Abstract
Background
Excessive alcohol consumption is associated with cardiomyopathy, but the influence of moderate alcohol use on cardiac structure and function is largely unknown.
Methods and Results
We studied 4466 participants from visit 5 of the Atherosclerosis Risk in Communities (ARIC) Study (76±5 years and 60% women) who underwent transthoracic echocardiography, excluding former drinkers and those with significant valvular disease. Participants were classified into 4 categories based on self-reported alcohol intake: non-drinkers, drinkers of up to 7 drinks per week, ≥7 to 14 and ≥ 14 drinks per week. We related alcohol intake to measures of cardiac structure and function, stratified by sex, and fully adjusted for covariates. In both genders, increasing alcohol intake was associated with larger left ventricular (LV) diastolic and systolic diameters and larger left atrial diameter (p values <0.05). In men, increasing alcohol intake was associated with greater LV mass (8.2 ± 3.8 g per consumption category, p = 0.029) and higher E/E’ ratio (0.82±0.33 per consumption category, p= 0.014). In women, increasing alcohol intake was associated with lower LV ejection fraction (−1.9% ± 0.6% per consumption category, p=0.002) and a tendency for worse LV global longitudinal strain (0.45% ±0.25% per consumption category, p=0.07).
Conclusions
In an elderly community-based population, increasing alcohol intake is associated with subtle alterations in cardiac structure and function, with women appearing more susceptible than men to the cardiotoxic effects of alcohol.
Keywords: alcohol, echocardiography, population, cardiac structure and function, heart failure, cardiomyopathy
Excessive alcohol consumption is associated with alcoholic cardiomyopathy, characterized by enlargement of the heart, increased left ventricular (LV) mass and ventricular dysfunction.1 Moreover, alcohol intake has been associated with hypertension, which also contributes to alterations in cardiac structure and function2. Conversely, numerous studies support a protective association between light to moderate drinking with the risk of coronary artery disease (CAD) and even the risk of heart failure (HF).3, 4 However, the cardiovascular mechanisms of the risks and potential benefits of alcohol are uncertain.5, 6 Furthermore, the variation in the toxic and protective effects of alcohol by sex remains controversial, as women may be more sensitive than men to the toxic effects of alcohol on cardiac function, developing alcoholic cardiomyopathy at a lower total lifetime dose of alcohol compared to men.7
Several echocardiographic morphologic and functional features are known to contribute to risk stratification for HF,8 but their association with alcohol consumption in the general population, independently of the effects over blood pressure and other factors, is unknown. We assessed the associations between alcohol intake and cardiac structure and function, in elderly men and women in the large, community-based Atherosclerosis Risk in Communities (ARIC) Study.
Methods
Study sample
The overall ARIC Study is an ongoing, prospective observational study. Detailed study rationale, design, and procedures have been previously published.9 The original cohort included 15 792 men and women aged 45 to 64 years recruited between 1987 and 1989 (visit 1), selected from 4 communities in the United States: Forsyth County, North Carolina; Jackson, Mississippi; Minneapolis, Minnesota; and Washington County, Maryland. Subsequently, three follow-up visits (visit 2 to 4) occurred at 3-year intervals, with annual telephone interviews conducted between visits. Between 2011 and 2013, 6118 surviving participants performed echocardiography during Visit 5, in all 4 ARIC field centers. Institutional review boards from each site approved the study, and informed consent was obtained from all participants.
Our analyses were restricted to the participants who were self-described as black or white (n=6102). We excluded participants without alcohol consumption data (n=149), those classified as former drinkers (n=1459) and those with moderate to severe valvular disease (n=28). A total of 4466 participants, 1781 men and 2685 women constitute the sample for the present analysis.
Measurements
Alcohol Consumption
Alcohol consumption was ascertained at all visits by means of an interviewer-administered questionnaire. Subjects were asked if they currently drank alcoholic beverages and, if not, whether they had done so in the past. Current drinkers were asked how often they usually drank wine, beer, or liquor per week. In calculating the amount of alcohol consumed (in grams per week), it was assumed that 4 ounces (118 ml) of wine contains 10.8 g, 12 ounces (355 ml) of beer contains 13.2 g, and 1.5 ounces (44 ml) of liquor contains 15.1 g of ethanol. Subsequently, grams of ethanol were converted to drinks per week (14 g of alcohol= 1 drink) and participants were classified into 4 categories, as in prior publications,6 according to their alcohol consumption at visit 5: non-drinkers, drinkers of up to 7 drinks per week, ≥7 to 14 and ≥ 14 drinks per week. Non drinking participants at visit 5, who had reported alcohol consumption in visits 1 to 4, were classified as former drinkers and excluded from the analysis. Alcohol consumption measured at visit 5 was used as primary exposure variable in the analysis. In addition, we estimated cumulative average alcohol intake using data acquired from questionnaire responses at all visits, for a mean time exposure of 23.6±0.9 years. The calculation of this variable included the estimation of 4 separate time intervals (visit 1 to visit 2; visit 2 to visit 3, etc.). For each time interval, we estimate the average alcohol consumption by averaging the consumption levels reported at the beginning and end of the interval. We then take a weighted average of the 4 interval estimates, weighting according the number of days elapsed between the two visits. If a participant was missing alcohol consumption for a certain visit, the measurements from the available years were averaged. Cumulative average alcohol intake was also converted to the previously described 4 categories of drinks per week.6
Echocardiography Protocol
The echocardiographic imaging and analysis protocol has been previously described in detail.10 All echocardiograms were performed using dedicated Philips iE33 Ultrasound systems with Vision 2011, using a preprogrammed acquisition protocol. All studies were acquired and stored digitally and transferred from field centers to a secure server at the Corelab, the Echocardiography Reading Center (ERC; Brigham and Women’s Hospital, Boston, MA), where echocardiographic measures were performed and over read, using proprietary validated echocardiographic analysis software.
