Abstract
Purpose
Gut microbiota regulate intestinal function and health. However, mounting evidence indicates that they can also influence the immune and nervous systems and vice versa. Here we reviewed the bidirectional relationship between the gut microbiota and the brain, termed microbiota-gut-brain (MGB) axis, and we discuss how it contributes to the pathogenesis of certain disorders, that may involve brain inflammation.
Methods
Articles were chosen from Medline since 1980 using the key words anxiety, attention-deficit hypersensitivity disorder (ADHD), autism, cytokines, depression, gut, hypothalamic-pituitary-adrenal (HPA) axis, inflammation, immune system, microbiota, nervous system, neurologic, neurotransmitters, neuroimmune conditions, psychiatric, stress.
Findings
Various afferent or efferent pathways are involved in the MGB axis. Antibiotics, environmental and infectious agents, intestinal neurotransmitters/neuromodulators, sensory vagal fibers, cytokines, essential metabolites, all convey information about the intestinal state to the CNS. Conversely, the HPA axis, the CNS regulatory areas of satiety and neuropeptides released from sensory nerve fibers affect the gut microbiota composition directly or through nutrient availability. Such interactions appear to influence the pathogenesis of a number of disorders in which inflammation is implicated such as mood disorder, autism-spectrum disorders (ASDs), attention-deficit hypersensitivity disorder (ADHD), multiple sclerosis (MS) and obesity.
Implications
Recognition of the relationship between the MGB axis and the neuroimmune systems provides a novel approach for better understanding and management of these disorders. Appropriate preventive measures early in life or corrective measures such as use of psychobiotics, fecal microbiota transplantation and flavonoids are discussed.
Keywords: gut, microbiota, immune disorders, nervous system diseases, MGB axis, cytokines
Introduction
Humans have up to 37% gene homology with Bacteria and Archae1. Great numbers of commensal microorganisms reside on both the external and the internal surfaces of our bodies, especially the gut, outnumbering human somatic cells by approximately 10:12. Our colonization starts at birth during vaginal delivery with a maternal signature followed by complex “adult” microbiota after the first year of age3, 4. As a result, the human body is considered as a super-complex ecosystem, a social network with the gut microbiota having formed a permanent symbiotic relationship rather than a temporary form of parasitism5. Normally, the gastrointestinal (GI) microbiota has a symbiotic relationship with our enteric cells and contributes to basic physiological processes including digestion, growth and self-defense (Table 1).
Table 1.
Beneficial Functions of Gut Microbiota
|
An individual’s gut microbiota composition depends on the mode of delivery at birth, genetic predisposition, age, nutrition, physical activity, environmental factors, stress, infections, other diseases and use of antibiotics. Brain function and psychological make-up are now increasingly considered to have a reciprocal relationship with the gut6.
Disruption of the gut microbiota (dysbiosis) balance is known to contribute, among others, to the pathogenesis of GI diseases, especially inflammatory bowel disorder (IBD)7 and irritable bowel syndrome (IBS)8, especially since the gut microbiome regulates immunity9–13. In fact, bacteria reported to directly induce inflammation and pain14
Accumulating evidence suggests that the gut microbiota maintain bidirectional interactions with critical parts of the central nervous system (CNS) and the immune system through direct and indirect pathways (Table 2 and Fig. 1). These involve the endocrine [hypothalamic-pituitary-adrenal (HPA) axis], immune (chemokines, cytokines), autonomic nervous system (ANS) and enteric nervous systems forming the microbiota-gut-brain (MGB) axis6.
Table 2.
