). In many studies, visual attention is defined not as the orienting of resources as here (Posner et al., 1980), but as the detection process itself (Juan et al., 2004; Buschman and Miller, 2007). In addition, attention is tightly entwined with saccade target selection, and covert attention and saccade selection may be mediated by highly overlapping circuits (Rizzolatti, 1983). Thus, criterion and sensitivity shifts are only a subset of the many mechanisms of selective processing associated with the term attention. Given its heterogeneity, future investigations into attention would be most fruitful when focusing the specific mechanism of selective processing rather than relying solely on the umbrella term attention.
An alternative view would be to limit the term attention to sensitivity changes and exclude criterion shifts and other processes. While that approach could be taken, it would exclude many phenomena commonly attributed to attention, including not only selection of external stimuli but also selection of internal representations in memory, task rules, and motor responses (Chun et al., 2011). Moreover, the current definitions of attention, which ascribe selective processing as a central property, can aptly describe mechanisms other than behavioral sensitivity (Carrasco, 2011). In particular, spatially specific shifts in criterion, which selectively improve performance at a visual location, are entirely consistent with these definitions.
Finally, it is likely that complex brain processes such as attention all consist of disparate neurobiological mechanisms. Memory, another complex process, is composed of different subprocesses that depend on separate brain structures (Squire, 2004). Other cognitive functions such as decision-making may also comprise distinct mechanisms. Experiments that can dissociate the components of such processes are likely to be needed for elaborating the circuits that mediate higher behaviors.
EXPERIMENTAL PROCEDURES
Criterion and Sensitivity
Criterion and sensitivity were measured using signal detection theory (Green and Swets, 1966; Macmillan and Creelman, 2004). Criterion was indexed as criterion location (c),
In this equation, Φ−1 is the inverse normal cumulative distribution function. When c = 0, the subject shows no bias towards reporting either targets or nontargets. In the signal detection model (Figure 1C), this is the x value where the two Gaussian distributions intersect. When c < 0, the subject exhibits a bias towards reporting targets, and when c > 0, a bias towards nontargets.
Sensitivity was indexed as d′,
In the signal detection model, d′ is the horizontal offset between the two Gaussian distributions. A larger d′ indicates better sensitivity. The index d′ characteristically ranges from zero to infinity, though negative d′ values can result from sampling errors.
The results here generalize for other indices in signal detection theory, such the likelihood ratio (β) and area under the ROC. The indices used here have the advantages that c is well-defined for d′ = 0, and that c and d′ have the same units to simplify comparison.
Behavioral Tasks and Neuronal Recording
Two rhesus monkeys F and L (Macaca mulatta, adult males, 9 and 10 kg) were first trained to perform a “standard attention task,” and then for the main experiment, a “dissociation task”. The standard attention task is described in the Supplemental Experimental Procedures, and the dissociation task is described below. Before training, each animal was implanted with a head post. Eye movements were tracked using a video system (EyeLink 1000, 500 Hz). After training in the dissociation task, we implanted a 10 × 10 array of microelectrodes (Blackrock Microsystems) in area V4 to record simultaneously from dozens of neurons in each daily session (median 66 units: 4 single units, 62 multiunits). Neurophysiological recording and analyses are described in the Supplemental Experimental Procedures.
All procedures were approved by the Institutional Animal Care and Use Committee of Harvard Medical School and complied with the US Public Health Policy on the humane use and care of laboratory animals.
Dissociation Task
The monkey began each trial by fixating for 400–600 ms within a 1.5° window on a video display (57 cm away, 100 Hz frame rate). Two sample stimuli (full contrast Gabors) appeared on opposite sides of the fixation point for 200 ms. After a delay of 200–300 ms, a single test stimulus appeared at one of the two sample locations for 200 ms. The monkey had to decide whether the test had a different orientation from the sample that had appeared at the same location. The location of the test was randomly selected, and the probability that the test was different was 0.5. If the test differed from the sample, the monkey had to saccade to it within 100–500 ms to receive a juice reward. If the test were the same as the sample, the monkey had to wait to saccade to a second test stimulus that appeared at the same location as the first test stimulus. The second test always differed from the sample, and it was used to ensure that the monkey was engaged during correct rejection trials. The monkey rarely failed to respond to the second test stimulus (< 1%), and these failures were not included in analyses.
Each trial was categorized as a hit, miss, false alarm, or correct rejection based on the response to the first test stimulus. A target trial was a hit if the monkey responded to the changed test stimulus and a miss otherwise. A nontarget trial was a false alarm if the monkey incorrectly responded to the unchanged first test stimulus, and it was a correct rejection if the monkey waited to respond to the changed second test stimulus.
Session Types
Each daily recording session was either a “sensitivity session” or a “criterion session.” In a sensitivity session, we maximized the behavioral difference in d′ while minimizing the difference in c. On other days, in criterion sessions, we maximized the behavioral difference in c while minimizing the difference in d′.