All echocardiograms were obtained in a manner most consistent with good clinical practice, recording ≥3 full cardiac cycles for each view for patients in sinus rhythm and 1 or more 5-second acquisitions per view, for subjects in atrial fibrillation. LV dimensions, wall thickness, anteriorposterior left atrial (LA) dimension, and outflow tract diameter were measured from the parasternal long-axis view according to the recommendations of the American Society of Echocardiography (ASE).11 LV mass was calculated from LV linear dimensions and indexed to body surface area as recommended by ASE guidelines. LV hypertrophy was defined as LV mass indexed to body surface area (LV mass index) >115 g/m2 in men or >95 g/m2 in women. Relative wall thickness was calculated from LV end-diastolic dimension and posterior wall thickness. LV volumes were calculated by the modified Simpson method using the apical 4- and 2-chamber views, and LV ejection fraction was derived from volumes in the standard manner. LA volume was measured by the method of disks using apical 4- and 2-chamber views at an end-systolic frame preceding mitral valve opening. Early transmitral velocity (E-wave) was measured by pulsed-wave Doppler from the apical 4-chamber view with the sample volume positioned at the tip of the mitral leaflets. Peak lateral and septal mitral annular relaxation velocities (E′) were assessed using tissue Doppler imaging12 and LV diastolic function classified according to Olmsted criteria.13 Right ventricular (RV) function was assessed using the tricuspid annular peak systolic velocity measured from the lateral tricuspid annulus, and RV fractional area change was calculated as the percent change in cavity area from end-diastolic to end-systolic tracings of the RV cavity in the apical 4-chamber view. Peak tricuspid regurgitation velocity was measured and peak RV-to-right atrial systolic gradient was calculated as 4×(peak tricuspid regurgitation velocity).
Deformation analysis was performed using the TomTec Cardiac Performance Analysis package. Analysis was performed on 2D images acquired at a frame rate of 50 to 80 frames per second. Longitudinal strain was measured by tracing the endocardial borders in the apical 4-chamber and 2-chamber views. Peak longitudinal strain and strain rate were computed automatically, generating regional data from 6 segments and an average value for each view.
Measurement of Other Baseline Covariates
Standardized and validated interviewer-administered questionnaires included assessment of smoking, current medication, the presence of CAD or diabetes. Interviews included assessment of annual income and educational level, with high education defined as any college or graduate or professional school attendance. Information on demographics, anthropometric measures, and blood pressure was obtained at the time of echocardiography. Established definitions for hypertension, obesity, diabetes mellitus, CAD, and smoking status were used as previously described in the ARIC study.14 Total cholesterol, high-density lipoprotein cholesterol (HDL) and triglycerides levels were measured in a centralized laboratory and subsequently low-density cholesterol (LDL) was calculated. The assays and their performance have been previously reported.15 For the purpose of this study, results from Visit 5 were considered.16
Statistical methods
The analyses were performed overall and separately for each sex. For the 4 groups (non drinkers, up to 7 drinks per week, ≥7 to 14 and ≥14 drinks per week), summary statistics for covariates were calculated as counts and percentages or means and standard deviation for categorical and continuous data, respectively. Comparisons of baseline characteristics between the 4 groups were made using trend tests by regression methods and chi-squared tests for trend for continuous and dichotomous variables, respectively.
Using multivariate linear and logistic models, we examined separately the association of both alcohol intake at visit 5 and cumulative average alcohol intake, with measures of cardiac structure and function, stratified by sex, and adjusted for three different models: Model 1, which included age, body mass index (BMI), diabetes, anti-hypertensive medication, systolic blood pressure and previously diagnosed myocardial infarction; Model 2, which included Model 1 variables, plus education, income level and cigarette smoking in all visits and Model 3, including Model 2 variables with body size represented by BSA instead of BMI. The primary analysis variable was the alcohol consumption measured at visit 5 and the main results are presented according to Model 2. The analysis with the cumulative average alcohol consumption was performed as sensitivity analysis. In addition to the test for trend across alcohol consumption categories, we also tested for multivariate linear and curvilinear association between the continuous measure of alcohol consumption at baseline and measures of cardiac measures of cardiac structure and function. In the presence of non-linear associations, we used a restricted cubic spline model with the number of knots chosen on the basis of maximizing goodness of fit (i.e. minimizing AIC). Tests for interaction were performed using the likelihood ratio test comparing models with and without interaction terms between either sex or race and the continuous visit 5 drinking status variable(s). Two-sided p values <0.05 were considered significant. Analyses were performed using Stata version 13.1 (Stata Corp., College Station, Texas).
Results
The final analysis dataset included 4466 participants with a mean age of 76±5 years and approximately 54% of subjects reported no alcohol consumption. Overall 2685 (60%) were women and 873 (20%) were black. Table 1 illustrates the characteristics of the study population according to categories of alcohol intake at visit 5. Non-drinkers were more likely to be women with higher BMI, and non drinkers of both genders were older, had lower education level and lower annual income, and were more likely to be diabetic, to have lower LDL and HDL cholesterol and higher TG/HDL ratio. Drinkers of ≥14 drinks per week of both sexes were more frequently smokers and had higher LDL and HDL cholesterol. The relationship between alcohol consumption and hypertension was U-shaped in both genders, with hypertension being more frequent among non-drinkers and in those participants drinking ≥14 drinks per week. There was no significant trend in the prevalence of CAD by category of alcohol intake.
Table 1.