Pathways Involved in Bidirectional Communication Between Gut Microbiota, the Brain and the Immune System
| Afferent arm | |
| Pathways | Effect |
| Change of the gut microbiota due to usage of antibiotics/infectious agents/probiotic bacteria | Alteration in the circulating levels of pro/anti-inflammatory cytokines that affect brain function |
| Modulation of various host metabolic reactions | Production of essential metabolites (bile acids, choline, short-chain fatty acids) |
| Generation of neurotransmitters or neuromodulators in the intestinal lumen | Induction of epithelial cell release of molecules that stimulate afferent axons |
| Changes in tryptophan metabolism | Effects on behavior |
| Activation of sensory vagal fibers | Conveyance of information about the state of intestine to the CNS |
| Efferent arm | |
| Pathways | Effect |
| HPA axis activation | Regulation of immune cells locally in the gut and systematically affecting gut permeability, motility, secretion, barrier function and gut microbiota composition |
| Anti-inflammatory cholinergic reflex and/or sympathetic activation | Release of neurotransmitters that may affect gut microbiota composition, intestinal permeability and local immunity |
| Activation of CNS regulatory areas of satiety | Impact on nutrient availability to intestinal microbiota and their composition |
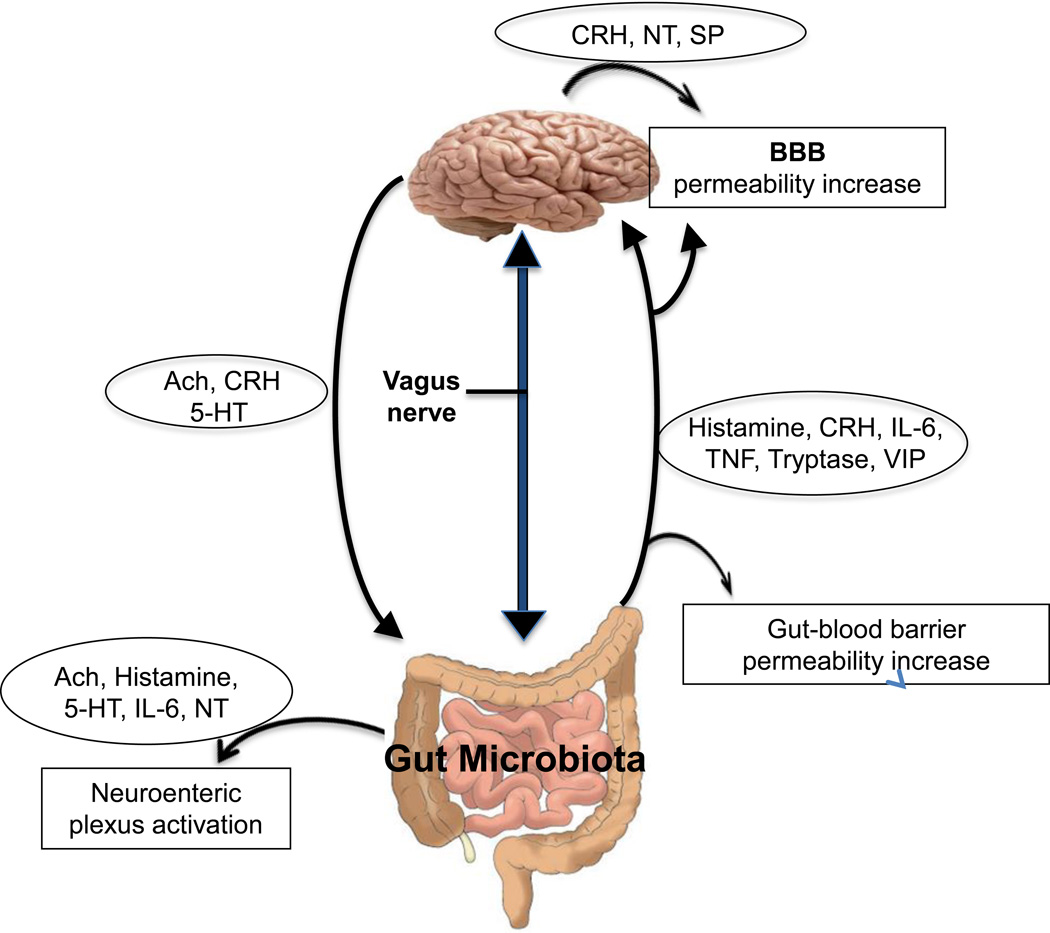
Figure 1. Diagrammatic representation of the M-G-B axis showing the proposed bidirectional communications.
Gut microbiota can release molecules that can: activate the neuroenteric plexus, stimulate brain production of neuropeptides, as well as increase gut-blood barrier and BBB permeability. The brain releases molecules that stimulate the neuroenteric plexus and gut function. The vagus nerve sends orthodromic and antidromic.
