Abstract
Respiratory sinus arrhythmia (RSA) has emerged as an indicator of how well the body maintains homeostasis and flexibly responds to environmental demands. Previous research has shown that smoking has both acute and chronic effects on RSA in adults. More recent work has focused on adolescent smokers because the natural decrease in RSA over the lifespan might be hastened by smoking at an early age. The goal of the current study, then, was to examine the acute effects of smoking on RSA and mean heart rate (HR) in a group of adolescent smokers. Participants completed two experimental sessions separated by 6–10 weeks, during which resting electrocardiogram (EKG) data were collected before and after smoking or not smoking a single cigarette ad libitum. Results indicate that smoking significantly decreased resting RSA and increased mean HR. In addition, those who smoked their first cigarette earlier in life (i.e., before age 8 or 10) evidenced a greater decrease in RSA during their smoking session relative to those who tried smoking after age 10. Importantly, these findings are largely consistent with the adult literature and suggest that smoking has acute effects on both RSA and HR in adolescence.
Keywords: Adolescent, smoking, respiratory sinus arrhythmia (RSA), heart rate, nicotine dependence, smoking behavior
1. INTRODUCTION
The negative health consequences of smoking are well-documented, as cigarette smokers experience greater rates of stroke, cancer, and emphysema than non-smokers (DHHS, 2004). Smoking also has serious and wide-ranging effects on cardiac health, including an increased risk for heart attack (Price et al., 1999) and greater dysfunction in the dilation of blood vessels in the heart, which is one of the first indicators of atherosclerosis (Ambrose & Barua, 2004). Examining the processes that may contribute to these health risks is crucial to prevention efforts. To that end, greater empirical investigation of non-invasive indicators of cardiovascular health, such as respiratory sinus arrhythmia (RSA), may be particularly valuable.
RSA is the rhythmic fluctuation of heart rate (HR) during the respiratory cycle and has emerged as a state and trait indicator of the extent to which the vagus nerve mediates parasympathetic influences on the heart so the body can flexibly respond to environmental demands (Porges, 1995; 1997; 2007; Thayer & Lane, 2000). More specifically, the sympathetic (SNS) and parasympathetic nervous systems (PNS) work together in a negative feedback loop to regulate numerous body systems, including cardiac functioning. The vagus nerve is specifically responsible for the PNS influences on HR, and a large body of evidence has indicated that these fast-acting vagal influences mediate more complex behaviors as well (Berntson et al., 1998; Porges, 2007). Thus, RSA is often conceptualized as a more general marker of self-regulatory capabilities (e.g., ability to modulate experience of emotion and arousal). Due to its close association with overall health, individual differences in RSA are impacted by various health-related behaviors, particularly smoking (e.g., Levenson & Ditto, 1981; Levin et al., 1992).
Previous research has shown that smoking has both acute and chronic effects on RSA in adults (Acharya et al., 2006). In laboratory studies, adult smokers typically manifest a decrease in RSA after smoking a single cigarette ad libitum (Hayano et al., 1990; Karakaya et al., 2013). Indeed, a small dose of nicotine (4mg) is enough to affect autonomic nervous system (ANS) function (Sjoberg & Saint, 2011), while chronic use results in more long-lasting cardiac effects that persist beyond acute nicotine ingestion. For instance, smokers show an increased or abnormal resting HR, as well as decreased RSA, compared with non-smokers (Levin et al., 1992). Further, when trained in biofeedback, smokers are less able to decrease their HR than non-smokers, in large part due to differences in their physical condition and respiratory health (Levenson & Ditto, 1981). The negative effects of smoking are not limited to current smokers. Non-smokers exposed to secondhand (i.e., environmental) or sidestream (i.e., given off by the cigarette itself) smoke show similarly increased HR and decreased RSA compared with those in a smoke-free setting (Felber Dietrich et al., 2007; Pope III et al., 2001; Valenti et al., 2013). Thus, the robust negative effects of smoking on cardiac health, as demonstrated by both acute and chronic decreases in RSA, are evident in a variety of settings.
It is important to note that the majority of existing studies on the acute (and chronic) effects of smoking on RSA have been conducted in adults. However, adolescence is considered a particularly sensitive time for smoking research and intervention as cigarettes have remained the substance most often used on a daily basis by high school students (Johnston et al., 2009). To our knowledge, only one previous study has focused on a younger sample, particularly young adults (below the age of 40). Results indicated that smokers and non-smokers had comparable levels of heart-rate variability (Erdem et al., 2015). Notably, this finding is discrepant with studies reporting group differences in RSA in older adult smokers relative to non-smokers (Levin et al., 1992), suggesting that age may indeed impact the association between smoking and cardiac functioning. Erdem et al. (2015) did not assess acute changes in RSA as a result of smoking, however, so this question remains unexplored.
This question is critical given that smoking’s effects on RSA have important health implications for adolescents. Data indicate that low RSA (i.e., increased sympathetic and decreased parasympathetic influences) leads to chronic energy expenditure that precipitates premature aging, immune dysfunction, inflammation, and disease (Brook & Julius, 2000). Deficits in vagal functioning also appear to increase risk for cardiac disease, hypertension (Schroeder et al., 2003), abnormal fasting blood glucose levels (Singh et al. 2000), increased cholesterol (Kupari et al., 1993), and inflammatory markers (Janszky et al., 2004). Therefore, although adolescents may not be at immediate risk for cardiac disease, even acute reductions in RSA during nicotine exposure may weaken immune functioning and increase vulnerability to long-lasting and pervasive negative health outcomes.
