Summary
Babesiosis is a worldwide tick-borne zoonosis caused by hemoprotozoan parasites of the genus Babesia. Babesia microti is the main etiologic agent of human babesiosis and is endemic in the northeastern and the upper Midwestern United States. The geographic expansion of babesiosis has followed that of Lyme disease, but has remained more restricted. The emergence of human babesiosis poses a serious health threat in highly endemic areas. Fever is the salient feature of babesiosis and often is accompanied by a series of non-specific symptoms, explaining why diagnosis may be delayed or missed. The diagnosis is confirmed by identification of babesia organisms on Giemsa stained blood smears, detection of babesia DNA by PCR, or a four-fold rise in anti-babesia antibody titers in acute and convalescent sera. The disease may be severe or fatal, particularly in patients who are otherwise healthy but older than 50 years of age, and in patients who are immunocompromised regardless of age. Most patients have complete recovery following a standard 7 to 10 day course of antimicrobial therapy.
Keywords: babesiosis, Babesia microti, protozoan, Apicomplexa, erythrocyte, tick, transfusion
Introduction
Babesiosis is caused by intraerythrocytic protozoan parasites that are transmitted by ticks, or less commonly through blood transfusion or transplacentally. The microorganisms that we now recognize as babesia were first described by Babes in 1888 when he investigated the cause of hemoglobinuria in febrile cattle. Five years later, Smith and Kilbourne identified a tick as the vector for Babesia bigemina that caused Texas cattle fever, thereby establishing for the first time transmission of an infectious agent by an arthropod vector. Human babesiosis was first recognized in a splenectomized patient in Europe but most cases have been reported from the northeastern and upper midwestern United States in people with an intact spleen and no history of immune impairment.1-3 Cases are sporadically reported in Asia, Africa, Australia, Europe, and South America. Babesiosis shares many clinical features with malaria and can be fatal, particularly in the elderly and the immunocompromised.
Epidemiology
The pathogens
Babesia species belong to the phylum Apicomplexa that is comprised of several important human pathogens such as species of Plasmodium, Toxoplasma, and Cryptosporidium. Of the large number of babesia species that infect wild and domestic animals, only a few are known to cause disease in humans, including Babesia microti and B. microti-like, Babesia duncani and B. duncani-type organisms, Babesia divergens and B. divergens–like organisms, Babesia venatorum, and KO1.2,4 The genome of B. microti recently has been sequenced. 5-6 Phylogenetic analyses indicate that B. microti is a species complex that is distant from all species of Babesidae and Theileridae and constitutes a new genus in the Apicomplexa phylum.5-6 B. microti has the smallest genome among all Apicomplexan parasites sequenced to date with ∼3,600 genes distributed on four nuclear chromosomes, one mitochondrial chromosome and one apicoplast chromosome. These genomic efforts have helped gain further understanding about the biology and phylogenetics of the parasite and have identified several targets for the development of novel therapies for human babesiosis.
Transmission
Ixodes ticks are the primary mode of transmission of babesia species to vertebrates, including humans (Figure 1). Babesia species are maintained in a wide range of vertebrate reservoirs; humans are incidental and terminal hosts. The primary reservoir for B. microti in the northeastern and upper Midwestern United States is the white-footed mouse (Peromyscus leucopus), but the parasite also has been found in shrews, chipmunks, voles, and rats.4 The primary vector is Ixodes scapularis, which also transmits Borrelia burgdorferi, the etiologic agent of Lyme disease, Anaplasma phagocytophilum, Borrelia miyamotoi, Ehrlichia muris-like agent, and Powassan virus.4,7 Humans can be infected with two or more of these pathogens. The prevalence of B. microti infection in nymphal I. scapularis ticks ranges from 1% in newly endemic areas to 20% in some well-established endemic areas.7 Initially identified on the coastal islands of southern New England, B. microti has spread north, west, and south to encompass much of the northeastern United States. This geographic expansion mimics that of Lyme disease but has proceeded more slowly. The reported incidence of babesiosis is lower than that of Lyme disease due to a more restricted geographic range, lower tick infection rate, greater proportion of asymptomatic infection, insufficient physician awareness, and greater difficulty in diagnosis 2,7 Carefully designed epidemiologic studies have shown that differences in the incidence of babesiosis and Lyme disease are small at certain sites that have long been endemic for both diseases.7-8
Figure 1. Transmission of Babesia microti by the Ixodes scapularis tick.