Ach= Acetylcholine
BBB= Blood-brain barrier
CRH= Corticotropin-releasing hormone
5-HT= 5-hydroxytryptamine
IL-6= Interleukin 6
NT= Neurotensin
SP= Substance P
TNF= Tumor necrosis factor
VIP= Vasoactive intestinal peptide
Neuro/immune-active substances derived from the intestinal lumen can penetrate the gut mucosa, be transported by blood, cross the blood-brain-barrier (BBB) and affect the CNS15. Gut microbiota can influence CNS function through their ability to synthesize or mimic a range of host-signaling neuroactive molecules, such as acetylcholine (Ach), catecholamines, gamma-aminobutyric acid (GABA), histamine, melatonin and 5-hydroxytryptamine (5-HT, serotonin)16. 5-HT is crucial in the regulation of peristalsis or modulation of sensation17.
Conversely the composition of gut microbiota is influenced by emotional and physiological stress18. One study found that healthy students during an extremely stressful time had fewer Lactobacilli present in their stool as compared to less stressful periods19. Maternal separation stress between 6–9 months of age in rhesus monkeys resulted in decreased faecal Lactobacilli20. Exposure to chronic stress in adult mice decreased the relative abundance of Bacteroides species and increased the Clostridium species in the caecum; moreover, it caused activation of the immune system as documented by increased IL-6 and CCL2 production21. Acute stress increased GI22, 23 and BBB24 permeability through activation of mast cells (MCs), which express high affinity receptors for CRH25. Moreover, chronic stress disrupted the intestinal barrier through MC activation and permitted penetration of luminal antigens, microflora metabolites, toxins and lipopolysaccharide (LPS) into the systemic circulation and the CNS26. In fact, stress-induced MC activation has been implicated in functional GI diseases27. Maternal separation stress in mice also increased intestinal MC-neuron communication28.
MCs communicate with pathogens29 and have been invoked as key modulatory cells in innate immunity30, as well as in inflammation31–34 and autoimmunity35. A new finding concerning MCs is their ability to secrete mitochondrial components, including DNA, extracellularly36. These components are then misconstrued by the body as “innate pathogens” and induce a strong auto-inflammatory response36 leading to inflammation and neuronal damage37. The microbiota can also modulate the immune system through other mechanisms38 And the increased use of antibiotics results in depletion of microbiota-derived metabolites, impairs immune homeostasis and contributes to chronic inflammation39.
Mood disorders
Genes involved in synapse formation between neurons in the brain and neurons in the GI tract are quite similar, and any mutations could possibly lead to both brain and GI abnormalities40. Recent studies analyzing the human genome in brains from diseased individuals with psychiatric disorders reported only two clusters of affected genes with: (a) increased inflammation and (b) decreased mitochondrial function41. Depression is associated with increased inflammatory biomarkers, such as interleukin (IL)-6, tumor necrosis factor (TNF)-α, and C reactive protein (CRP)42. Schizophrenia has been linked to intestinal inflammation43 and gastrojejunal ulcers44.
“Psychobiotics”, which are live organisms, when ingested may produce health benefits in patients suffering from mood disorders45. In a study of 124 healthy volunteers (mean age 61.8 years), those who consumed a mix of specific psychobiotics (Lactobacillus helveticus and bifidobacterium longum) exhibited less anxiety and depression19. Symptoms of “depression” were reported to decrease following probiotic treatment in the rat46. Additional studies showed beneficial effects of probiotics in animal models with altered behavioral phenotypes, as they reduced vagal-dependent activation of GABA receptors in response to physical and psychological stress46–51.
Studies in animals showed that certain bacterial species could reduce mood changes. For inastance, when Citrobacter rodentium was administrated orally to CF-1 mice, there was an increase in anxious-like behavior 7–8 hours following the infection, through activation of vagal pathways52. Postnatal colonization of germ-free (GF) mice by orally feeding them with different probiotics programmed the HPA for a stress response; for instance, when Campylobacter jejuni was given orally, it increased anxious-like behavior 7 hours after the infection53. Furthermore, a corresponding increase in brain-derived neurotrophic factor (BDNF) in the hippocampus and amygdala was evident and was eliminated after administration of antibiotic therapy in the mice53. Of note, BDNF is involved in the pathology of depression54 and Autism Spectrum Disorders (ASDs)55, while it is also considered a biomarker for gastric hypersensitivity56.
Attention-Deficit Hypersensitivity Disorder (ADHD) and Autism Spectrum Disorders (ASDs)
ADHD is a neurodevelopmental disorder characterized by lack of attention, impulsiveness and hyperactivity. Its cause is considered multifactorial, involving genetic pre-disposition, somatic mutations, epigenetic changes, perinatal factors (e.g. low birth weight, prematurity and prenatal exposure to alcohol and/or smoke), as well as environmental and socioeconomic factors57.