The goal of the current study, then, was to examine the acute effects of smoking on RSA and mean HR in a group of adolescent smokers using a two-session (smoke vs. no-smoke) within-subjects design. The two experimental sessions were separated by 6–10 weeks and resting electrocardiogram (EKG) data were collected before and after smoking or not smoking a single cigarette ad libitum. We examined the effects of various individual difference variables (e.g., smoking behavior, nicotine dependence) on RSA and mean HR because it is unclear whether they modulate smoking’s effects on cardiac functioning. With regard to the acute effects of smoking, we anticipated that responses would parallel those of adult smokers: RSA would decrease after smoking, as compared with not smoking, whereas mean HR would increase.
2. MATERIAL AND METHODS
2.1 Participants
The current study draws from a cohort of adolescents who participated in a larger program project that examined the social and emotional contexts of smoking using various methodologies. Participants from 16 Chicago-area high schools were recruited via survey, which gathered information about smoking behavior, intentions for continued smoking, demographics, and parental smoking status. Based on their responses to this screening questionnaire, they were oversampled for current and past smoking behavior. Participants were excluded only if they were former regular users (i.e., smoked 100 cigarettes in their lifetime but none in the past 30 days) or former occasional smokers (i.e., smoked less than 100 cigarettes but none in the past 12 months); current high rate regular users (smoked 5 or more cigarettes daily in the past 30 days); or provided inconsistent smoking information during screening. Over 1400 eligible students and their parents were invited to participate in the longitudinal study and subsequent follow-up assessments. Of those, 1263 9th and 10th graders enrolled in the overall program project and have been followed longitudinally through a seven year follow-up.
Between 2006 and 2008, a subgroup of 217 adolescents participated in the lab-based study from which the current sample originates, “Smoking’s Effect on Emotion in Adolescent Smokers.” Though gender was not of particular interest in the current study, it was a focus of the larger project, and recruitment was targeted to obtain a roughly equal number of male and female participants. The goals of this lab-based study were to determine whether 1) adolescent smokers derive affective benefit from smoking a cigarette, 2) smoking deprivation results in nicotine withdrawal symptoms, and 3) individual differences in smoking’s effect on emotional response and withdrawal reduction are predictive of subsequent developmental smoking behavior and patterns (see main results in Conrad, 2015 and Kassel et al., 2015). All participants completed two experimental sessions separated by 6–10 weeks, and participants who reported smoking at least one cigarette in the last two weeks without a desire to quit qualified as “smokers.” They were offered the chance to smoke ad libitum at only one of their visits, which was chosen randomly. Of those who chose to smoke, 49 participants smoked during Visit 1 and 45 smoked during Visit 2. Of interest in the current study is how acute smoking might affect RSA. Therefore, we initially narrowed the sample to include only those participants who chose to smoke at one of these two visits; that is, they had both a smoking and non-smoking session (n=94). The analytic approach used for the current study also required complete data for all participants, which meant we had to exclude those with missing or incomplete data from either session due to experimenter or equipment error (n=21). Thus, the analyses reported here include 73 participants. Of note, those with incomplete data did not differ significantly from those with complete data on any demographic or smoking variables.
2.2 Procedure
Upon arrival in the lab for each study session (Time 1), all participants provided an expired breath carbon monoxide (CO) reading (Vitalograph EC 50 CO monitor, Vitalograph, Lenexa, KS). Prior to each CO reading, participants were instructed to take a deep breath, hold it for 15 seconds, and then blow forcefully into a mouthpiece so that all the air left their lungs. This procedure generally ensures the most accurate CO reading possible. They also completed various self-report questionnaires before being fitted with psychophysiological electrodes. Participants then sat quietly for 3–5 minutes while the research assistant set up recording software. EKG data were collected for a total of two minutes while participants were seated in an upright position and instructed to view a blank computer screen. This time period is consistent with American Psychological Association (APA) Record Keeping Guidelines when studying stress as measured via psychophysiology (APA, 1993). Of note, respiration was not controlled or monitored in the present study. During the session in which they chose to smoke (“smoking session”), participants then lit a cigarette before placing it in a CReSS device, which examines many different aspects of smoking topography, including both volume and duration of each puff (results reported elsewhere; see Veilleux et al., 2011). They were told to smoke normally ad libitum (i.e., as much or as little of the cigarette as they desired) and remove the cigarette butt when finished. During the session in which they did not smoke (“non-smoking session”), participants were offered a magazine and asked to sit and relax for nine minutes. This amount of time was chosen to approximately match the average amount of time it took participants to light a cigarette, insert it into the CReSS machine, smoke ad libitum, remove the cigarette from the CReSS machine, and extinguish the cigarette during the smoking session. Immediately after smoking (Time 2), participants provided a second CO reading; no CO reading was taken after relaxing during the non-smoking session. EKG data were collected for an additional two minutes, and participants completed a set of self-report questionnaires. At the end of each session, participants were debriefed, compensated for their time, and provided with referrals to aid in smoking cessation. All procedures were approved by the Institutional Review Board of the University of Illinois at Chicago.
2.3 Respiratory Sinus Arrhythmia
EKG data were recorded by electrodes placed on the right and left inner forearms using a Schmitt trigger that interrupted the computer each time it detected the cardiac R-wave, providing a record of interbeat intervals (IBI) to the nearest millisecond. Data were then recorded and displayed using AcqKnowledge 4.0 data acquisition software (BIOPAC Systems, Inc., Goleta, CA) installed on a Pentium IV computer, with a resolution of 1000 Hz.