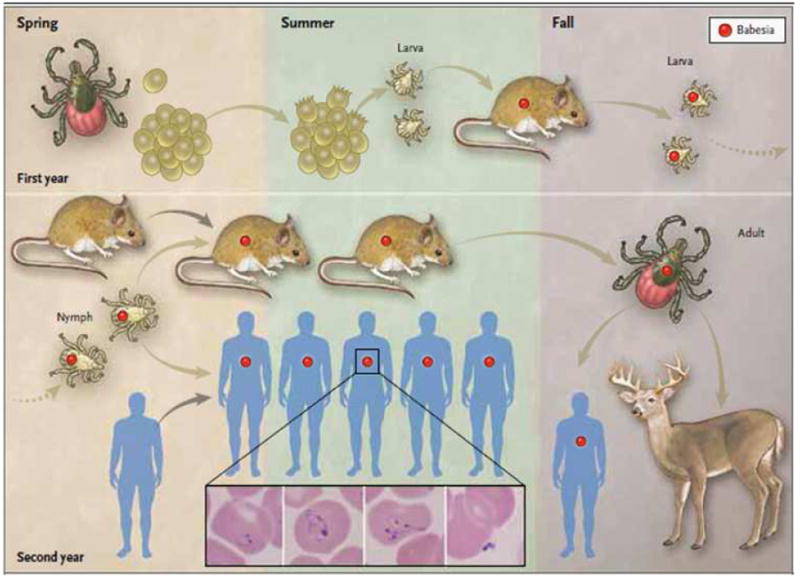
Adult female ticks lay eggs in the spring (first year, top left panel).4 Larvae hatch in the early summer and become infected with B. microti (red circle) as they take a blood meal from infected white-footed mice (Peromyscus leucopus) in late summer. White-footed mice are the primary reservoir host, but other small rodents may carry B. microti. Larvae molt to nymphs the following spring (second year, bottom left panel). When infected nymphs feed on mice or humans in late spring or early summer, these hosts may become infected. Humans are incidental hosts. In the fall, nymphs molt to adults that feed on white-tailed deer (Odocoileus virginianus) but rarely on humans. White-tailed deer do not become infected with B. microti but amplify the tick population by providing a blood meal for adult ticks. The following spring, adult female ticks lay eggs and the cycle is repeated. B. microti are obligate parasites of erythrocytes and typically are visualized on a Giemsa stained thin blood smear (see inset).2-3 The inset panels from left to right show a ring form with a non-staining vacuole surrounded by cytoplasm (in blue) and a small nucleus (in purple), an amoeboid form, a tetrad (also referred to as Maltese Cross), and an extracellular form. From Vannier E, Krause PJ. Human babesiosis. New Engl J Med 2012;366:2397-407 (reference 2).
Each of the three active stages in the life cycle of I. scapularis (larva, nymph, and adult) takes a blood meal from a vertebrate host to mature to the next stage (Figure 1).2,4 The tick transmission cycle begins in late summer when newly hatched larvae ingest the parasite during a blood meal obtained from an infected host. B. microti eventually cross the tick gut epithelium to reach the hemolymph and travel to the salivary glands. The parasites remain dormant sporoblasts when larvae molt into nymphs. Upon attachment of a B. microti infected nymphal tick to a vertebrate host, sporozoites are produced and released into the dermis of the host within tick saliva. Nymphs transmit B. microti sporozoites to vertebrate hosts in late spring and early summer of the following year.4 Once fed, nymphs molt into adults in late summer and early fall. Adults feed on the white-tailed deer (Odocoileus virginianus), which is an important host for survival of the adult tick but is not a reservoir for B. microti.4 Larvae, nymphs, and adults all feed on humans, but the nymph is the primary vector because of its summer questing activity and its small size. B. microti transmission from I. scapularis to vertebrate hosts requires 36 to 72 hours because sporozoites are not readily available in the salivary glands and are generated following activation of dormant sporoblasts upon tick exposure to warm blooded hosts.9 The continuous expansion of the deer population is thought to be a major factor in the increased density of I. scapularis and increased incidence of human babesiosis.4
Babesiosis also may be acquired through blood transfusion and transplacental transmission. More than 170 transfusion-transmitted cases have been reported.10-11 B. microti is the most common transfusion-transmitted pathogen reported in the United States. Although most cases occur during or shortly after summer in highly endemic areas, transfusion-transmitted babesiosis also occurs year round and in non-endemic areas. About a fifth of the cases acquired through blood transfusion have had a fatal outcome.10-11 Most cases of babesiosis in neonates have been acquired through blood transfusion whereas at least one case has been acquired by transplacental transmission.2,12,13
Human epidemiology
The incidence of human babesiosis has exponentially increased in the United States over the past five decades.8,14-16 Most cases occur in seven states, five of which are located in the Northeast (MA, CT, RI, NY, NJ) and two in the upper Midwest (MN, WI). The geographic range of human babesiosis has expanded beyond these highly endemic areas, and cases of human babesiosis caused by B. microti are now reported all along the northeastern seaboard and inland, ranging from Maine to Maryland (Figure 2).2,14-17 Most cases of human babesiosis occur in the summer and in areas where the tick vector, vertebrate reservoirs, and deer are in close proximity to humans. At some sites and in certain years of high transmission, babesiosis may impose a significant public health burden.8 Cases caused by B. duncani or B. duncani-type organisms have been sporadic on the Pacific Coast from northern California to Washington State. Three cases due to B. divergens-like organisms have been documented in Missouri, Kentucky, and Washington State.
Figure 2. Geographic distribution of human cases of babesiosis in the United States.