Increasing evidence from clinical and epidemiological studies suggests that children and adults with food allergies, eczema or asthma are associated with behavioral problems and neuropsychiatric disorders, including ADHD58–63. The gut microbiota are known to participate in susceptibility to allergies64, 65, especially food allergens66.
One meta-analysis reported that the Kaiser-Permanente (K-P) diet using elimination of salicylates, artificial food colors (AFC) and flavors, as well as the preservative butylated hydroxytoluene, could decrease the hyperactivity of ADHD children57. Children with ADHD were substantially improved on either an AFC-free diet67, or by dietary supplementations with polyunsaturated fatty acids (PUFA), iron and zinc68. In fact, PUFA levels in plasma of ADHD children were reported low69. Food-based treatments in children with allergic disorders significantly reduced ADHD-like behavior70.
Autism spectrum disorders (ASDs) are neurodevelopmental disorders characterized by deficits in social interactions and communication, along with repetitive and stereotyped behaviors71.
Many children with ASDs present with GI symptoms72–74 and altered GI flora71. Increasing evidence indicates that ASD pathogenesis may involve brain inflammation75 especially activation of microglia76, 77. Moreover, about 30% of children with ASDs have auto-antibodies against brain proteins78 and the presence of such antibodies strongly correlated with allergic symptoms79.
We recently showed that levels of the neuropeptide neurotensin (NT), found both in the brain and the gut and CRH were increased in the serum of children with ASDs; moreover, NT was significantly correlated with the presence of GI symptoms80. We also reported elevated levels of mitochondrial DNA in the serum of children with ASDs81 and CRH augmented the stimulatory effect of mitochondrial DNA on MCs82. A paper recently reported increased amount of mitochondrial DNA in peripheral mononuclear cells (PBMC) from patients with ASDs83. Extracellular mitochondrial DNA could derive either from MCs, PBMC, intestinal cells or bacteria and is misconstrued as “innate pathogens” leading to auto-inflammatory reactions84.
About 30% of ASD children are characterized by hyperserotonemia85 and a serotonin re-uptake transporter (SERT) gene mutation (SERT Ala56) was identified in some ASD children with hyperserotenemia86. Introduction of this mutation in mice resulted in communication delays and repetitive behaviors similar to those in children with ASDs86. In fact, 5-HT can affect the immune system28, and autoimmune neuroinflammation was treated with a tryptophan metabolite87.
The SERT Ala56 mice were also constipated and had bacterial intestinal overgrowth similar to what is often seen in children with ASDs88.
Increased intestinal permeability would permit bacterial products, cytokines and chemokines to enter the circulation and cross the BBB89 influencing brain and behavior. For example, children with ASDs had higher levels of immunoglobulins (IgA, IgG, IgM) against cow’s milk-derived allergens, and milk intake by these patients significantly worsened some of their behavioral symptoms70. Elimination of caseinomorphin, gliadomorphin, colorings, sweeteners and preservatives led to significant benefit70. The gut microbiota composition appears to differ between healthy children and those with ASDs71. For example, there was a higher prevalence of Bifidobacteria in healthy controls as compared to ASD patients90. In contrast, Bacteroides vulgatus and Desulfovibrio species were more commonly found in stools of ASDs children; however, only D. desulfuricans, D. fairfielddensis and D. piger were associated with regressive ASD. Clostridium species were increased at the expense of Bifidobacterium in ASD children with food allergies and pediatric IBD as compared to sex-matched controls children91. ASD children treated with oral vancomycin had significant improvement in behavioral, cognitive and GI symptoms92. Such findings are discussed in detail in another manuscript in this issue.
Such findings have led to the gut-to-brain connections being proposed as target for treatment of ASDs93.