Data were initially processed using Acqknowledge and all artifacts were identified and corrected by hand by research assistants blind to condition. After initial correction, the IBI series were extracted and entered into CardioEdit (Brain-Body Center, University of Illinois at Chicago) for further artifact correction. After data were processed, average RSA was calculated based on methods developed by Porges and Bohrer (1990), using CardioBatch (Brain-Body Center, University of Illinois at Chicago). The data were first resampled into 30-second epochs and the IBI of each segment was calculated by identifying and averaging the time (in miliseconds) between R-peaks. Next, RSA was calculated by applying the adolescent CardioBatch filter to the data, which extracts the variance in the 0.12–1.0 Hz frequency band associated with heart-rate variability. Mean HR was similarly calculated using CardioBatch.
2.4 Self-Report Smoking Measures
At Visit 1, participants reported the number of days they smoked out of the past 30 (frequency), the average number of cigarettes smoked on those days (quantity), and when they last smoked (recency). Note that this provided a broader picture of their general smoking behavior than the study definition of “smoker” (i.e., that they had smoked in the last two weeks without expressing any interest in quitting). To assess nicotine dependence, participants completed the seven-item version of the Fagerström Tolerance Questionnaire, modified for use with adolescent smokers (mFTQ; Prokhorov et al., 1996), as part of a battery given by the larger program project. The mFTQ was designed to correlate with physiological measures of dependence, and the measure demonstrated adequate internal consistency within the current sample (coefficient alpha = 0.66). Finally, participants also reported the age at which they first tried a cigarette.
2.5 Statistical Analyses
Demographic variables were summarized by mean and frequency, when appropriate. In the primary analyses, RSA and mean HR were examined in separate 2 (Time 1 vs. Time 2) x 2 (smoking session vs. non-smoking session) within-subjects analyses of variance (ANOVAs). To examine the unique variance explained by individual differences, current smoking behavior (i.e., smoking frequency, quantity, and recency) and nicotine dependence were entered as separate within-subjects covariates in these analyses. Change in CO level from Time 1 to Time 2 during the smoking session was also considered, to control for individual differences in the physical experience of smoking. Finally, age of smoking initiation was entered as a between-subjects factor, to examine any interactions with time and session. All covariates were examined in the same ANOVA model. In separate analyses, the individual difference variables were also correlated with Time 1 RSA and mean HR to examine their effects on baseline physiology.
3. RESULTS
3.1 Participant Characteristics
Table 1 illustrates general characteristics for all participants. Participants were between 15 and 16 years old, Caucasian, and non-Hispanic/Latino. About half of all participants were male. At Visit 1, most smokers reported smoking one-third to all the days in a month, an average of two to three cigarettes per day, and within the last 24 hours, with scores on the mFTQ indicating low levels of nicotine dependence. Finally, most reported starting to smoke when they were between 12 and 14 years old. With regard to ad libitum smoking, Veilleux et al. (2011) shows that participants took an average of 16–17 puffs per cigarette, which is above average for a single cigarette. Puff volume and duration were variable, however, which helps explain the high puff number. Therefore, it is unclear how many participants smoked the full cigarette.
Table 1.
Participant Characteristics
| n = 73 | |
|---|---|
| General Characteristics | |
| Age (years) | 15.70 (0.61) |
| Gender (male) | 35 (47.9%) |
| Race (Caucasian) | 60 (82.2%) |
| Ethnicity (not Hispanic/Latino) | 62 (84.9%) |
| Smoking Behaviora | |
| Smoking frequency (out of the last 30 days) | |
| 1 day | 2 (2.7%) |
| 2 to 3 days | 8 (11.0%) |
| 4 to 5 days | 2 (2.7%) |
| 6 to 7 days | 1 (1.4%) |
| 8 to 10 days | 13 (17.8%) |
| 11 to 20 days | 14 (19.1%) |
| 21 to 29 days | 16 (21.9%) |
| All 30 days | 17 (23.3%) |
| Smoking quantity (cigarettes/day) | |
| Less than one | 6 (8.2%) |
| 1 | 10 (13.7%) |
| 2 | 15 (20.5%) |
| 3 | 12 (16.4%) |
| 4 | 8 (11.0%) |
| 5 | 5 (6.8%) |
| 6 to 10 | 14 (19.2%) |
| 11 to 19 | 3 (4.1%) |
| Smoking recency | |
| Last 24 hours | 44 (60.3%) |
| Past 7 days | 23 (31.5%) |
| Past 14 days | 2 (2.7%) |
| Past 30 days | 2 (2.7%) |
| 1 to 4 years ago | 1 (1.4%) |
| 5 or more years ago | 1 (1.4%) |
| Nicotine Dependenceb | 2.70 (1.44) |
| Carbon Monoxide Levelc | |
| Time 1 (Pre-Smoking) | 4.79 (3.46) |
| Time 2 (Post-Smoking) | 15.44 (12.38) |
Note: Data are Mean (SD) or n (%).
All smoking behavior was gathered using a series of multiple-choice questions.
Assessed using the Modified Fagerström Tolerance Questionnaire (mFTQ)
Values reported are from the smoking session only. The Time 1 value from the smoking session was not significantly different from the Time 1 value during the non-smoking session (Time 2 value for the non-smoking session not recorded).
3.2 RSA and Mean HR
Bivariate correlations revealed that smoking behavior (frequency, quantity, and recency), nicotine dependence, and Time 1 CO reading were not significantly related to Time 1 RSA or mean HR (rs ≤ 0.12, ps = ns).