Participants Characteristics According to Alcohol Consumption* and Sex
| N drinks/week | Non-drinker | 0 to 7 | 7 to 14 | ≥14 | P Value | |
|---|---|---|---|---|---|---|
| Total Participants (n, %) | 2402 (53.7) | 1467 (32.8) | 402 (9.0) | 195 (4.4) | ||
| Men (M) | 665 (37.3) | 707 (39.7) | 261 (14.7) | 148 (8.3) | ||
| Women (W) | 1737 (64.7) | 760 (28.3) | 141 (5.3) | 47 (1.8) | ||
| Age (years) | M | 76.9 ± 5.1 | 76.2 ± 5.4 | 75.7 ± 4.6 | 75.4 ± 4.8 | <0.001 |
| W | 76.1 ± 5.2 | 74.9 ± 4.8 | 75.4 ± 4.8 | 74.9 ± 4.5 | <0.001 | |
| White (n, %) | M | 530 (79.7) | 628 (88.8) | 239 (91.6) | 134 (90.5) | <0.001 |
| W | 1203 (69.3) | 678 (89.2) | 137 (97.2) | 44 (93.6) | <0.001 | |
| BMI (kg/m2) | M | 28.8 ± 5.1 | 28.4 ± 4.3 | 28.1 ± 4.3 | 28.2 ± 4.6 | 0.028 |
| W | 29.4 ± 6.4 | 27.3 ± 5.4 | 26.2 ± 4.4 | 27.4 ± 6.5 | <0.001 | |
| Hypertension (n,%) | M | 560 (84.2) | 573 (81.0) | 206 (78.9) | 128 (86.5) | 0.244 |
| W | 1497 (86.2) | 582 (76.6) | 109 (77.3) | 41 (87.2) | <0.001 | |
| DM (n,%) | M | 257 (38.8) | 212 (30.2) | 66 (25.5) | 47 (31.8) | 0.001 |
| W | 596 (34.8) | 153 (20.3) | 22 (15.7) | 7 (15.2) | <0.001 | |
| CAD (n,%) | M | 67 (10.5) | 68 (10.2) | 22 (9.2) | 12 (8.6) | 0.411 |
| W | 84 (5.1) | 29 (4.1) | 7 (5.4) | 0 (0.0) | 0.204 | |
| Current smoker (n,%) | M | 21 (3.2) | 33 (4.7) | 18 (6.9) | 17 (11.5) | <0.001 |
| W | 65 (3.7) | 56 (7.4) | 17 (12.1) | 7 (14.9%) | 0.246 | |
| LDL Cholesterol (mg/dl) | M | 94.4 ± 32.5 | 97.3 ± 32.7 | 98.9 ± 37.9 | 99.5 ± 32.5 | 0.026 |
| W | 108.6 ± 34.2 | 112.8 ± 33.2 | 115.4 ± 33.2 | 116.1 ± 35.9 | <0.001 | |
| HDL Cholesterol (mg/dl) | M | 43.4 ± 9.7 | 46.2 ± 10.1 | 50.1 ± 12.7 | 54.3 ± 15.4 | <0.001 |
| W | 54.8 ± 12.8 | 59.8 ± 14.6 | 67.5 ± 17.9 | 67.5 ± 13.8 | <0.001 | |
| Triglycerides (mg/dl) | M | 127.6 ± 68.8 | 123.4 ± 60.5 | 123.7 ± 74.7 | 130.4 ± 82.4 | 0.8755 |
| W | 129.0 ± 65.3 | 124.2 ± 57.3 | 118.6 ± 56.8 | 126.4 ± 57.7 | 0.0354 | |
| TG/HDL | M | 3.2 ± 2.1 | 2.9 ± 1.8 | 2.7 ± 2.0 | 2.7 ± 2.4 | 0.0004 |
| W | 2.6 ± 1.7 | 2.3 ± 1.7 | 2.0 ± 1.5 | 2.0 ± 1.0 | <0.001 | |
| High educ. level (n,%) | M | 275 (41.4) | 430 (60.8) | 169 (64.8) | 80 (54.1) | <0.001 |
| W | 603 (34.7) | 396 (52.1) | 81 (57.4) | 25 (53.2) | <0.001 | |
| Income> 50,000/yr (n,%) | M | 243 (39.1) | 404 (59.6) | 166 (65.1) | 75 (52.8) | <0.001 |
| W | 373 (24.0) | 338 (49.1) | 68 (54.0) | 23 (50.0) | <0.001 | |
Categories defined according to alcohol consumption at visit 5. P value for trend, considering alcohol consumption at visit 5. BMI, body mass index; DM, diabetes mellitus; CAD, coronary artery disease
Increasing alcohol intake was associated with larger LV diastolic and systolic diameter and larger LA diameter in men and in women (Table 2). Among men, increasing alcohol intake was associated with greater LV mass, higher E/E’ ratio and tricuspid annulus peak systolic velocity and a tendency for larger RV diastolic area. In woman, increasing alcohol intake was associated with lower LV ejection fraction and a propensity towards worse peak longitudinal LV strain and increased time to peak longitudinal LV strain (Table 3). In spite of increasing LV end diastolic diameter, no significant increase in LV volumes was observed in either men or women according to alcohol intake.
Table 2.
Echocardiographic Morphological Characteristics According to Alcohol Consumption and Sex
| Model 1- alcohol consumption Visit 5 |
Model 2- alcohol consumption Visit 5 |
Model 2- cumulative average alcohol consumption |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N drinks/week | Non-drinker | 0 to 7 | 7 to 14 | ≥14 | Coef. (SE) | P Value | Coef. (SE) | P Value | Coef. (SE) | P Value | |
| Total Participants (n, %) | 2402 (53.7) | 1467 (32.8) | 402 (9.0) | 195 (4.4) | |||||||
| Men (M) | 665 (37.3) | 707 (39.7) | 261 (14.7) | 148 (8.3) | |||||||
| Women (W) | 1737 (64.7) | 760 (28.3) | 141 (5.3) | 47 (1.8) | |||||||
| LV end diastolic diameter (cm) | M | 4.6 ± 0.5 | 4.7 ± 0.5 | 4.7 ± 0.5 | 4.7 ± 0.5 | .19 (.04) | <0.001 | .18 (.04) | <0.001 | .19 (.04) | <0.001 |
| W | 4.2 ± 0.5 | 4.2 ± 0.4 | 4.3 ± 0.4 | 4.3 ± 0.4 | .10 (.03) | 0.004 | .11 (.04) | 0.008 | .15 (.04) | 0.001 | |
| LV end systolic diameter (cm) | M | 2.8 ± 0.6 | 2.9 ± 0.6 | 2.9 ± 0.5 | 2.9 ± 0.5 | .12 (.04) | 0.003 | .11 (.04) | 0.014 | .13 (.04) | 0.005 |