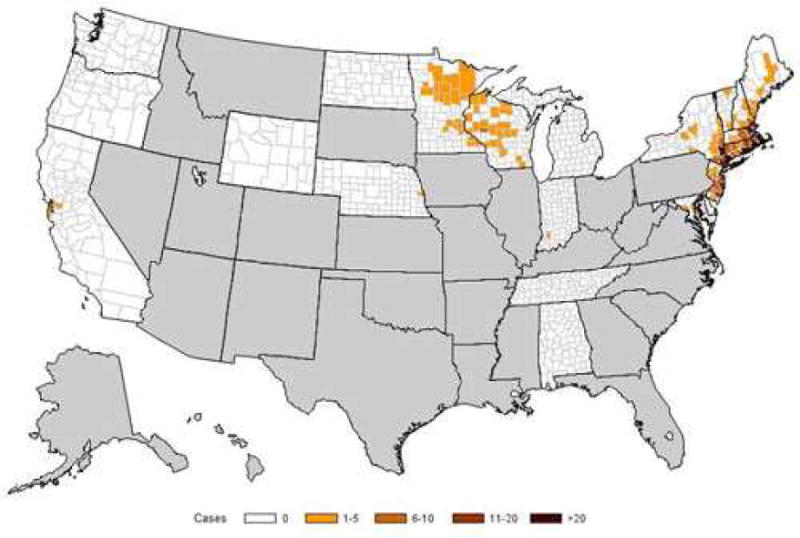
Babesiosis became a nationally notifiable condition in January 2011. As of 2012, babesiosis is reportable in 22 states and the District of Columbia. The figure shows the incidence of babesiosis (number of cases per 100,000 persons) by county of residence in 2012. Human babesiosis caused by B. microti has long been reported from the Northeast, particularly from Massachusetts to New Jersey, and recently became endemic in northern New England (Maine and New Hampshire) and in the northern Mid-Atlantic States (from Pennsylvania to Maryland). B. microti also causes disease in the upper Midwest, particularly in Wisconsin and Minnesota. B. duncani has been the etiologic agent along the northwest Pacific Coast. Cases of B. divergens-like infection have been reported from Washington State, Kentucky and Missouri. Adapted from Centers for Disease Control and Prevention (http://www.cdc.gov/parasites/babesiosis/data-statistics.html).
Cases of human babesiosis have been reported throughout the world. B. divergens is the most common cause of babesiosis in Europe where a few cases of B. microti and B. venatorum infections also have been described. Human cases have been reported in Africa, Asia, Australia and South America.2
Clinical Manifestations
Asymptomatic infection
Asymptomatic B. microti infection has been recorded in about a fifth of adults and half of children in a decade long cohort study on Block Island, Rhode Island, a highly endemic area.8 Serosurveys in other highly endemic areas have confirmed the prevalence of asymptomatic infection as shown by the disparity between seroprevalence rates and the number of reported cases of babesiosis.8,11,15 In New England, seroprevalence has varied between 0.5% and 16%.11,15
Mild to moderate illness
Clinical manifestations of babesiosis range from mild to fulminating illness resulting in death. Following an incubation period of about 1 to 4 weeks after a tick bite or 1 to 9 weeks (but up to 6 months) following transfusion of contaminated blood products, a gradual onset of malaise and fatigue is accompanied by fever and one or more of the following: chills, sweats, anorexia, headache, myalgia, nausea, nonproductive cough and arthralgia (Table 1).14,16-23 Also reported are emotional liability and depression, hyperesthesia, sore throat, abdominal pain, vomiting, conjunctival injection, photophobia, and weight loss.
Table 1. Symptoms of babesiosis.
| Symptoms | Outpatients (%) (n=41) | Inpatients (%) (n=249) | All patients (%) (n=290) |
|---|---|---|---|
| Fever | 88 | 89 | 89 |
| Fatigue | 85 | 82 | 82 |
| Chills | 63 | 68 | 67 |
| Sweats | 73 | 58 | 60 |
| Anorexia | 56 | 53 | 53 |
| Headache | 68 | 44 | 47 |
| Myalgia | 66 | 39 | 43 |
| Nausea | 32 | 44 | 42 |
| Cough | 34 | 27 | 28 |
| Arthralgia | 46 | 22 | 25 |
Fever is the salient feature on physical examination, but splenomegaly and hepatomegaly occasionally are noted. Less common are mild pharyngeal erythema, jaundice, and retinopathy with splinter hemorrhages and retinal infarcts.14,16,19-22 Rash seldom is noted and if present should raise the possibility of concurrent Lyme disease. Laboratory abnormalities that reflect the hemolytic anemia caused by invasion and lysis of red blood cells by babesia organisms include low hemoglobin, low hematocrit and elevated lactic dehydrogenase.14,16,19,22 Serum liver enzyme concentrations often are elevated. The leukocyte count is variable. Thrombocytopenia is common.16 Elevated serum levels of blood urea nitrogen and creatinine may occur, especially in severe illness and often are accompanied by proteinuria. Symptoms usually resolve in a week or two but anemia and fatigue may persist for several months.19,24 Parasitemia (as measured by parasite DNA) has been detected for more than a year even after resolution of symptoms and completion of treatment.24
Severe disease
Some patients, especially those who are older than 50 years, are immunocompromised, have comorbidities, or experience B. divergens infection may experience severe babesiosis that requires hospital admission. Complications include adult respiratory distress syndrome, pulmonary edema, disseminated intravascular coagulation, congestive heart failure, renal failure, coma, splenic rupture, or a prolonged relapsing course of illness despite standard antibiotic therapy.14,16,22-23,25 Death occurs in up to a tenth of patients hospitalized for B. microti infection.16,22 The fatality rate is even higher among those who are immunocompromised or acquired the infection through blood transfusion.2-3,22,25 Concurrent infection with B. microti and one or several tick-transmitted pathogens can occur and may increase the number and duration of acute symptoms.26
Pathogenesis
Red cell invasion and lysis
After attachment and entry into the erythrocyte, babesia organisms mature into trophozoites that eventually undergo asexual budding to yield two or four daughter cells known as merozoites. Released into the bloodstream, free merozoites quickly invade nearby red blood cells to ensure persistence of the infection in the host.