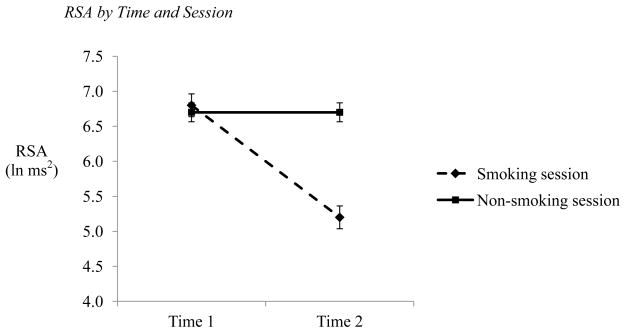
In the analysis of RSA, there was a main effect of time, F(1,68) = 111.39, p < 0.001, η2 = 0.604, such that RSA decreased from Time 1 to Time 2 (Time 1: M = 6.74, SE = 0.12; Time 2: M = 5.92, SE = 0.14). There was also a main effect of session, F(1,68) = 38.08, p < 0.001, η2 = 0.343, as participants showed differences between smoking sessions (Smoking session: M = 5.96, SE = 0.15; Non-smoking session: M = 6.71, SE = 0.13). These effects were driven by the hypothesized time by session interaction, F(1,68) = 59.51, p < 0.001, η2 = 0.449 (see Figure 1). At Time 1, participants showed similar RSA regardless of session type, t(72) = −0.30, p = 0.76, Cohen’s d = 0.035 (Smoking session: M = 6.72, SE = 0.15; Non-smoking session: M = 6.76, SE = 0.14). After smoking, however, participants evidenced decreased RSA, whereas their RSA remained stable when they did not smoke, t(72) = −8.39, p < 0.001, Cohen’s d = 0.938 (Smoking session: M = 5.13, SE = 0.18; Non-smoking session: M = 6.70, SE = 0.14). Finally, neither smoking behavior (i.e., frequency, quantity, and recency), nor nicotine dependence, nor change in CO level during the smoking session contributed unique variance as covariates, Fs(1,68) ≤ 0.95, p = ns. Age at first cigarette did interact significantly with time and session, however, F(6,63) = 2.23, p = 0.051, η2 = 0.168, driven mostly by a session by age at first cigarette interaction. Specifically, those who started smoking before the age of 8 or 10 had lower RSA during their smoking session than those who began smoking at 11 or later, F(6,63) = 3.16, p < 0.01, η2 = 0.223, though the time pattern was the same (i.e., decrease in RSA from Time 1 to Time 2).
Figure 1. RSA by Time and Session.
NOTE: Time 1 measurement taken prior to smoking (smoking session) or relaxing (non-smoking session) period. Time 2 measurement taken after smoking or relaxing period. Error bars reflect standard error of the mean.
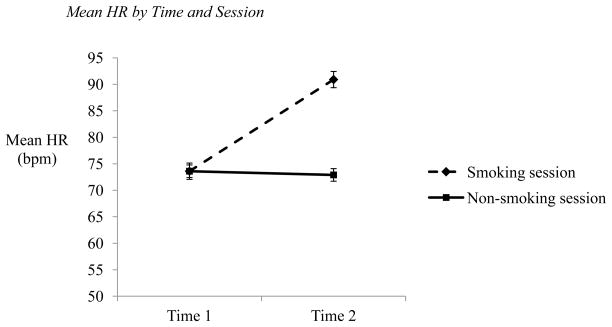
These findings are summarized in Table 2. In the analysis of mean HR, there was a main effect of time, F(1,68) = 148.13, p < 0.001, η2 = 0.670 such that mean HR increased from Time 1 to Time 2 (Time 1: M = 73.58, SE = 1.15; Time 2: M = 82.03, SE = 1.26). There was also a main effect of session, F(1,68) = 45.40, p < 0.001, η2 = 0.383, as participants experienced greater overall mean HR during their smoking session compared to their nonsmoking session (Smoking session: M = 82.39, SE = 1.43; Non-smoking session: M = 73.21, SE = 1.18). These effects were driven by the hypothesized time by session interaction, F(1,68) = 148.04, p < 0.001, η2 = 0.670 (see Figure 2). At Time 1, participants manifested similar mean HR regardless of session type, t(72) = 0.02, p = 0.98, Cohen’s d = 0.028 (Smoking session: M = 73.60, SE = 1.34; Non-smoking session: M = 73.56, SE = 1.31). After smoking, however, participants showed increased mean HR, whereas their mean HR remained stable when they did not smoke, t(72) = 10.43, p < 0.001, Cohen’s d = 1.166 (Smoking session: M = 91.29, SE = 1.83; Non-smoking session: M = 72.77, SE = 1.19). Finally, neither smoking behavior (i.e., frequency, quantity, and recency), nor nicotine dependence, nor change in CO level during the smoking session contributed unique variance as covariates, Fs(1,68) ≤ 0.94, p = ns), and age at first cigarette was not related to mean HR, F(6,63) = 1.64, p = ns. These findings are summarized in Table 3.
Table 2.