Multiple Sclerosis (MS) and Neuromyelitis optica (NMO)
Multiple sclerosis (MS), an autoimmune disease characterized by progressive demyelination and deterioration of neurological function94, 95. It has been suggested that gut microbiota may contribute to the pathogenesis of MS96. One study showed that germ-free mice had delayed induction of experimental autoimmune encephalomyelitis (EAE), probably due to the attenuation of Th17 and auto-reactive B cell responses96. In another study, mice genetically predisposed to develop EAE spontaneously did not develop EAE when housed under germ-free conditions; however, this was reversed upon colonization with conventional microbiota in adulthood97. Even the presence of commensal microbiota promoted the induction of EAE in germ-free B6 mice due to decreased IFN-γ and IL-17 responses98. High-fat diet was found to increase EAE severity in mice, while caloric restriction attenuated EAE symptoms99.
Patients with NMO have aquaporin (AQP) autoantibodies (AQP4-seropositive) against the optic nerve and spinal cord, but also more antibodies against GI antigens than healthy controls100. Specifically, 37% of these patients had increased levels of antibodies at least against one of the following: gliadin, tissue transglutaminase (tTG), intrinsic factor (IF), parietal cells (PC) and Saccharomyces cerevisiae compared to 8% of healthy controls, with anti-gliadin and ASCA being the most frequent in AQP4-seropositive NMO (P=0.01 and P<0.05, respectively100. In addition, the AQP4-specific T-cells in NMO patients showed cross-reactivity to a protein of Clostridium perfrigens, supporting a microbiota-related molecular mimicry process in NMO pathogenesis101. MS102 and EAE103 are precipitated or worsen by stress, which is known to also affect the gut104. In fact, stress-induced gut alterations can impact the brain and behavior105.
5. Obesity
Obesity has been called a psychiatric disease106 and is associated with depression107 and other neuropsychiatric disorders43Adipocytokines can influence both the brain and the gut106. Recent evidence suggests that gut microbiota influence energy balance and weight68. Increased energy harvesting from diet, regulation of biologically active fatty acid tissue composition, chronic low-grade endotoxemia and modulation of gut-derived peptide secretion are some of the proposed routes that link gut microbiota with obesity108.
Gut microbiota may also contribute to low-grade inflammation in obesity109. Increased fat intake has been associated with increased serum levels of LPS in normal humans110 and mice111. This endotoxin can potentially trigger toll-like receptors (TLRs) in adipose or on pancreatic β-cells, contributing to both insulin resistance and β-cell damage112, 113. Experimental endotoxemia induced adipose inflammation and insulin resistance in lean human subjects114. Modulation of gut microbiota by using probiotics in obese mice was found to decrease high-fat-diet-induced LPS endotoxemia, as well as systemic and liver inflammation111, 112.
There are many studies with contradictory results concerning the types of bacteria that predominate in obese as compared to lean individuals115–117. For instance, metabolically obese mice with mutated leptin gene had different microbiota than mice without the mutation115. The same researchers later reported altered gut microbiota composition (reduction of Bacteroidetes and increase of Firmicute phyla) in obese human subjects compared to lean human subjects118. In contrast, other authors reported higher proportion of Bacteroidetes in overweight and obese subjects119. These conflicting results may be due to the variable methods of analysis and to the different profile of subjects.
Gut microbiota can convert undigested carbohydrates into short-chain-fatty acids (SCFA), like acetate, propionate and butyrate. These SCFAs are able to bind and activate two G-protein-coupled receptors (GPR41 and GPR43) on gut epithelial cells, leading to secretion of petide YY (PYY), which suppresses gut motility and retards intestinal transit108. It is interesting that propionate could induce an autistic-like phenotype in rats120.
Modulation of gut microbiota may have therapeutic potential in the management of metabolic disorders121.
Treatment and Future Directions
Therapeutic modulation of gut microbiota possibly by the use of pPre- and probiotics may be helpful in disorders involving MGB axis disturbances122. Prebiotics can benefit both intestinal mucosa and systemic immunity as they reach the large intestine non-hydrolyzed and stimulate the growth of beneficial intestinal microbiota123. Probiotics could restore intestinal permeability by improving mucosal barrier function124. Administration of different probiotics has been reported to be beneficial in humans with abdominal pain125, 126 and increased the pain threshold in rats127. Lactobacillus acidophilus induced the expression of the cannabinoid 2 and μ-opioid 1 receptors in the colonic epithelium128, while Lactobacillus farciminis inhibited stress-induced visceral hypersensitivity129. However, use of probiotics may result in both beneficial and detrimental effects. For example, there were beneficial effects in IBS with the use of probiotics Bifidobacterium infantis 35624130, 131 and Bifidobacterium lactis and animalis DN173010132 and of probiotic mixtures, such as Escherichia coli (DSM 17252) and Enterococcus faecalis (DSM 16440)133 or Lactobacillus rhamnosus GG, L. rhamnosus LC705, Bifidobacterium breve Bb99 and Propionibacterium freudenreichii ssp. shermanii JS134, 135. On the contrary, use of other probiotic mixtures, such as Lactobacillus paracasei spp. Paracasei F19, L. acidophilus La5 and Bifidobacterium lactis Bb12136, 137 or Lactobacillus plantarum MF1298138 had negative effects in IBS. Non-absorbable antibiotics (e.g. oral rifaximin) was shown to be beneficial in IBS139.