Erythrocyte lysis is associated with many of the clinical manifestations and complications of babesiosis, including fever, anemia, jaundice, hemoglobinemia, hemoglobinuria, and renal insufficiency. The absence of synchrony in asexual reproduction of the parasite may explain the lack of massive hemolysis associated with babesiosis and therefore the milder clinical presentation of babesiosis when compared with malaria.
Host immune response
The pathogenesis of babesia infection is closely linked to the host response, and studies of babesiosis in animal models suggest that disease results from the excessive production of proinflammatory cytokines.27-28 The release of proinflammatory cytokines may be initiated by contact of immune cells with the glycosylphosphatidylinositol (GPI) anchors of babesia proteins expressed at the surface of the parasite or the surface of infected red blood cells.5 Proinflammatory cytokines subsequently stimulate production of downstream mediators, such as nitric oxide, which may kill parasites, but also cause cellular damage when produced in excess. Obstruction of blood vessels due to adherence of parasitized erythrocytes to vascular endothelium with ensuing tissue ischemia and necrosis has been hypothesized to contribute to pathology.28 Although cytoadherence occurs in cattle infected with Babesia bovis, it has not been observed in human babesiosis.29
The immune response in babesiosis involves macrophages and their products, such as tumor necrosis factor-α and interleukin-12, B-lymphocytes and antibody secretion, T lymphocytes and cytokine production such interferon-γ, and complement factors.2, 27-28, 30 Most fatal cases of human babesiosis occur in splenectomized individuals, although asplenia does not always result in death or even severe illness. The spleen plays a critical role in protection against Babesia spp. because resident phagocytes and histiocytes ingest and clear infected red blood cells. Given that anemia often is more severe than predicted by parasitemia, uninfected red blood cells likely are removed. Whether caused by hemolysis of infected red blood cells or by non-specific removal of uninfected red blood cells, the anemia of babesiosis elicits stress-induced erythropoiesis and reticulocytosis. The splenic immune response and the extramedullary erythropoiesis explain why patients who experience high-grade parasitemia tend to present with splenomegaly.
Aging is an important risk factor for severe babesiosis. Most clinically apparent cases are reported in adults, although seroprevalence studies indicate that children are equally exposed to Babesia infected ticks. Most severe cases of babesiosis occur in people over 50 years of age even if otherwise healthy16 Almost all of the pediatric cases reported have been in neonates and caused by transfusion of blood products.23,31 Data from a murine model of human babesiosis suggest that resistance to B. microti infection conferred by the adaptive immune system is genetically determined and altered by aging.31-32 Sterile immunity may be slow to develop as parasite DNA (a marker of parasitemia) may persist for months to years after recovery from the initial illness.20
Diagnosis
Clinical diagnosis
The diagnosis of babesiosis is based on epidemiological and medical history, physical examination, and confirmatory laboratory tests (Figure 3).2 A diagnosis of babesiosis should be considered when a patient has a history of travel to or residence in a babesia endemic area or has received a blood transfusion in the previous six months and presents with symptoms that are consistent with babesiosis. A history of tick exposure is useful but may be absent as the bite often is unnoticed.
Figure 3. Algorithm for diagnosis of human babesiosis caused by Babesia microti.

The laboratory diagnosis of babesiosis should only be initiated for patients who are at risk of infection and for whom there is strong suspicion of infection.2, 10 Laboratory testing is required for definitive diagnosis of babesiosis. From Vannier E, Krause PJ. Human babesiosis. New Engl J Med 2012;366:2397-407 (reference 2).
Laboratory diagnosis
The diagnosis of babesiosis is confirmed by laboratory testing, most commonly by identification of the organism on Giemsa or Wright stained thin blood smears (Figure 1).33 Trophozoites of Babesia spp. are round, oval or pear-shaped and have a blue cytoplasm with a red chromatin. Ring forms are most common and may resemble the rings of early stage Plasmodium falciparum trophozoites. Babesia are distinguished from Plasmodia by the absence of hemozoin deposit in the ring form, the lack of banana-shaped gametocytes, and the presence of tetrads (Maltese cross).2 Multiple thin blood smears should be examined in the early stage of the illness, as parasitemia often is low at presentation.