| W | 2.5 ± 0.4 | 2.5 ± 0.4 | 2.6 ± 0.5 | 2.5 ± 0.5 | .12 (.03) | 0.001 | .14 (.03) | 0.001 | .17 (.04) | <0.001 | |
| LV end diastolic volume (ml) | M | 98.5 ± 26.3 | 99.4 ± 26.0 | 100.8 ± 23.8 | 99.9 ± 23.1 | 1.8 (1.9) | 0.344 | .95 (2.1) | 0.654 | 1.7 (2.1) | 0.414 |
| W | 70.8 ± 17.7 | 69.3 ± 15.7 | 69.0 ± 15.3 | 68.9 ± 14.8 | .40 (1.4) | 0.782 | .37 (1.6) | 0.820 | .48 (1.7) | 0.782 | |
| LV mass (g) | M | 170.3 ± 51.4 | 169.9 ± 44.7 | 172.5 ± 45.9 | 175.6 ± 50.1 | 9.7 (3.5) | 0.006 | 8.2 (3.8) | 0.029 | 9.7 (3.7) | 0.010 |
| W | 135.4 ± 37.4 | 128.3 ± 34.4 | 126.5 ± 30.6 | 129.3 ± 27.1 | −.07 (2.8) | 0.980 | .49 (3.2) | 0.877 | 2.9 (3.3) | 0.391 | |
| LV mass index (g/m2) | M | 85.2 ± 24.5 | 84.5 ± 20.7 | 85.3 ± 20.5 | 86.1 ± 21.2 | 2.7 (1.7) | 0.111 | 2.5 (1.8) | 0.166 | 3.9 (1.8) | 0.033 |
| W | 76.7 ± 19.1 | 74.2 ± 18.1 | 74.3 ± 17.1 | 75.1 ± 14.2 | −.61 (1.5) | 0.701 | .06 (1.7) | 0.969 | 1.5 (1.8) | 0.410 | |
| Relative wall thickness (cm) | M | 0.43 ± 0.08 | 0.41 ± 0.07 | 0.41 ± 0.07 | 0.42 ± 0.08 | −.01 (.001) | 0.011 | −.01 (.001) | 0.009 | −.01 (.00) | 0.006 |
| W | 0.43 ± 0.08 | 0.42 ± 0.07 | 0.42 ± 0.05 | 0.41 ± 0.06 | −.01 (.001) | 0.010 | −.01 (.001) | 0.047 | −.01 (.00) | 0.025 | |
| LV hypertrophy (n, %) | M | 68 (10.2) | 64 (9.1) | 26 (10.0) | 20 (13.5) | 1.4 (.39) | 0.140 | 1.5 (.45) | 0.131 | 1.6 (.46) | 0.090 |
| W | 258 (14.9) | 78 (10.3) | 16 (11.3) | 5 (10.6) | .75 (.22) | 0.347 | .83 (.27) | 0.593 | .95 (.33) | 0.885 | |
| LA maximal AP diameter (cm) | M | 3.7 ± 0.6 | 3.8 ± 0.5 | 3.8 ± 0.5 | 3.8 ± 0.5 | .17 (.03) | <0.001 | .16 (.04) | <0.001 | .18 (.04) | <0.001 |
| W | 3.4 ± 0.5 | 3.4 ± 0.4 | 3.4 ± 0.5 | 3.4 ± 0.5 | .11 (.04) | 0.005 | .12 (.04) | 0.008 | .14 (.04). | 0.002 | |
| LA maximal volume (ml) | M | 56.2 ± 26.2 | 54.7 ± 21.3 | 56.7 ± 21.5 | 58.1 ± 20.3 | 3.6 (1.8) | 0.043 | 3.2 (1.9) | 0.096 | 3.3 (1.9) | 0.093 |
| W | 45.2 ± 15.7 | 42.6 ± 14.3 | 42.4 ± 13.7 | 41.5 ± 11.7 | −.46 (1.2) | 0.722 | −.7 (1.4) | 0.618 | −.14 (1.5) | 0.924 | |
| RV end diastolic area (cm2) | M | 22.2 ± 5.4 | 22.5 ± 5.4 | 23.5 ± 5.6 | 23.2 ± 5.5 | 1.5 (.45) | 0.001 | 1.1 (.48) | 0.021 | .80 (.49) | 0.100 |
| W | 17.8 ± 4.4 | 17.4 ± 4.0 | 17.1 ± 3.8 | 17.2 ± 3.3 | .23 (.31) | 0.545 | .001 (.40) | 0.999 | −.12 (.46) | 0.788 | |
Model 1: adjusted for age, body mass index, diabetes, previously diagnosed myocardial infarction, anti-hypertensive treatment and systolic blood pressure. Model 2: adjusted for Model 1 + education and income level and cigarette smoking in all visits.Coef. (SE),estimated difference per consumption category (standard error)
Table 3.
Echocardiographic Functional Characteristics According to Alcohol Consumption and Sex
| Model 1- alcohol consumption Visit 5 |
Model 2- alcohol consumption Visit 5 |
Model 2- cumulative average alcohol consumption |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N drinks/week | Non-drinker | 0 to 7 | 7 to 14 | ≥14 | Coef. (SE) | P Value | Coef. (SE) | P Value | Coef. (SE) | P Value | |
| Total Participants (n, %) | 2402 (53.7) | 1467 (32.8) | 402 (9.0) | 195 (4.4) | |||||||
| Men (M) | 665 (37.3) | 707 (39.7) | 261 (14.7) | 148 (8.3) | |||||||
| Women (W) | 1737 (64.7) | 760 (28.3) | 141 (5.3) | 47 (1.8) | |||||||
| LV ejection fraction (%) | M | 63.6 ± 7.1 | 63.6 ± 7.5 | 63.1 ± 6.3 | 64.3 ± 6.5 | .17 (.55) | 0.752 | .11 (.59) | 0.845 | −.13 (.60) | 0.818 |
| W | 66.5 ± 5.9 | 66.2 ± 5.7 | 65.6 ± 6.9 | 65.5 ± 6.5 | −1.2 (.54) | 0.020 | −1.9 (.62) | 0.002 | −2.1 (.66) | 0.001 | |
| Global long. LV strain (%) | M | −17.3 ± 2.9 | −17.5 ± 2.6 | −17.4 ± 2.5 | −17.4 ± 2.7 | −.005(.21) | 0.808 | .001 (.23) | 0.983 | .05 (.23) | 0.823 |
| W | −18.3 ± 2.5 | −18.6 ± 2.4 | −18.1 ± 2.5 | −18.1 ± 2.4 | .45 (.22) | 0.044 | .45 (.25) | 0.075 | .42 (.27) | 0.121 | |
| SD Time to peak longitudinal LV strain (msec) | M | 66.9± 43.6 | 66.9 ± 39.0 | 61.1 ± 32.4 | 63.1 ± 48.4 | −4.2 (3.3) | 0.206 | −2.4 (3.7) | 0.521 | −5.4 (3.7) | 0.145 |
| W | 59.9 ± 33.2 | 57.6 ± 28.5 | 52.2 ± 26.8 | 58.7 ± 25.3 | −6.0 (2.9) | 0.042 | −6.4 (3.3) | 0.055 | −6.2 (3.5) | 0.078 | |
| E/A ratio | M | 0.9 ± 0.4 | 0.9 ± 0.3 | 0.9 ± 0.3 | 0.9 ± 0.4 | .04 (.02) | 0.100 | .01 (.02) | 0.593 | −.001 (.02) | 0.895 |
| W | 0.8 ± 0.3 | 0.9 ± 0.3 | 0.9 ± 0.3 | 0.9 ± 0.3 | .11 (.02) | <0.001 | .08 (.02) | 0.005 | .06 (.03) | 0.030 | |