Natural flavonoids may be useful because they have immunoregulatory actions140. For instance, the quercetin glycoside rutin is cleaved by gut bacteria to liberate quercetin, which has anti-inflammatory actions141. Both quercetin, luteolin and tetramethoxyluteolin are potent inhibitors of MCs142.
Fecal microbiota transplantation (FMT) from a healthy donor can re-establish intestinal flora balance and could be used for specific GI diseaseS143, especially the treatment of Clostridium difficile infection144 and possibly be efficacious in IBD145.
Acknowledgments
Aspects of our work described above were funded by NIH grants NS38326 and AR47652, as well as the Autism Collaborative, the Autism Research Institute, National Autism Association, Safe Minds, the Johnson B. Johnson Fnd., the Michael and Margaret Johnson Family Fnd, The Nancy Laurie Marks Fnd, and Theta Biomedical Consulting and Development Co., Inc. (Brookline, MA, USA).
Footnotes
Disclosures
TCT is the inventor of US patents No. 6,624,148; 6,689,748; 6,984,667, and EPO 1365777, which cover methods and compositions of mast cell blockers, including flavonoids, as well as US patents No 7,906,153 and 8,268,365 for treatment of brain inflammation.
Conflicts of interests
The authors declare no conflicts.
Reference List
- 1.Fall-Ngai M, Hadfield MG, Bosch TC, et al. Animals in a bacterial world, a new imperative for the life sciences. Proc Natl Acad Sci U S A. 2013;110(9):3229–3236. doi: 10.1073/pnas.1218525110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belkaid Y, Naik S. Compartmentalized and systemic control of tissue immunity by commensals. Nat Immunol. 2013;14(7):646–653. doi: 10.1038/ni.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grenham S, Clarke G, Cryan JF, Dinan TG. Brain-gut-microbe communication in health and disease. Front Physiol. 2011;2:94. doi: 10.3389/fphys.2011.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5(7):e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7(7):688–693. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. 2011;12(8):453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J, Butcher J, Mack D, Stintzi A. Functional impacts of the intestinal microbiome in the pathogenesis of inflammatory bowel disease. Inflamm Bowel Dis. 2014;21(1):139–153. doi: 10.1097/MIB.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 8.Lee KN, Lee OY. Intestinal microbiota in pathophysiology and management of irritable bowel syndrome. World J Gastroenterol. 2014;20(27):8886–8897. doi: 10.3748/wjg.v20.i27.8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat Rev Immunol. 2006;6(4):318–328. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maranduba CM, De Castro SB, de Souza GT, et al. Intestinal Microbiota as Modulators of the Immune System and Neuroimmune System: Impact on the Host Health and Homeostasis. J Immunol Res. 2015;2015:931574. doi: 10.1155/2015/931574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salzman NH. The role of the microbiome in immune cell development. Ann Allergy Asthma Immunol. 2014;113(6):593–598. doi: 10.1016/j.anai.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 12.Spasova DS, Surh CD. Blowing on embers: commensal microbiota and our immune system. Front Immunol. 2014;5:318. doi: 10.3389/fimmu.2014.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar R, Parsad D, Kanwar AJ. Role of apoptosis and melanocytorrhagy: a comparative study of melanocyte adhesion in stable and unstable vitiligo. Br J Dermatol. 2011;164(1):187–191. doi: 10.1111/j.1365-2133.2010.10039.x. [DOI] [PubMed] [Google Scholar]
- 14.Chiu IM, Heesters BA, Ghasemlou N, et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nature. 2013;501(7465):52–57. doi: 10.1038/nature12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theoharides TC, Weinkauf C, Conti P. Brain cytokines and neuropsychiatric disorders. J Clin Psychopharmacol. 2004;24(6):577–581. doi: 10.1097/01.jcp.0000148026.86483.4f. [DOI] [PubMed] [Google Scholar]