PCR is a more sensitive test for B. microti infection than blood smear and provides a molecular characterization of the babesia species.33 The advent of real-time PCR has considerably lowered the limit of detection. 34-36 Endpoint B. microti PCR has been widely available for about two decades whereas real-time B. microti PCR has recently been developed and is now available from several commercial diagnostic laboratories. The diagnosis also can be confirmed and the organism amplified for genetic sequencing by injecting patient blood into small laboratory animals, although this approach is less sensitive and more time-consuming and costly than PCR.33
Serology is useful for supporting or confirming the diagnosis. A four-fold rise in babesia IgG titer in acute and convalescent sera confirms recent infection whereas a single positive antibody titer is not confirmatory as it cannot distinguish recent from past infection. The indirect immunofluorescence assay (IFA) is the most common babesia serologic test. Others include ELISA and western blot.37-38 The antigen used in babesia antibody testing should be specific for the babesia species that is locally prevalent. For example, B. duncani antibody testing would not be informative in areas where B. microti is endemic and B. duncani absent. Furthermore, the antibody titer that defines the threshold for a positive serology often differs from one babesia species antibody assay to another and occasionally from one laboratory to another for the same babesia species. Babesia antibody testing is of limited use in fulminant infection such as B. divergens illness because initial antibody testing often is negative. During the acute phase of the illness, B. microti IgG titers usually exceed 1:1024, but typically decline to 1:64 or less within 8 to 12 months.24 A positive serology for B. divergens does not exclude B. venatorum infection as antibodies against B. divergens antigen cross-react with B. venatorum antigen. Cases of B. duncani infection have been too few to validate the IFA for this pathogen.
Treatment
Asymptomatic infection
A one-week course of atovaquone plus azithromycin should be considered for any person identified with asymptomatic babesial infection (as determined by blood smear or PCR) for longer than three months.24,39-40 People who have a positive serology but in whom parasites cannot be detected by blood smears and by PCR should not be treated, as they likely have resolved the infection.
Mild to moderate illness
The recommended treatment for mild to moderate babesiosis consists of atovaquone plus azithromycin for 7 to 10 days (Table 2).18,40 This regimen was compared with clindamycin plus quinine in the only prospective trial of antibabesial therapy in humans.15 Both drug combinations were equally effective in clearing symptoms and parasitemia. Adverse effects were reported in 15 percent of subjects who received atovaquone plus azithromycin compared with 72 percent of subjects who received clindamycin plus quinine. Drug reactions were so severe that treatment had to be stopped or drug dosage decreased in approximately one third of subjects who took clindamycin plus quinine but in only 2 percent of subjects who received atovaquone plus azithromycin. Although rare cases of resistance to atovaquone plus azithromycin have been reported, this combination has been proven effective in the vast majority of children and adults.18,40
Table 2. Treatment of human babesiosis.
| Antimicrobials | Dose | Frequency |
|---|---|---|
| Atovaquone plus azithromycin | ||
| Atovaquone | Adult: 750 mg | Every 12 hours |
| Child: 20 mg/kg (maximum 750 mg/dose) | Every 12 hours | |
| Azithromycin | Adult: 500 to 1000 mg | On day 1 |
| 250 to 1000 mg | On subsequent days | |
| Child: 10 mg/kg (maximum 500 mg/dose) | On day 1 | |
| 5 mg/kg (maximum 250 mg/dose) | On subsequent days | |
| Clindamycin plus quinine | ||
| Clindamycin | Adult: 600 mg | Every 8 hours |
| Child: 7-10 mg/kg hours (maximum 600 mg/dose) | Every 6-8 | |
| Intravenous administration | ||
| Adult: 300-600 mg | Every 6 hours | |
| Child: 7-10 mg/kg hours (maximum 600 mg/dose) | Every 6-8 | |
| Quinine | Adult: 650 mg | Every 6-8 |
| hours Child: 8 mg/kg (maximum 650 mg/dose) | Every 8 hours | |
All antibiotics are administered by mouth unless otherwise specified. All doses administered for 7 to 10 days except for persistent relapsing infection (see text).
Severe disease
Severe disease typically develops in patients who have one or more of the following risk factors: age >50 years, splenectomy, malignancy, HIV infection, or immunosuppressive therapy. Clindamycin plus quinine is the combination of choice for these patients because of cumulative experience with this combination for severe disease.23,40 Oral quinine can be replaced with intravenous quinidine, but a patient treated with intravenous quinine or quinidine must be closely monitored in a hospital setting for possible Q-T prolongation.
Persistent or relapsing babesiosis despite a standard course of antimicrobial therapy has occurred in patients who present with two or more of the risk factors listed above. In these patients, resolution of infection may require at least 6 weeks of antimicrobial therapy, including 2 weeks after babesia parasites are no longer detected on blood smears.25 In addition to clindamycin plus quinine, several antibiotic combinations have been reported to be effective in a few highly immunocompromised patients. These alternative regimens have consisted of atovaquone-proguanil; atovaquone, clindamycin, plus doxycycline; atovaquone, azithromycin, plus doxycycline; artemisinin, atovaquone, plus doxycycline; atovaquone, azithromycin, plus clindamycin; and azithromycin plus quinine.24 Close clinical follow-up with repeat blood smears, babesia PCR, and complete blood counts should be performed immediately if symptoms recur.