| E/E’ ratio | M | 9.4 ± 3.8 | 9.5 ± 3.8 | 9.7 ± 4.6 | 10.1 ± 3.7 | 1.1 (.30) | <0.001 | .82 (.33) | 0.014 | .59 (.33) | 0.077 |
| W | 10.8 ± 4.1 | 10.6 ± 4.0 | 10.3 ± 3.8 | 11.14 ± 4.0 | −.02 (.35) | 0.948 | −.19 (.41) | 0.643 | −.24 (.43) | 0.577 | |
| LV diastolic function classification (n, %) | M | −.01 (.02) | 0.508 | −.03 (.09) | 0.704 | −.02 (.09) | 0.816 | ||||
| Unclassified | 82 (13.3) | 97 (14.8%) | 33 (13.7) | 20 (14.8) | .03 (.02) | 0.212 | .02 (.03) | 0.460 | .02 (.03) | 0.389 | |
| Normal | 202 (32.7) | 236 (35.9%) | 100 (41.5) | 53 (39.3) | .04 (.03) | 0.212 | .04 (.04) | 0.251 | .01 (.04) | 0.828 | |
| Mild | 194 (31.4) | 181 (27.5%) | 51 (21.2) | 23 (17.0) | −.14 (.03) | <0.001 | −.13 (.04) | 0.001 | −.07 (.04) | 0.054 | |
| Moderate-severe | 140 (22.7) | 143 (21.8%) | 57 (23.7) | 39 (28.9) | .65 (.35) | 0.063 | .06 (.03) | 0.114 | .04 (.03) | 0.270 | |
| LV diastolic function classification (n, %) | W | −.01 (.10) | 0.865 | −.10 (.12) | 0.401 | −.11 (.12) | 0.387 | ||||
| Unclassified | 387 (23.0) | 145 (19.8%) | 32 (23.9) | 12 (26.1) | .02 (.03) | 0.790 | .02 (.04) | 0.528 | .01 (04) | 0.664 | |
| Normal | 371 (22.0) | 213 (29.0%) | 40 (29.9) | 12 (26.1) | .04 (.03) | 0.274 | .06 (.04) | 0.172 | .08 (.04) | 0.061 | |
| Mild | 405 (24.0) | 139 (18.9%) | 22 (16.4) | 5 (10.9) | −.09 (.03) | 0.011 | −.10 (.04) | 0.019 | −.12 (.04) | 0.007 | |
| Moderate-severe | 521 (30.9) | 237 (32.3%) | 40 (29.9) | 17 (37.0) | .04 (.04) | 0.303 | .01 (.04) | 0.792 | .01 (.05) | 0.760 | |
| RV fractional area change | M | 0.50 ± 0.08 | 0.50 ± 0.08 | 0.51 ± 0.07 | 0.51 ± 0.08 | .01 (.001) | 0.083 | .001 (.001) | 0.382 | −.001 (.001) | 0.935 |
| W | 0.54 ± 0.08 | 0.53 ± 0.08 | 0.53 ± 0.08 | 0.53 ± 0.07 | −.01 (.01) | 0.049 | −.00(.00) | 0.275 | −.01 (.001) | 0.240 | |
| Tricuspid annulus peak systolic velocity (cm/s) | M | 11.3 ± 2.8 | 11.5 ± 3.1 | 11.8 ± 3.1 | 12.3 ± 3.1 | .86 (.23) | <0.001 | .86 (.23) | 0.001 | .84 (.26) | 0.012 |
| W | 11.7 ± 2.9 | 11.8 ± 2.7 | 11.5 ± 2.8 | 11.7 ± 2.9 | −.12 (.25) | 0.618 | −.12 (.25) | 0.719 | −.10 (.28) | 0.969 | |
Model 1: adjusted for age, body mass index, diabetes, previously diagnosed myocardial infarction, anti-hypertensive treatment and systolic blood pressure. Model 2: adjusted for Model 1 + education and income level and cigarette smoking in all visits.Coef. (SE), estimated difference per consumption category (standard error)
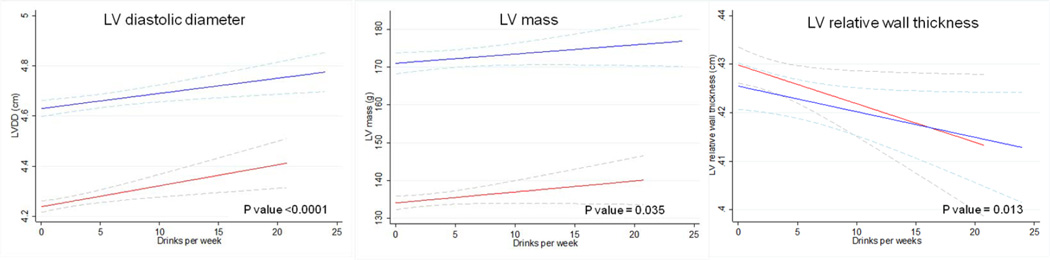
The linear association between the continuous measurement of alcohol consumption and LV end diastolic diameter and LV mass confirmed the results obtained by the multivariate linear model (Figure 1). In addition we observed a significant reduction in LV relative wall thickness, by increasing alcohol intake and a higher prevalence of LV hypertrophy in men with higher alcohol consumption (Table 2).
Figure 1. Multivariate analysis of morphological echocardiography characteristics, as a function of alcohol intake, by sex.
Multiple linear regression analysis models with 95% confidence intervals indicated by the dashed lines. Models are adjusted for age, body mass index, diabetes, previously diagnosed myocardial infarction, anti-hypertensive treatment, systolic blood pressure, education, income level and cigarette smoking in all visits. P for overall relationship is shown. Blue lines represent men, red lines represent women. In men and women, LV end diastolic diameter and LV mass increase and LV relative wall thickness reduces, by alcohol intake.
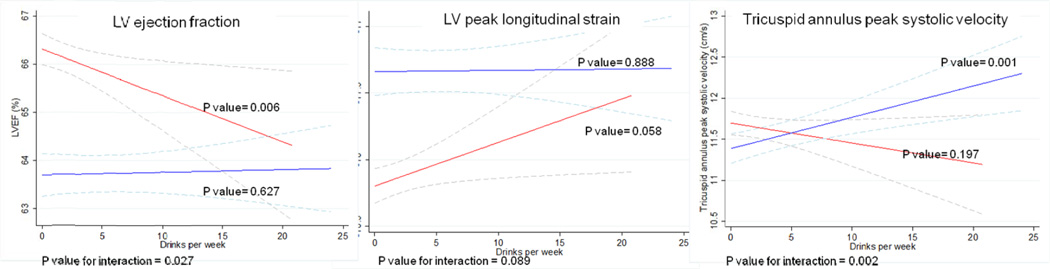
We observed a significant interaction between sex and alcohol intake with respect to LV ejection fraction (p = 0.03) and tricuspid annulus peak systolic velocity (p=0.002), in addition to a nominal association in the interaction with respect to peak systolic longitudinal strain (p=0.08) (Table 3 and Figure 2), with women demonstrating worse LV and RV function for any given degree of alcohol consumption, compared with men. We found no significant differences in diastolic function categories classification, according to alcohol consumption, but drinkers of ≥14 drinks per week were less likely to have mild diastolic dysfunction (DD) and more prone to have moderate-severe (DD). However, 19% of participants were unclassifiable according to Olmsted criteria.