Each daily session had two different task conditions. In a sensitivity session, throughout one task condition, the animal performed at high d′ for one stimulus location and at low d′ for the other location. In the other task condition, performance was reversed for the two locations. For both conditions, criterion was controlled to be unbiased (c = 0 or, equivalently, β = 1).
On a separate day, in a criterion session, the animal performed at low c for one location and high c for other location and switched performance for the two locations between task conditions. Sensitivity was similar across task conditions for each location.
The animal alternated between two task conditions in blocks of 240–360 trials. Each task condition was termed high d′, low d′, low c, or high c according to the animal’s performance at the stimulus location represented by the recorded neurons.
Reward Manipulations
To control criterion and sensitivity, we adjusted reward sizes for hits and correct rejections for each stimulus location (average reward ~150 μL). At each location, criterion was controlled primarily by the ratio of the reward given for hits and correct rejections (H:CR reward ratio) at that location. The difference in sensitivity between the two locations was controlled primarily by the relative difference in the overall reward size (across H and CR) between locations.
In criterion sessions, the H:CR reward ratio was > 1 at the low c location (on average 1.5) and < 1 at the high c location (on average 0.5). The overall reward at each location (across H and CR) was adjusted to maintain similar d′ across task conditions. The overall reward at the low c location averaged 90% of the overall reward at the high c location.
In sensitivity sessions, reward at the high d′ location was set to be 2 to 6 times larger than the reward at the low d′ location (on average 5 times larger). The H:CR reward ratio was adjusted independently for each location to control criterion to be unbiased at that location. The H:CR reward ratio averaged 0.7 at the high d′ location and 1.1 at the low d′ location.
To achieve clear behavioral dissociation within each session, reward values were titrated throughout the session, and priming trials, which were excluded from analysis, were used at the beginning of each block to stabilize behavior. See Supplemental Experimental Procedures.
Supplementary Material
Acknowledgments
We thank Mark H. Histed, Gabriel Kreiman, J. Patrick Mayo, Bram-Ernst Verhoef, and Jeremy M. Wolfe for discussions and comments, and Steven J. Sleboda for technical assistance. This work was supported by grants from the US National Institute of Health (R01EY005911, R01EY021550, and F31MH103895).
Footnotes
AUTHOR CONTRIBUTIONS
T.Z.L. and J.H.R.M. designed the experiments, performed the surgeries, and wrote the paper.
T.Z.L. performed the experiments and analyzed the data.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armstrong KM, Chang MH, Moore T. Selection and maintenance of spatial information by frontal eye field neurons. J Neurosci. 2009;29:15621–15629. doi: 10.1523/JNEUROSCI.4465-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashinski HS, Bacharach VR. Enhancement of perceptual sensitivity as the result of selectively attending to spatial locations. Percept and Psychophys. 1980;28:241–248. doi: 10.3758/bf03204380. [DOI] [PubMed] [Google Scholar]
- Boudreau CE, Williford TH, Maunsell JHR. Effects of task difficulty and target likelihood in area V4 of macaque monkeys. J Neurophysiol. 2006;96:2377–2387. doi: 10.1152/jn.01072.2005. [DOI] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- Carrasco M. Visual attention: The past 25 years. Vision Research. 2011;51:1484–1525. doi: 10.1016/j.visres.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Ling S, Read S. Attention alters appearance. Nat Neurosci. 2004;7:308–313. doi: 10.1038/nn1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelazzi L, Miller EK, Duncan J, Desimone R. Responses of neurons in macaque area V4 during memory-guided visual search. Cereb Cortex. 2001;11:761–772. doi: 10.1093/cercor/11.8.761. [DOI] [PubMed] [Google Scholar]
- Chun MM, Golomb JD, Turk-Browne NB. A taxonomy of external and internal attention. Annu Rev Psychol. 2011;62:73–101. doi: 10.1146/annurev.psych.093008.100427. [DOI] [PubMed] [Google Scholar]
- Cohen MR, Maunsell JHR. Attention improves performance primarily by reducing interneuronal correlations. Nat Neurosci. 2009;12:1594–1600. doi: 10.1038/nn.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Downing CJ. Expectancy and visual-spatial attention: effects on perceptual quality. J Exp Psychol Hum Percept Perform. 1988;14:188–202. doi: 10.1037//0096-1523.14.2.188. [DOI] [PubMed] [Google Scholar]