Partial or complete red blood cell exchange transfusion is recommended for patients with parasitemia ≥10%, severe anemia (hemoglobin <10 g/dL), or pulmonary, liver or renal impairment. Exchange transfusion rapidly decreases parasitemia, corrects anemia, and removes toxic byproducts of babesia infections.2,41 Exchange transfusion and treatment with clindamycin plus quinine also is recommended for any patient infected with B. divergens because the illness often is fulminant. A combination of intravenous pentamidine plus oral trimethoprim-sulfamethoxazole was effective in treating a case of B. divergens infection.42
Prevention
Babesiosis can be prevented by avoiding areas where ticks, deer, and mice are known to thrive. It is especially important for asplenic individuals and other immunocompromised patients who are at risk of severe babesiosis, and who live or travel in endemic areas, to avoid tall grass, brush, and forested areas where I. scapularis ticks may abound. Use of protective clothing that is sprayed or impregnated with diethyltoluamide (DEET), dimethyl phthalate, or permethrin is recommended for individuals who travel into the foliage of endemic areas.43-44 A search for ticks should be carried out and the ticks removed as soon as possible.44-45 Embedded ticks are best removed by use of tweezers to grasp the mouth parts without squeezing the body of the tick.
Landscape-management approaches such as keeping grass mowed, clearing leaf litter, and spraying property with acaricides where tick density is high may help reduce the risk of infection. The risk of Lyme disease and presumably other tick borne diseases can be decreased by limiting the amount of edge between lawn and shrub on private properties.43,46 Acaricide application to deer using a 4-poster device decreases the number of I. scapularis ticks.47 Reduction in the number of deer on several islands has decreased both the number of I. scapularis ticks and the incidence of Lyme disease.48-49
Prospective blood donors who have had a history of babesiosis are permanently deferred from donating. There is no FDA approved test for screening of the blood supply for B. microti, but some states in highly endemic regions have instituted screening that combines serology and PCR.50-51
Although B. bovis and B. bigemina vaccines are available for use in cattle, no human babesiosis vaccine has been developed.
Exchange transfusion
Partial or complete exchange transfusion should be considered for treatment of severe cases of babesiosis. Exchange transfusion is recommended for any patient who is infected with B. divergens.
Key points.
Human babesiosis is an emerging infectious disease caused by hemoprotozoan parasites that are transmitted by tick vectors and less frequently through blood transfusion or transplacentally.
Babesia microti, the most frequent cause of human babesiosis, is endemic in the northeastern and upper midwestern United States and is sporadic throughout the rest of the world.
Clinical manifestations consist most commonly of a viral-like illness but range from asymptomatic infection to severe illness resulting in complications or death.
Diagnosis is confirmed by identification of babesia organisms on blood smear, detection of babesia DNA by PCR, or a four-fold rise in babesia antibody titers in acute and convalescent sera.
Treatment with atovaquone plus azithromycin is used for mild to moderate babesiosis whereas clindamycin plus quinine is recommended for severe disease.
Acknowledgments
The authors are supported by grants from the Gordon and Llura Gund Foundation (Drs. Krause and Vannier) and the National Institutes of Health (R01 GM105246 to Dr. Diuk-Wasser and R21 AI09486 to Drs. Ben Mamoun and Krause).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Edouard G. Vannier, Email: evannier@tuftsmedicalcenter.org, Tufts Medical Center and Tufts University School of Medicine Boston, Massachusetts, 617-636-8526.
Maria A. Diuk-Wasser, Email: mad2256@columbia.edu, Columbia University New York, New York, 212-854-3355.
Choukri Ben Mamoun, Email: choukri.benmamoun@yale.edu, Yale School of Medicine New Haven, Connecticut, 203-737-1972.
Peter J. Krause, Email: peter.krause@yale.edu, Yale School of Public Health and Yale School of Medicine New Haven, Connecticut, 203-785-3223.
References
- 1.Skrabalo A, Deanovic A. Piroplasmosis in man: Report on a case. Doc Med Geogr Trop. 1957;9:11–16. [PubMed] [Google Scholar]
- 2.Vannier E, Krause PJ. Human babesiosis. New Engl J Med. 2012;366:2397–407. doi: 10.1056/NEJMra1202018. [DOI] [PubMed] [Google Scholar]
- 3.Hunfeld KP, Hildebrandt A, Gray JS. Babesiosis: recent insights into an ancient disease. Int J Parasitol. 2008;38:1219–37. doi: 10.1016/j.ijpara.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Spielman A, Wilson ML, Levine JF, et al. Ecology of Ixodes dammini borne human babesiosis and Lyme disease. Ann Rev Entomol. 1985;30:439–60. doi: 10.1146/annurev.en.30.010185.002255. [DOI] [PubMed] [Google Scholar]