Summary of Analyses with RSA (n=73)
| F | df | p | η2 | |
|---|---|---|---|---|
| Main Analyses | ||||
| Timea | 111.39 | 1,68 | < 0.001 | 0.604 |
| Session | 38.08 | 1,68 | < 0.001 | 0.343 |
| Time x Session | 59.51 | 1,68 | < 0.001 | 0.449 |
|
| ||||
| Covariatesb | ||||
| Smoking frequencyc | 0.01 | 1,68 | 0.95 | 0.01 |
| Smoking quantity | 0.25 | 1,68 | 0.62 | 0.01 |
| Smoking recency | 0.26 | 1,68 | 0.61 | 0.01 |
| Nicotine dependenced | 0.88 | 1,68 | 0.36 | 0.03 |
| Change in carbon monoxide (CO) levele | 0.18 | 1,68 | 0.70 | 0.01 |
|
| ||||
| Between-Subjects Factor | ||||
| Age at first cigarette | 2.23 | 6,63 | 0.051 | 0.168 |
Time 1 measurement taken prior to smoking (smoking session) or relaxing (non-smoking session) period. Time 2 measurement taken after smoking or relaxing period.
All covariates were examined in the same ANOVA.
Number of smoking days out of the last 30.
Assessed via the Modified Fagerström Tolerance Questionnaire.
CO measured before and after smoking.
Figure 2. Mean HR by Time and Session.
NOTE: Time 1 measurement taken prior to smoking (smoking session) or relaxing (non-smoking session) period. Time 2 measurement taken after smoking or relaxing period. Error bars reflect standard error of the mean.
Table 3.
Summary of Analyses with Mean HR (n=73)
| F | df | p | η2 | |
|---|---|---|---|---|
| Main Analyses | ||||
| Timea | 148.13 | 1,68 | < 0.001 | 0.670 |
| Session | 45.40 | 1,68 | < 0.001 | 0.383 |
| Time x Session | 148.04 | 1,68 | < 0.001 | 0.670 |
|
| ||||
| Covariatesb | ||||
| Smoking frequencyc | 0.05 | 1,68 | 0.82 | 0.01 |
| Smoking quantity | 0.46 | 1,68 | 0.50 | 0.02 |
| Smoking recency | 0.79 | 1,68 | 0.38 | 0.03 |
| Nicotine dependenced | 0.01 | 1,68 | 0.94 | 0.01 |
| Change in carbon monoxide (CO) levele | 2.31 | 1,68 | 0.14 | 0.09 |
|
| ||||
| Between-Subjects Factor | ||||
| Age at first cigarette | 1.64 | 6,63 | 0.149 | 0.130 |
Time 1 measurement taken prior to smoking (smoking session) or relaxing (non-smoking session) period. Time 2 measurement taken after smoking or relaxing period.
All covariates were examined in the same ANOVA.
Number of smoking days out of the last 30.
Assessed via the Modified Fagerström Tolerance Questionnaire.
CO measured before and after smoking.
4. DISCUSSION
The goals of the current study were to examine the acute effects of smoking on cardiac functioning in a group of high-risk adolescent smokers. We were also interested in the unique contributions of smoking behavior (frequency, quantity, and recency), nicotine dependence, and change in CO level after smoking to this relationship. We also examined how age at first cigarette might moderate smoking’s effects. Results indicate that RSA responded as hypothesized: RSA significantly decreased in response to smoking. Age at first cigarette also interacted significantly with time and session, as those who started smoking earlier (i.e., before age 8 or 10) had a greater decrease in RSA during their smoking session as compared with those who began smoking later (i.e., after age 10). When considering mean HR, the opposite general pattern was observed: HR significantly increased in response to smoking. Importantly, these findings are largely consistent with the adult literature (Hayano et al., 1990; Karakaya et al., 2013; Sjoberg & Saint, 2011), suggesting that regardless of developmental maturity, nicotine pharmacologically diminishes vagal influences on HR.
These results may have important implications for adolescents. In addition to negatively impacting physical health, changes in vagal functioning may exert important influences on emotional functioning. A large body of research has demonstrated that low RSA is associated with deficits in emotion regulation, including increased affective reactivity (Butler et al., 2006; Gorka et al., 2013b) and an inability to modulate affective responses over time (Gorka et al., 2013a; Sack et al., 2004). Numerous studies have also demonstrated that adolescent smokers experience a wide range of negative emotional and behavioral consequences including increased rates of depression and anxiety (Munafó et al., 2007; Pederson & von Soset, 2009) and substance use disorders (Brown et al., 1996; Lindsay & Rainey, 1997). Therefore, it is possible that decreased RSA is a marker of decreased emotion regulation capabilities, which could increase rates of psychological disorders moving forward (e.g., Hofmann et al., 2012). Within our own lab, we have tested this hypothesis, and recent analyses show that low resting RSA in adolescence predicts increased substance use five years later (Unpublished data). Therefore, even acute nicotine-induced deficits in RSA may exacerbate psychological distress and maladaptive behaviors. Given that this link between acute changes in RSA and subsequent mental and physical health consequences is still relatively speculative, future research is critically needed to elucidate these relationships.
It is also important to consider the role that individual differences play in moderating the effects of smoking on RSA, and perhaps, more general health outcomes. In the current study, smoking for the first time at a younger age was associated with a greater decrease in RSA during the smoking session. It is unlikely that this is due to greater lifetime exposure to smoking, as neither smoking behavior nor nicotine dependence had a comparable effect. It may, however, be due to increased sensitization to nicotine. Previous studies have shown that RSA naturally decreases with age (Acharya et al., 2004a; 2004b). This decline starts in childhood, with a rapid decrease in sympathetic activity between the ages of 5 and 10 years old (Finley et al., 1987). If children smoke during this time, they expose themselves to nicotine, which is a stimulant that increases SNS activity. This combination of influences could make adolescent smokers more physiologically sensitive to nicotine’s effects later in life. In contrast, smoking for the first time after age 10 could result in relatively less sensitization, which would explain why age at first cigarette moderates smoking’s effects on RSA in the current study. Interestingly, neither smoking behavior, nor nicotine dependence, nor CO change during the smoking session affected smoking’s effect on RSA and mean HR. It is possible that none of these individual differences varied enough between participants to contribute any significant variance to the model, as most participants showed similar smoking patterns and experience of nicotine dependence. Indeed, these variables are likely correlated as well, and their inclusion in the same model might have obscured the contributions of any one in particular.