Figure 2. Multivariate analysis of functional echocardiography characteristics, as a function of alcohol intake by sex.
Multiple linear regression analysis models with 95% confidence intervals indicated by the dashed lines. Models are adjusted for age, body mass index, diabetes, previously diagnosed myocardial infarction, anti-hypertensive treatment, systolic blood pressure, education, income level and cigarette smoking in all visits. P values represented for overall relationship by sex and for interaction between sex and alcohol intake. Blue lines represent men, red lines represent women. Women present worse LV and RV function for any given degree of alcohol consumption, compared with men.
We observed a significant interaction between race and alcohol intake in men only for LV end-diastolic diameter, with greater increases in LV end-diastolic diameter in black men than in white men, for the same amount of alcohol intake (p = 0.008) (Supplemental Figure). However, the limited number of black participants, particularly in the highest drinking category, 14 (9.5%) men and 3 (6.4%) women, might have limited the power to estimate potential differences by race.
Discussion
In the present study alcohol intake was independently associated with larger LV diastolic and systolic diameter and larger LA diameter in men and in women. Among men, increasing alcohol intake was associated with greater LV mass, higher E/E’ ratio and higher tricuspid annulus peak systolic velocity. In women, increasing alcohol intake was linearly associated with lower LV ejection fraction, and there was a propensity for worse peak longitudinal LV strain.
Alcohol is a known dose-dependent cardiac toxin, but myocardial damage may be a consequence of direct toxic effects of alcohol or its metabolites by ethanol induced apoptosis;17 associated hypertension,18 coexisting nutritional deficiencies, or, rarely, toxic additives to alcoholic beverages.19 Alcoholic cardiomyopathy is characterized by enlargement of the heart, increased LV mass and ventricular dysfunction,1 besides, total lifetime dose of ethanol correlates inversely with LV ejection fraction and directly with LV mass.20 Subtle signs of cardiac abnormalities as LV dilatation and impaired LV relaxation have also been reported in chronic asymptomatic heavy drinkers21 and prior small studies have described depression of LV contractility by acute alcohol consumption, evaluated by ejection fraction22 or by global longitudinal strain, which may allow detection of earlier stages of LV dysfunction.23 Similarly, reversible myocardial injury has been reported after binge drinking in healthy volunteers, with myocardial hyperenhancement demonstrated on magnetic resonance imaging.23 However, in spite of the awareness of alcohol induced LV injury and dysfunction by heavy alcohol intake, associations between alcohol consumption and measures of cardiac structure and function in the general population are mostly unknown.
In this study, of an elderly community-based cohort, we found that alcohol intake was independently associated with larger LA and LV diameters, in both men and women, and with increasing LV mass among men. In patients with CAD, heavier alcohol consumption has been associated with a 5-year increase in LA volume,24 but the association between LA dimensions and alcohol consumption in the community was generally unknown. Left atrial enlargement is a robust predictor of cardiovascular outcomes25 and it has been associated with the incidence and prevalence of HF with reduced and preserved LV ejection fraction.26 Similarly, LV mass is strongly associated with incidence of cardiovascular disease and death.27 Our results are consistent with those reported with respect to LV mass in the Framingham Study, as alcohol intake was positively associated with LV mass in men but not in women.28 Moreover, alcohol intake was associated with higher E/E’ ratio among men, suggesting increasing LV diastolic pressures.
Men had better measurements of RV systolic function with increasing alcohol intake, in contrast to women. There is limited data on the effects of alcohol on RV function, but it has been reported that low doses of alcohol in normal healthy individuals may lead to dilation of RV end-diastolic diameter and to an acute increase in some indices of RV function.29 Conversely, studies on dogs reported that moderate or high doses of alcohol were associated with RV myocardial dysfunction in a dose-dependent manner.30
The sex-related differences in the association between alcohol intake and echocardiography measures of structure and function cannot be explained by differences in age, body mass index or body surface area, current anti-hypertensive treatment, systolic blood pressure, prevalence of diabetes or CAD, socio-economic status or smoking. They also cannot be plausibly explained by gender differences in alcohol intake reporting, as we observed consistent positive associations between alcohol intake and HDL levels, a biological marker of alcohol consumption,31 in both men and women. Similarly, the TG/HDL ratio, a predictor of cardiovascular disease and known to be inversely associated with alcohol drinking,32 decreased with alcohol consumption in both men and women. There are a number of different mechanisms by which the effects of alcohol on the heart may differ by sex. Women absorb and metabolize alcohol differently than men33 and women seem to be more sensitive than men to the toxic effects of alcohol on cardiac function, developing alcoholic cardiomyopathy with a lower total lifetime dose of alcohol compared with men.7,34 Consistent with these findings, our study shows associations between alcohol intake and subclinical changes in cardiac structure in both sexes; although only among women increasing alcohol intake was associated with reduction of LV and RV systolic function. Interestingly, while we observed the classic U-shaped relationship between alcohol consumption and hypertension,2 there was no threshold for alcohol protection, when associated with measures of cardiac structure and function in this cohort. However, the possibility that the effects of alcohol consumption may be partly mediated by increased blood pressure cannot be ruled out. In addition, alcohol affects sex hormones metabolism,35 which have been associated with cardiac morphology and function, particularly, higher levels of androgens were associated with greater RV mass and volumes in both sexes. Thus, the part of sex hormones in the sex-related differences in the association between alcohol intake and cardiac structure and function cannot be excluded.36
A number of limitations should be noted. This is an observational study and alcohol consumption was self reported in a questionnaire administered by an interviewer, thus participants may have underreported their consumption level. Our population only includes participants older than 65 years old, and the number of black participants was too small for interpretation of race related differences. Therefore our observations may not be generalizable to other populations. As in any observational study, potential confounding by unmeasured factors can contribute to biased estimates, and the associations observed between alcohol consumption and subclinical changes of cardiac structure and function cannot be assumed to be causal, hence our results should be interpreted accordingly.