- 5.Cornillot E, Hadj-Kaddour K, Dassouli A, et al. Sequencing of the smallest Apicomplexan genome from the human pathogen Babesia microti. Nucleic Acids Res. 2012:1–13. doi: 10.1093/nar/gks700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornillot E, Dassouli A, Garg A, et al. Whole genome mapping and re-organization of the nuclear and mitochondrial genomes of Babesia microti isolates. Plos One. 2013;8 doi: 10.1371/journal.pone.0072657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diuk-Wasser M, Liu L, Steeves T, et al. Monitoring human babesiosis emergence through vector surveillance New England USA. Emerg Infect Dis. 2014;20:225–31. doi: 10.3201/eid2002.130644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krause PJ, McKay K, Gadbaw J, et al. Increasing health burden of human babesiosis in endemic sites. Am J Trop Med Hyg. 2003;68:431–6. [PubMed] [Google Scholar]
- 9.Piesman J, Spielman A. Human babesiosis on Nantucket Island: Prevalence of Babesia microti in ticks. Am J Trop Med Hyg. 1980;29:742–6. doi: 10.4269/ajtmh.1980.29.742. [DOI] [PubMed] [Google Scholar]
- 10.Herwaldt BL, Linden JV, Bosserman E, et al. Transfusion-associated babesiosis in the United States: a description of cases. Ann Intern Med. 2011;155:509–19. doi: 10.7326/0003-4819-155-8-201110180-00362. [DOI] [PubMed] [Google Scholar]
- 11.Leiby DA. Transfusion-transmitted Babesia spp.: bull's-eye on Babesia microti. Clin Microbiol Rev. 2011;24:14–28. doi: 10.1128/CMR.00022-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornett JK, Malhotra A, Hart D. Vertical transmission of babesiosis from a pregnant, splenectomized mother to her neonate. Infect Dis Clin Practice. 2012;20:408–410. [Google Scholar]
- 13.Joseph JT, Purtill K, Wong SJ, et al. Vertical transmission of Babesia microti, United States. Emerg Infect Dis. 2012;18:1318–21. doi: 10.3201/eid1808.110988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joseph JT, Roy SS, Shams N, et al. Babesiosis in lower Hudson Valley, New York, USA. Emerg Infect Dis. 2011;17:843–7. doi: 10.3201/eid1705.101334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krause PJ, Telford SR, Ryan R, et al. Geographical and temporal distribution of babesial infection in Connecticut. J Clin Microbiol. 1991;29:1–4. doi: 10.1128/jcm.29.1.1-4.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White DJ, Talarico J, Chang HG, et al. Human babesiosis in New York State: Review of 139 hospitalized cases and analysis of prognostic factors. Arch Intern Med. 1998;158:2149–54. doi: 10.1001/archinte.158.19.2149. [DOI] [PubMed] [Google Scholar]
- 17.Herwaldt BL, Montgomery S, Woodhall D, Bosserman EA. Babesiosis surveillance – 18 States, 2011. Morbidity Mortality Weekly Report. 2012;61:505–9. [PubMed] [Google Scholar]
- 18.Krause PJ, Lepore T, Sikand VJ, et al. Atovaquone and azithromycin for the treatment of human babesiosis. N Engl J Med. 2000;343:1454–8. doi: 10.1056/NEJM200011163432004. [DOI] [PubMed] [Google Scholar]
- 19.Ruebush TK, 2nd, Cassaday PB, Marsh HJ, et al. Human babesiosis on Nantucket Island: clinical features. Ann Intern Med. 1977;86:6–9. doi: 10.7326/0003-4819-86-1-6. [DOI] [PubMed] [Google Scholar]
- 20.Krause PJ, Telford SR, 3rd, Spielman A, et al. Concurrent Lyme disease and babesiosis. Evidence for increased severity and duration of illness. JAMA. 1996;275:1657–60. [PubMed] [Google Scholar]
- 21.Krause PJ, McKay K, Thompson CA, et al. Disease-specific diagnosis of coinfecting tickborne zoonoses: babesiosis, human granulocytic ehrlichiosis, and Lyme disease. Clin Infect Dis. 2002;34:1184–91. doi: 10.1086/339813. [DOI] [PubMed] [Google Scholar]
- 22.Hatcher JC, Greenberg PD, Antique J, et al. Severe babesiosis in Long Island: Review of 34 cases and their complications. Clin Infect Dis. 2001;32:1117–2. doi: 10.1086/319742. [DOI] [PubMed] [Google Scholar]
- 23.Wittner M, Rowin KS, Tanowitz HB, et al. Successful chemotherapy of transfusion babesiosis. Ann Intern Med. 1982;96:601–4. doi: 10.7326/0003-4819-96-5-601. [DOI] [PubMed] [Google Scholar]
- 24.Krause PJ, Spielman A, Telford S, et al. Persistent parasitemia after acute babesiosis. N Engl J Med. 1998;339:160–5. doi: 10.1056/NEJM199807163390304. [DOI] [PubMed] [Google Scholar]
- 25.Krause PJ, Gewurz BE, Hill D, et al. Persistent and relapsing babesiosis in immunocompromised patients. Clin Infect Dis. 2008;46:370–6. doi: 10.1086/525852. [DOI] [PubMed] [Google Scholar]
- 26.Thompson C, Spielman A, Krause PJ. Coinfecting deer associated zoonoses: Lyme disease, babesiosis, and ehrlichiosis. Clin Inf Dis. 2001;33:676–85. doi: 10.1086/322681. [DOI] [PubMed] [Google Scholar]
- 27.Clark IA, Wills EJ, Richmond JE, et al. Immunity to intraerythrocytic protozoa. Lancet. 1973;2:1128–9. doi: 10.1016/s0140-6736(75)91010-7. [DOI] [PubMed] [Google Scholar]