Despite the strengths of the current study design, there are limitations. First, the impact of respiration on the calculation of RSA is an unresolved debate within the literature: several studies have demonstrated that ignoring indices of respiration leads to biased or inaccurate measures of cardiac vagal tone (e.g., Grossman & Taylor, 2007), whereas others have presented data refuting these conclusions (e.g., Denver et al., 2007). Within the present study, we did not collect respiration data and thus are unable to assess the potential impact of respiration on the present indices of RSA. Second, adolescents were instructed to smoke as much or as little of a cigarette as they desired during the smoking condition. Thus, nicotine levels likely varied across participants. Future studies would greatly benefit by examining the effects of nicotine on RSA in a dose-dependent manner. Lastly, the current sample of adolescents had relatively low levels of nicotine dependence and consequently, the present findings may not extend to more dependent adolescent smokers. In addition, use of caffeine and psychostimulants was not captured, and it is unclear to what extent use of these substances affected study results.
In sum, the results of the present study extend the literature on the acute effects of smoking on vagal functioning and suggest that nicotine reduces RSA, while simultaneously increasing HR, in adolescent smokers. These cardiac effects are clinically important given that low RSA is associated with cardiac disease and affective dysregulation. Moreover, these acute deleterious effects may prove even more salient in adolescence. There is evidence to suggest that vagal functioning may improve upon smoking cessation (Harte & Meston, 2014; Lewis et al., 2010; Minami et al., 1999; Munjal et al., 2009; Stein et al., 1996; Yotsukura et al., 1998), and thus, adolescent smoking interventions are crucial in the effort to stave off the adverse health outcomes associated with altered cardiac function. As this is an important avenue of future research, further studies are needed to continue elucidating the relationship between smoking and cardiac functioning in youth, as well as examine potential mediators and moderators of this association.
HIGHLIGHTS.
Adolescent smokers provided RSA readings before and after smoking or relaxing.
Participants showed similar RSA/mean HR at before smoking/relaxing.
Participants showed decreased RSA/increased mean HR after smoking (vs. relaxing).
Smoking’s effects on RSA were moderated by age at first cigarette.
Acknowledgments
This research was supported by the National Cancer Institute of the National Institutes of Health under award number 5P01CA09862.
The authors wish to thank Justin Greenstein, Margaret Wardle, Jenn Veilleux, Adrienne Heinz, and Ashley Braun for their work during the data collection and management portions of this study.
Footnotes
Work was performed at the Substance Use Research Laboratory, University of Illinois at Chicago. Findings were previously presented via poster at the 20th Annual Meeting of the Society for Research on Nicotine and Tobacco, Seattle, WA.
The authors report no conflicts of interest.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acharya UR, Joseph KP, Kannathal N, Lim CM, Suri JS. Heart rate variability: A review. Med Biol Eng Comput. 2006;4412:1031–51. doi: 10.1007/s11517-006-0119-0. [DOI] [PubMed] [Google Scholar]
- Acharya UR, Kannathal N, Krishnan SM. Comprehensive analysis of cardiac health using heart rate signals. Physiol Meas. 2004a;J25:1130–1151. doi: 10.1088/0967-3334/25/5/005. [DOI] [PubMed] [Google Scholar]
- Acharya UR, Kannathal N, Seng OW, Ping LY, Chua T. Heart rate analysis in normal subjects of various age groups. Biomed Eng Online. 2004b;3:24. doi: 10.1186/1475-925X-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: An update. J Am Coll Cardiol. 2004;4310:1731–7. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- American Psychological Association. Record keeping guidelines. Am Psychol. 1993;48:984–986. doi: 10.1037/0003-066X.62.9.993. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Sarter M, Cacioppo JT. Anxiety and cardiovascular reactivity: The basal forebrain cholinergic link. Behav Brain Res. 1998;942:225–48. doi: 10.1016/s0166-4328(98)00041-2. [DOI] [PubMed] [Google Scholar]
- Brook RD, Julius S. Autonomic imbalance, hypertension, and cardiovascular risk. 2000. Am J Hypertens. 2000;136(Pt 2):112S–122S. doi: 10.1016/s0895-7061(00)00228-4. [DOI] [PubMed] [Google Scholar]
- Brown RA, Lewinsohn PM, Seeley JR, Wagner EF. Cigarette smoking, major depression, and other psychiatric disorders among adolescents. J Am Acad Child Psy. 1996;3512:1602–10. doi: 10.1097/00004583-199612000-00011. [DOI] [PubMed] [Google Scholar]
- Butler EA, Wilhelm FH, Gross JJ. Respiratory sinus arrhythmia, emotion, and emotion regulation during social interaction. Psychophysiology. 2006;436:612–622. doi: 10.1111/j.1469-8986.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- Conrad M. Unpublished dissertation. University of Illinois at Chicago, Department of Psychology; Chicago: 2015. Smoking’s acute effects on emotional response in adolescent smokers. [Google Scholar]