The strengths of this study include its large size, the ability to control for cardiovascular risk factors, CAD, socio-economic status and demographic factors. Moreover, we analyzed associations by time updated cumulative average alcohol consumption during 23.6±0.9 years, we excluded former drinkers, minimizing the potential bias due to drinking cessation because of illness, and we were able to analyze sex specific differences in the association of alcohol intake and measures of cardiac structure and function. In addition, we followed a rigorous echocardiographic protocol utilizing modern echocardiographic techniques.
In summary, we found that increasing alcohol intake in among the elderly is associated with mild alterations in cardiac structure and function. In women, moderate alcohol consumption was associated with modest reduction in systolic function, potentially contributing to a higher risk of alcoholic cardiomyopathy, for any given level of alcohol intake.
Supplementary Material
Clinical Perspective.
In this elderly community-based population, increasing alcohol intake was associated with subtle alterations in cardiac structure and function. In women, moderate alcohol consumption was associated with modest reduction in systolic function, potentially contributing to a higher risk of alcoholic cardiomyopathy, for any given level of alcohol intake. There are a number of different mechanisms by which the effects of alcohol on the heart may differ by sex. Women absorb and metabolize alcohol differently than men33 and women seem to be more sensitive than men to the toxic effects of alcohol on cardiac function, developing alcoholic cardiomyopathy with a lower total lifetime dose of alcohol compared with men.7,34 Consistent with these findings, our study shows associations between alcohol intake and subclinical changes in cardiac structure in both sexes; although only among women increasing alcohol intake was associated with mild reduction of ventricular systolic function. In consequence, this study gives strength to the differences in the sensitivity of toxic alcohol effects by sex, which is in agreement with the international recommendations for a lower threshold of alcohol consumption in women compared to men.
Acknowledgments
Sources of Funding
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The authors thank the staff and participants of the ARIC study for their important contributions.
Alexandra Gonçalves receives funds from Portuguese Foundation for Science and Technology Grant HMSP-ICS/007/2012
Footnotes
Disclosures
None.
References
- 1.Mathews EC, Jr, Gardin JM, Henry WL, Del Negro AA, Fletcher RD, Snow JA, Epstein SE. Echocardiographic abnormalities in chronic alcoholics with and without overt congestive heart failure. The American journal of cardiology. 1981;47:570–578. doi: 10.1016/0002-9149(81)90540-3. [DOI] [PubMed] [Google Scholar]
- 2.Fuchs FD, Chambless LE, Whelton PK, Nieto FJ, Heiss G. Alcohol consumption and the incidence of hypertension: The atherosclerosis risk in communities study. Hypertension. 2001;37:1242–1250. doi: 10.1161/01.hyp.37.5.1242. [DOI] [PubMed] [Google Scholar]
- 3.Walsh CR, Larson MG, Evans JC, Djousse L, Ellison RC, Vasan RS, Levy D. Alcohol consumption and risk for congestive heart failure in the framingham heart study. Annals of internal medicine. 2002;136:181–191. doi: 10.7326/0003-4819-136-3-200202050-00005. [DOI] [PubMed] [Google Scholar]
- 4.Goncalves A, Claggett B, Jhund PS, Rosamond W, Deswal A, Aguilar D, Shah AM, Cheng S, Solomon SD. Alcohol consumption and risk of heart failure: The atherosclerosis risk in communities study. European heart journal. 2015 doi: 10.1093/eurheartj/ehu514. pii: ehu514. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Djousse L, Gaziano JM. Alcohol consumption and risk of heart failure in the physicians' health study i. Circulation. 2007;115:34–39. doi: 10.1161/CIRCULATIONAHA.106.661868. [DOI] [PubMed] [Google Scholar]
- 6.Bryson CL, Mukamal KJ, Mittleman MA, Fried LP, Hirsch CH, Kitzman DW, Siscovick DS. The association of alcohol consumption and incident heart failure: The cardiovascular health study. Journal of the American College of Cardiology. 2006;48:305–311. doi: 10.1016/j.jacc.2006.02.066. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez-Sola J, Estruch R, Nicolas JM, Pare JC, Sacanella E, Antunez E, Urbano-Marquez A. Comparison of alcoholic cardiomyopathy in women versus men. The American journal of cardiology. 1997;80:481–485. doi: 10.1016/s0002-9149(97)00399-8. [DOI] [PubMed] [Google Scholar]
- 8.Stevens SM, Farzaneh-Far R, Na B, Whooley MA, Schiller NB. Development of an echocardiographic risk-stratification index to predict heart failure in patients with stable coronary artery disease: The heart and soul study. JACC. Cardiovascular imaging. 2009;2:11–20. doi: 10.1016/j.jcmg.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The atherosclerosis risk in communities (aric) study: Design and objectives. The aric investigators. American journal of epidemiology. 1989;129:687–702. [PubMed] [Google Scholar]
- 10.Shah AM, Cheng S, Skali H, Wu J, Mangion JR, Kitzman D, Matsushita K, Konety S, Butler KR, Fox ER, Cook N, Ni H, Coresh J, Mosley TH, Heiss G, Folsom AR, Solomon SD. Rationale and design of a multicenter echocardiographic study to assess the relationship between cardiac structure and function and heart failure risk in a biracial cohort of community-dwelling elderly persons: The atherosclerosis risk in communities study. Circulation. Cardiovascular imaging. 2014;7:173–181. doi: 10.1161/CIRCIMAGING.113.000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J, Solomon S, Spencer KT, St John Sutton M, Stewart W American Society of Echocardiography's N, Standards C, Task Force on Chamber Q, American College of Cardiology Echocardiography C, American Heart A, European Association of Echocardiography ESoC. Recommendations for chamber quantification. European journal of echocardiography : the journal of the Working Group on Echocardiography of the European Society of Cardiology. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. European journal of echocardiography : the journal of the Working Group on Echocardiography of the European Society of Cardiology. 2009;10:165–193. doi: 10.1093/ejechocard/jep007. [DOI] [PubMed] [Google Scholar]