- 28.Krause PJ, Daily J, Telford SR, et al. Shared features in the pathobiology of babesiosis and malaria. Trends Parasitol. 2007;23:605–10. doi: 10.1016/j.pt.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Clark IA, Budd AC, Hsue G, et al. Malaria Journal. 2006;5:69. doi: 10.1186/1475-2875-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Terkawi MA, Cao S, Herbas MS, et al. Macrophage is the determinant of resistance to and outcome of non-lethal Babesia microti infection in mice. Infect Immun. 2014 doi: 10.1128/IAI.02128-14. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krause PJ, Telford SR, Pollack RJ, et al. Babesiosis: An underdiagnosed disease of children. Pediatrics. 1992;89:1045–8. [PubMed] [Google Scholar]
- 32.Vannier E, Borggraefe I, Telford SR, et al. Age-associated decline in resistance to Babesia microti is genetically determined. J Infect Dis. 2004;189:1721–8. doi: 10.1086/382965. [DOI] [PubMed] [Google Scholar]
- 33.Krause PJ, Telford SR, Spielman A, et al. Comparison of PCR with blood smear and inoculation of small animals for diagnosis of Babesia microti parasitemia. J Clin Microbiol. 1996;34:2791–4. doi: 10.1128/jcm.34.11.2791-2794.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teal AE, Habura A, Ennis J, et al. A new real-time PCR assay for improved detection of the parasite Babesia microti. J Clin Microbiol. 2012;50:903–8. doi: 10.1128/JCM.05848-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rollend L, Bent SJ, Krause PJ, et al. Quantitative PCR for detection of Babesia microti in Ixodes scapularis ticks and in human blood. Vector-Borne Zoonotic Dis. 2013;13:784–90. doi: 10.1089/vbz.2011.0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bloch EM, Lee TH, Krause PJ. Development of a real-time PCR assay for sensitive detection and quantitation of Babesia microti infection. Transfusion. 2013;53:2299–306. doi: 10.1111/trf.12098. [DOI] [PubMed] [Google Scholar]
- 37.Krause PJ, Telford S, Ryan R, et al. Diagnosis of babesiosis: Evaluation of a serologic test for the detection of Babesia microti antibody. J Infect Dis. 1994;169:923–6. doi: 10.1093/infdis/169.4.923. [DOI] [PubMed] [Google Scholar]
- 38.Levin AE, Williamson PC, Erwin JL, et al. Determination of Babesia microti seroprevalence in blood donor populations using an investigational enzyme immunoassay. Transfusion. 2014;54:2237–44. doi: 10.1111/trf.12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kjemtrup AM, Conrad PA. Human babesiosis: an emerging tick-borne disease. Int J Parasitol. 2000;30:1323–37. doi: 10.1016/s0020-7519(00)00137-5. [DOI] [PubMed] [Google Scholar]
- 40.Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: Clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089–94. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 41.Spaete J, Patrozou E, Rich JD, et al. Red cell exchange transfusion for babesiosis in Rhode Island. J Clin Apheresis. 2009;24:97–105. doi: 10.1002/jca.20197. [DOI] [PubMed] [Google Scholar]
- 42.Raoult D, Soulayrol L, Toga B, Dumon H, Casanova P. Babesiosis, pentamidine, and cotrimoxazole. Ann Intern Med. 1987;107:944. doi: 10.7326/0003-4819-107-6-944_2. [DOI] [PubMed] [Google Scholar]
- 43.Finch C, Al-Damluji MS, Krause PJ, et al. Integrated assessment of behavioral and environmental risk factors for Lyme disease infection on Block Island, Rhode Island. PLOS One. 2014;9:e84758. doi: 10.1371/journal.pone.0084758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vazquez M, Muehlenbein C, Cartter M, et al. Effectiveness of personal protective measures to prevent Lyme disease. Emerg Infect Dis. 2008;14:210–16. doi: 10.3201/eid1402.070725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Connally NP, Durante AJ, Yousey-Hindes KM, et al. Peridomestic Lyme disease prevention: results of a population-based case-control study. Am J Prev Med. 2009;37:201–6. doi: 10.1016/j.amepre.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 46.Frank, DH, Fish D, Moy FH. Landscape features associated with Lyme disease risk in a suburban residential environment. Landscape Ecol. 1998;13:27–36. [Google Scholar]
- 47.Fish D, Childs JE. Community-based prevention of Lyme disease and other tick-borne diseases through topical application of acaricide to white-tailed deer: background and rationale. Vector Borne Zoonotic Dis. 2009;9:357–64. doi: 10.1089/vbz.2009.0022. [DOI] [PubMed] [Google Scholar]
- 48.Wilson ML, Telford SR, Piesman J, Spielman A. Reduced abundance of immature Ixodes dammini (Acari: Ixodidae) following elimination of deer. J Med Entomol. 1988;25:224–8. doi: 10.1093/jmedent/25.4.224. [DOI] [PubMed] [Google Scholar]
- 49.Kilpatrick HJ, Labonte AM, Stafford K. The relationship between deer density, tick abundance, and human cases of Lyme disease in a residential community. J Med Entomol. 2014;51:777–84. doi: 10.1603/me13232. [DOI] [PubMed] [Google Scholar]
- 50.Young C, Chawla A, Berardi V, et al. Preventing transfusion transmitted babesiosis: Preliminary experience of the first laboratory-based blood donor screening program. Transfusion. 2012;52:1523–29. doi: 10.1111/j.1537-2995.2012.03612.x. [DOI] [PubMed] [Google Scholar]
- 51.Goodell A, Bloch EM, Krause PJ, et al. Costs, consequences, and cost-effectiveness of strategies for Babesia microti blood donor screening strategies the US blood supply. Transfusion. 2014;54:2245–57. doi: 10.1111/trf.12805. [DOI] [PubMed] [Google Scholar]