- Denver JW, Reed SF, Porges SW. Methodological issues in the quantification of respiratory sinus arrhythmia. Biol Psychol. 2007;742:286–94. doi: 10.1016/j.biopsycho.2005.09.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health and Human Services (DHHS) 2004 Surgeon General’s report: The health consequences of smoking. Washington, D.C: 2004. [Google Scholar]
- Erdem A, Ayhan SS, Oztürk S, Ozlü MF, Alcelik A, Sahin S, Tosun M, Erdem FH, Gumustekin K, Yazici M. Cardiac autonomic function in healthy young smokers. Toxicol Ind Health. 2015;31:67–72. doi: 10.1177/0748233712468024. [DOI] [PubMed] [Google Scholar]
- Felber Dietrich D, Schwartz J, Schindler C, Gaspoz JM, Barthélémy JC, Tschopp JM, Roche F, von Eckardstein A, Brändli O, Leuenberger P, Gold DR, Ackermann-Liebrich U. Effects of passive smoking on heart rate variability, heart rate and blood pressure: An observational study. Int J Epidemiol. 2007;364:834–40. doi: 10.1093/ije/dym031. [DOI] [PubMed] [Google Scholar]
- Finley JP, Nungent ST, Hellenbrand W. Heart rate variability in children. Spectral analysis of developmental changes between 5 and 25 years. Can J Physiol Pharm. 1987;65:2048–2052. doi: 10.1139/y87-320. [DOI] [PubMed] [Google Scholar]
- Gorka SM, McGowan SK, Campbell ML, Nelson BD, Sarapas C, Bishop JR, Shankman SA. Respiratory sinus arrhythmia as a predictor of startle habituation in three independent samples. Biol Psychol. 2013a;932:334–341. doi: 10.1016/j.biopsycho.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Nelson BD, Sarapas C, Campbell ML, Lewis GF, Bishop JR, Porges SW, Shankman SA. Relation between respiratory sinus arrhythmia and startle response during predictable and unpredictable threat. J Psychophysiol. 2013b;272:95–104. doi: 10.1027/0269-8803/a000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman P, Taylor EW. Toward understanding respiratory sinus arrhythmia: Relations to cardiac vagal tone, evolution and biobehavioral functions. Biol Psychol. 2007;742:263–85. doi: 10.1016/j.biopsycho.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Harte CB, Meston CM. Effects of smoking cessation on heart rate variability among long-term male smokers. Int J Behav Med. 2014;21:302–309. doi: 10.1007/s12529-013-9295-0. [DOI] [PubMed] [Google Scholar]
- Hayano J, Yamada M, Sakakibara Y, Fujinami T, Yokoyama K, Watanabe Y, Takata K. Short- and long-term effects of cigarette smoking on heart rate variability. Am J Cardiol. 1990;651:84–8. doi: 10.1016/0002-9149(90)90030-5. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Sawyer AT, Fang A, Asnaani A. Emotion dysregulation model of mood and anxiety disorders. Depress Anxiety. 2012;29:409–416. doi: 10.1002/da.21888. [DOI] [PubMed] [Google Scholar]
- Janszky I, Ericson M, Lekander M, Blom M, Buhlin K, Georgiades A, Ahnve S. In ammatory markers and heart rate variability in women with coronary heart disease. J Intern Med. 2004;2565:421–428. doi: 10.1111/j.1365-2796.2004.01403.x. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. NIH Publication No. 09-7402. Bethesda, MD: National Institute on Drug Abuse; 2009. Monitoring the Future national survey results on drug use, 1975–2008. Volume I: Secondary school students. [Google Scholar]
- Karakaya O, Barutcu I, Kaya D, Esen AM, Saglam M, Melek M, Onrat E, Turkmen M, Esen OB, Kaymaz C. Acute effect of cigarette smoking on heart rate variability. Angiology. 2013;585:620–4. doi: 10.1177/0003319706294555. [DOI] [PubMed] [Google Scholar]
- Kupari M, Virolainen J, Koskinen P, Tikkanen MJ. Short-term heart rate variability and factors modifying the risk of coronary artery disease in a population sample. Am J Cardiol. 1993;72:897–903. doi: 10.1016/0002-9149(93)91103-o. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Wardle MC, Veilleux JC, Greenstein JE, Heinz AJ, Evatt DP, Roesch L, Braun AR, Segawa E, Hedeker D, Berbaum M, Mermelstein R, Blumenthal TD. The acute effects of cigarette smoking on emotional response in adolescent smokers: A multidimensional analysis. University of Illinois at Chicago, Department of Psychology; Chicago: 2015. Unpublished manuscript. [Google Scholar]
- Levenson RW, Ditto WB. Individual differences in ability to control heart rate: Personality, strategy, physiological and other variables. Psychophysiology. 1981;182:91–100. doi: 10.1111/j.1469-8986.1981.tb02918.x. [DOI] [PubMed] [Google Scholar]
- Levin FR, Levin HR, Nagoshi C. Autonomic functioning and cigarette smoking: Heart rate spectral analysis. Biol Psychiat. 1992;316:639–43. doi: 10.1016/0006-3223(92)90254-w. [DOI] [PubMed] [Google Scholar]
- Lewis MJ, Balaji G, Dixon H, Syed Y, Lewis KE. Influence of smoking abstinence and nicotine replacement therapy on heart rate and QT time-series. Clin Physiol Funct I. 2010;301:43–50. doi: 10.1111/j.1475-097X.2009.00902.x. [DOI] [PubMed] [Google Scholar]