- 13.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. JAMA : the journal of the American Medical Association. 2006;296:2209–2216. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 14.Folsom AR, Yamagishi K, Hozawa A, Chambless LE Atherosclerosis Risk in Communities Study I. Absolute and attributable risks of heart failure incidence in relation to optimal risk factors. Circulation. Heart failure. 2009;2:11–17. doi: 10.1161/CIRCHEARTFAILURE.108.794933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharrett AR, Patsch W, Sorlie PD, Heiss G, Bond MG, Davis CE. Associations of lipoprotein cholesterols, apolipoproteins a-i and b, and triglycerides with carotid atherosclerosis and coronary heart disease. The atherosclerosis risk in communities (aric) study. Arteriosclerosis and thrombosis : a journal of vascular biology/American Heart Association. 1994;14:1098–1104. doi: 10.1161/01.atv.14.7.1098. [DOI] [PubMed] [Google Scholar]
- 16.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. The American journal of clinical nutrition. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-Sola J, Fatjo F, Sacanella E, Estruch R, Bosch X, Urbano-Marquez A, Nicolas JM. Evidence of apoptosis in alcoholic cardiomyopathy. Human pathology. 2006;37:1100–1110. doi: 10.1016/j.humpath.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 18.Cheng CP, Cheng HJ, Cunningham C, Shihabi ZK, Sane DC, Wannenburg T, Little WC. Angiotensin ii type 1 receptor blockade prevents alcoholic cardiomyopathy. Circulation. 2006;114:226–236. doi: 10.1161/CIRCULATIONAHA.105.596494. [DOI] [PubMed] [Google Scholar]
- 19.Alexander CS. Cobalt-beer cardiomyopathy. A clinical and pathologic study of twenty-eight cases. The American journal of medicine. 1972;53:395–417. doi: 10.1016/0002-9343(72)90136-2. [DOI] [PubMed] [Google Scholar]
- 20.Urbano-Marquez A, Estruch R, Navarro-Lopez F, Grau JM, Mont L, Rubin E. The effects of alcoholism on skeletal and cardiac muscle. The New England journal of medicine. 1989;320:409–415. doi: 10.1056/NEJM198902163200701. [DOI] [PubMed] [Google Scholar]
- 21.Lazarevic AM, Nakatani S, Neskovic AN, Marinkovic J, Yasumura Y, Stojicic D, Miyatake K, Bojic M, Popovic AD. Early changes in left ventricular function in chronic asymptomatic alcoholics: Relation to the duration of heavy drinking. Journal of the American College of Cardiology. 2000;35:1599–1606. doi: 10.1016/s0735-1097(00)00565-9. [DOI] [PubMed] [Google Scholar]
- 22.Delgado CE, Gortuin NJ, Ross RS. Acute effects of low doses of alcohol on left ventricular function by echocardiography. Circulation. 1975;51:535–540. doi: 10.1161/01.cir.51.3.535. [DOI] [PubMed] [Google Scholar]
- 23.Reant P, Chasseriaud W, Pillois X, Dijos M, Arsac F, Roudaut R, Lafitte S. Early detection of left ventricular systolic dysfunction using two-dimensional speckle tracking strain evaluation in healthy subjects after acute alcohol intoxication. Echocardiography. 2012;29:927–932. doi: 10.1111/j.1540-8175.2012.01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh KJ, Cohen BE, Na B, Regan M, Schiller NB, Whooley MA. Alcohol consumption and 5-year change in left atrial volume among patients with coronary heart disease: Results from the heart and soul study. Journal of cardiac failure. 2013;19:183–189. doi: 10.1016/j.cardfail.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Benjamin EJ, D'Agostino RB, Belanger AJ, Wolf PA, Levy D. Left atrial size and the risk of stroke and death. The framingham heart study. Circulation. 1995;92:835–841. doi: 10.1161/01.cir.92.4.835. [DOI] [PubMed] [Google Scholar]
- 26.Gottdiener JS, Kitzman DW, Aurigemma GP, Arnold AM, Manolio TA. Left atrial volume, geometry, and function in systolic and diastolic heart failure of persons > or =65 years of age (the cardiovascular health study) The American journal of cardiology. 2006;97:83–89. doi: 10.1016/j.amjcard.2005.07.126. [DOI] [PubMed] [Google Scholar]
- 27.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the framingham heart study. The New England journal of medicine. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 28.Manolio TA, Levy D, Garrison RJ, Castelli WP, Kannel WB. Relation of alcohol intake to left ventricular mass: The framingham study. Journal of the American College of Cardiology. 1991;17:717–721. doi: 10.1016/s0735-1097(10)80189-5. [DOI] [PubMed] [Google Scholar]
- 29.Cameli M, Ballo P, Garzia A, Lisi M, Bocelli A, Mondillo S. Acute effects of low doses of ethanol on left and right ventricular function in young healthy subjects. Alcoholism, clinical and experimental research. 2011;35:1860–1865. doi: 10.1111/j.1530-0277.2011.01530.x. [DOI] [PubMed] [Google Scholar]
- 30.Kettunen R, Timisjarvi J, Saukko P. The acute dose-related effects of ethanol on right ventricular function in anesthetized dogs. Alcohol. 1992;9:149–153. doi: 10.1016/0741-8329(92)90026-7. [DOI] [PubMed] [Google Scholar]
- 31.Volcik KA, Ballantyne CM, Fuchs FD, Sharrett AR, Boerwinkle E. Relationship of alcohol consumption and type of alcoholic beverage consumed with plasma lipid levels: Differences between whites and african americans of the aric study. Annals of epidemiology. 2008;18:101–107. doi: 10.1016/j.annepidem.2007.07.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wakabayashi I. Alcohol intake and triglycerides/high-density lipoprotein cholesterol ratio in men with hypertension. American journal of hypertension. 2013;26:888–895. doi: 10.1093/ajh/hpt033. [DOI] [PubMed] [Google Scholar]
- 33.Frezza M, di Padova C, Pozzato G, Terpin M, Baraona E, Lieber CS. High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. The New England journal of medicine. 1990;322:95–99. doi: 10.1056/NEJM199001113220205. [DOI] [PubMed] [Google Scholar]
- 34.Urbano-Marquez A, Estruch R, Fernandez-Sola J, Nicolas JM, Pare JC, Rubin E. The greater risk of alcoholic cardiomyopathy and myopathy in women compared with men. JAMA : the journal of the American Medical Association. 1995;274:149–154. doi: 10.1001/jama.1995.03530020067034. [DOI] [PubMed] [Google Scholar]
- 35.Gordon GG, Altman K, Southren AL, Rubin E, Lieber CS. Effect of alcohol (ethanol) administration on sex-hormone metabolism in normal men. The New England journal of medicine. 1976;295:793–797. doi: 10.1056/NEJM197610072951501. [DOI] [PubMed] [Google Scholar]
- 36.Ventetuolo CE, Ouyang P, Bluemke DA, Tandri H, Barr RG, Bagiella E, Cappola AR, Bristow MR, Johnson C, Kronmal RA, Kizer JR, Lima JA, Kawut SM. Sex hormones are associated with right ventricular structure and function: The mesa-right ventricle study. American journal of respiratory and critical care medicine. 2011;183:659–667. doi: 10.1164/rccm.201007-1027OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.