- Ferrera VP, Rudolph KK, Maunsell JHR. Responses of neurons in the parietal and temporal visual pathways during a motion task. J Neurosci. 1994;14:6171–6186. doi: 10.1523/JNEUROSCI.14-10-06171.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrera VP, Yanike M, Cassanello C. Frontal eye field neurons signal changes in decision criteria. Nat Neurosci. 2009;12:1458–1462. doi: 10.1038/nn.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal Detection Theory and Psychophysics. New York: Wiley; 1966. [Google Scholar]
- Gregoriou GG, Gotts SJ, Zhou H, Desimone R. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science. 2009;324:1207–1210. doi: 10.1126/science.1171402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins HL, Hillyard SA, Luck SJ, Mouloua M, Downing CJ, Woodward DP. Visual attention modulates signal detectability. J Exp Psychol Hum Percept Perform. 1990;16:802–811. doi: 10.1037//0096-1523.16.4.802. [DOI] [PubMed] [Google Scholar]
- Herrington TM, Assad JA. Temporal sequence of attentional modulation in the lateral intraparietal area and middle temporal area during rapid covert shifts of attention. J Neurosci. 2010;30:3287–3296. doi: 10.1523/JNEUROSCI.6025-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan CH, Shorter-Jacobi SM, Schall JD. Dissociation of spatial attention and saccade preparation. Proc Natl Acad Sci USA. 2004;101:15541–15544. doi: 10.1073/pnas.0403507101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinchla RA. Attention. Annu Rev Psychol. 1992;43:711–742. doi: 10.1146/annurev.ps.43.020192.003431. [DOI] [PubMed] [Google Scholar]
- Lu ZL, Dosher BAB. Spatial attention: different mechanisms for central and peripheral temporal precues? J Exp Psychol Hum Percept Perform. 2000;26:1534–1548. doi: 10.1037//0096-1523.26.5.1534. [DOI] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD. Detection Theory: A User’s Guide. Mahwah, NJ: Lawrence Erlbaum Associates; 2004. [Google Scholar]
- McPeek RM, Keller EL. Deficits in saccade target selection after inactivation of superior colliculus. Nat Neurosci. 2004;7:757–763. doi: 10.1038/nn1269. [DOI] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH. Differential attention-dependent response modulation across cell classes in macaque visual area V4. Neuron. 2007;55:131–141. doi: 10.1016/j.neuron.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH. Spatial attention decorrelates intrinsic activity fluctuations in macaque area V4. Neuron. 2009;63:879–888. doi: 10.1016/j.neuron.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller HJ, Findlay JM. Sensitivity and criterion effects in the spatial cuing of visual attention. Percept and Psychophys. 1987;42:383–399. doi: 10.3758/bf03203097. [DOI] [PubMed] [Google Scholar]
- Müller HJ, Humphreys GW. Luminance-increment detection: capacity-limited or not? J Exp Psychol Hum Percept Perform. 1991;17:107–124. doi: 10.1037//0096-1523.17.1.107. [DOI] [PubMed] [Google Scholar]
- Pagan M, Urban LS, Wohl MP, Rust NC. Signals in inferotemporal and perirhinal cortex suggest an untangling of visual target information. Nat Neurosci. 2013;16:1132–1139. doi: 10.1038/nn.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, Robinson DL, Keys W. Pulvinar nuclei of the behaving rhesus monkey: visual responses and their modulation. J Neurophysiol. 1985;54:867–886. doi: 10.1152/jn.1985.54.4.867. [DOI] [PubMed] [Google Scholar]
- Posner MI, Snyder CR, Davidson BJ. Attention and the detection of signals. J Exp Psychol Gen. 1980;109:160–174. [PubMed] [Google Scholar]
- Reynolds JH, Pasternak T, Desimone R. Attention increases sensitivity of V4 neurons. Neuron. 2000;26:703–714. doi: 10.1016/s0896-6273(00)81206-4. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G. Mechanisms of selective attention in mammals. In: Ewert JP, Capranica RR, Ingle DJ, editors. Advances in Vertebrate Neuroethology. London: Plenum Press; 1983. pp. 261–297. [Google Scholar]
- Robinson DL, Kertzman C. Covert orienting of attention in macaques. III Contributions of the superior colliculus. J Neurophysiol. 1995;74:713–721. doi: 10.1152/jn.1995.74.2.713. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory systems of the brain: A brief history and current perspective. Neurobiology of Learning and Memory. 2004;82:171–177. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- White CN, Mumford JA, Poldrack RA. Perceptual criteria in the human brain. J Neurosci. 2012;32:16716–16724. doi: 10.1523/JNEUROSCI.1744-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyart V, Nobre AC, Summerfield C. Dissociable prior influences of signal probability and relevance on visual contrast sensitivity. Proc Natl Acad Sci USA. 2012;109:3593–3598. doi: 10.1073/pnas.1120118109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeshurun Y, Carrasco M. Attention improves or impairs visual performance by enhancing spatial resolution. Nature. 1998;396:72–75. doi: 10.1038/23936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zénon A, Krauzlis RJ. Attention deficits without cortical neuronal deficits. Nature. 2012;489:434–437. doi: 10.1038/nature11497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.