- Lindsay GB, Rainey J. Psychosocial and pharmacologic explanations of nicotine’s “Gateway Drug” function. J School Health. 1997;674:123–126. doi: 10.1111/j.1746-1561.1997.tb03430.x. [DOI] [PubMed] [Google Scholar]
- Minami J, Ishimitsu T, Matsuoka H. Effects of smoking cessation on blood pressure and heart rate variability in habitual smokers. Hypertension. 1999;331:586–590. doi: 10.1161/01.hyp.33.1.586. [DOI] [PubMed] [Google Scholar]
- Munafó MR, Hitsman B, Rende R, Metcalfe C, Niaura R. Effects of progression to cigarette smoking on depressed mood in adolescents: Evidence from the National Longitudinal Study of Adolescent Health. Addiction. 2007;103:162–71. doi: 10.1111/j.1360-0443.2007.02052.x. [DOI] [PubMed] [Google Scholar]
- Munjal S, Koval T, Muhammad R, Jin Y, Demmel V, Roethig HJ, Mendes P, Unverdorben M. Heart rate variability increases with reductions in cigarette smoke exposure after 3 days. J Cardiovasc Pharm T. 2009;143:192–8. doi: 10.1177/1074248409340340. [DOI] [PubMed] [Google Scholar]
- Pederson W, von Soset T. Smoking, nicotine dependence and mental health among young adults: A 13-year population-based longitudinal study. Addiction. 2009;104:129–37. doi: 10.1111/j.1360-0443.2008.02395.x. [DOI] [PubMed] [Google Scholar]
- Pope CA, III, Eatough DJ, Gold DR, Pang Y, Nielsen KR, Nath P, Verrier RL, Kanner RE. Acute exposure to environmental tobacco smoke and heart rate variability. Environ Health Persp. 2001;109:711–716. doi: 10.1289/ehp.01109711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW. Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A Polyvagal Theory. Psychophysiology. 1995;324:301–18. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. Emotion: An evolutionary by-product of the neural regulation of the autonomic nervous system. Ann Ny Acad Sci. 1997;807:62–77. doi: 10.1111/j.1749-6632.1997.tb51913.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biol Psychol. 2007;742:116–43. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, Bohrer RE. Analyses of periodic processes in psycho physiological research. In: Cacioppo JT, Tassinary LG, editors. Principles of Psychophysiology: Physical, Social, and Inferential Elements. Cambridge University Press; New York: 1990. pp. 708–753. [Google Scholar]
- Price JF, Mowbray PI, Lee AJ, Rumley A, Lowe GD, Fowkes FG. Relationship between smoking and cardiovascular risk factors in the development of peripheral arterial disease and coronary artery disease: Edinburgh Artery Study. Eur Heart J. 1999;205:344–53. doi: 10.1053/euhj.1998.1194. [DOI] [PubMed] [Google Scholar]
- Prokhorov AV, Pallonen UE, Fava JL, Ding L, Niaura R. Measuring nicotine dependence among high-risk adolescent smokers. Addict Behav. 1996;211:117–127. doi: 10.1016/0306-4603(96)00048-2. [DOI] [PubMed] [Google Scholar]
- Sack M, Hopper JW, Lamprecht F. Low respiratory sinus arrhythmia and prolonged psychophysiological arousal in posttraumatic stress disorder: Heart rate dynamics and individual differences in arousal regulation. Biol Psychiat. 2004;553:284–290. doi: 10.1016/s0006-3223(03)00677-2. [DOI] [PubMed] [Google Scholar]
- Schroeder EB, Duanping L, Chambless LE, Prineas RJ, Evans GW, Heiss G. Hypertension, blood pressure, and heart rate variability. The atherosclerosis risk in communities ARIC study. Hypertension. 2003;42:1106–1111. doi: 10.1161/01.HYP.0000100444.71069.73. [DOI] [PubMed] [Google Scholar]
- Singh JP, Larson MG, O’Donnell CJ, Wilson PF, Tsuji H, Lloyd-Jones DM, Levy D. Association of hyperglycemia with reduced heart rate variability the Framingham heart study. Am J Cardiol. 2000;863:309–312. doi: 10.1016/s0002-9149(00)00920-6. [DOI] [PubMed] [Google Scholar]
- Sjoberg N, Saint DA. A single 4 mg dose of nicotine decreases heart rate variability in healthy nonsmokers: Implications for smoking cessation programs. Nicotine Tob Res. 2011;135:369–72. doi: 10.1093/ntr/ntr004. [DOI] [PubMed] [Google Scholar]
- Stein PK, Rottman JN, Kleiger RE. Effect of 21 mg transdermal nicotine patches and smoking cessation on heart rate variability. Am J Cardiol. 1996;779:701–705. doi: 10.1016/s0002-9149(97)89203-x. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disorders. 2000;613:201–16. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Valenti VE, Vanderlei LC, Ferreira C, Fonseca FL, Oliveira FR, Sousa FH, Rodrigues LM, Monteiro CB, Adami F, Wajnsztejn R, de Abreu LC. Sidestream cigarette smoke and cardiac autonomic regulation. Int Arch Med. 2013;6:11. doi: 10.1186/1755-7682-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veilleux JC, Kassel JD, Heinz AJ, Braun A, Wardle MC, Greenstein J, Evatt D, Conrad M. Predictors and sequelae of smoking topography over the course of a single cigarette in adolescent light smokers. J Adolescent Health. 2011;48:176–181. doi: 10.1016/j.jadohealth.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotsukura M, Koide Y, Fujii K, Tomono Y, Katayama A, Ando H, Suzuki J, Ishikawa K. Heart rate variability during the first month of smoking cessation. Am Heart J. 1998;1356(Pt 1):1004–1009. doi: 10.1016/s0002-8703(98)70065-1. [DOI] [PubMed] [Google Scholar]