- 16.Barrett E, Ross RP, O'Toole PW, Fitzgerald GF, Stanton C. gamma-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol. 2012;113(2):411–417. doi: 10.1111/j.1365-2672.2012.05344.x. [DOI] [PubMed] [Google Scholar]
- 17.Talley NJ. Serotoninergic neuroenteric modulators. Lancet. 2001;358(9298):2061–2068. doi: 10.1016/S0140-6736(01)07103-3. [DOI] [PubMed] [Google Scholar]
- 18.Dinan TG, Cryan JF. Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology. 2012;37(9):1369–1378. doi: 10.1016/j.psyneuen.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Dinan TG, Cryan JF. Melancholic microbes: a link between gut microbiota and depression? Neurogastroenterol Motil. 2013;25(9):713–719. doi: 10.1111/nmo.12198. [DOI] [PubMed] [Google Scholar]
- 20.Bailey MT, Lubach GR, Coe CL. Prenatal stress alters bacterial colonization of the gut in infant monkeys. J Pediatr Gastroenterol Nutr. 2004;38(4):414–421. doi: 10.1097/00005176-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun. 2011;25(3):397–407. doi: 10.1016/j.bbi.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Theoharidess TC. Irritable Bowel Syndrome and Ulcerative Colitis: Mast Cell Numbers are Increased, but Activation is more important. Dig Dis Sc. 2014;59(5):897–898. doi: 10.1007/s10620-013-2988-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Overman EL, Rivier JE, Moeser AJ. CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-alpha. PloS One. 2012;7(6):e39935. doi: 10.1371/journal.pone.0039935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esposito P, Chandler N, Kandere-Grzybowska K, et al. Corticotropin-releasing hormone (CRH) and brain mast cells regulate blood-brain-barrier permeability induced by acute stress. J Pharmacol Exp Ther. 2002;303:1061–1066. doi: 10.1124/jpet.102.038497. [DOI] [PubMed] [Google Scholar]
- 25.Cao J, Papadopoulou N, Kempuraj D, et al. Human mast cells express corticotropin-releasing hormone (CRH) receptors and CRH leads to selective secretion of vascular endothelial growth factor. J Immunol. 2005;174(12):7665–7675. doi: 10.4049/jimmunol.174.12.7665. [DOI] [PubMed] [Google Scholar]
- 26.Santos J, Yang PC, Soderholm JD, Benjamin M, Perdue MH. Role of mast cells in chronic stress induced colonic epithelial barrier dysfunction in the rat. Gut. 2001;48(5):630–636. doi: 10.1136/gut.48.5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rijnierse A, Nijkamp FP, Kraneveld AD. Mast cells and nerves tickle in the tummy: implications for inflammatory bowel disease and irritable bowel syndrome. Pharmacol Ther. 2007;116(2):207–235. doi: 10.1016/j.pharmthera.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Idzko M, Panther E, Stratz C, et al. The serotoninergic receptors of human dendritic cells: identification and coupling to cytokine release. J Immunol. 2004;172(10):6011–6019. doi: 10.4049/jimmunol.172.10.6011. [DOI] [PubMed] [Google Scholar]
- 29.Marshall JS. Mast-cell responses to pathogens. Nat Rev Immunol. 2004;4(10):787–799. doi: 10.1038/nri1460. [DOI] [PubMed] [Google Scholar]
- 30.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6(2):135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 31.Theoharides TC, Alysandratos KD, Angelidou A, et al. Mast cells and inflammation. Biochim Biophys Acta. 2010;1822(1):21–33. doi: 10.1016/j.bbadis.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kinet JP. The essential role of mast cells in orchestrating inflammation. Immunol Rev. 2007;217:5–7. doi: 10.1111/j.1600-065X.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- 33.Sismanopoulos N, Delivanis DA, Alysandratos KD, et al. Mast cells in allergic and inflammatory diseases. Curr Pharm Des. 2012;18(16):2261–2277. doi: 10.2174/138161212800165997. [DOI] [PubMed] [Google Scholar]
- 34.Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454(7203):445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rottem M, Mekori YA. Mast cells and autoimmunity. Autoimmun Rev. 2005;4:21–27. doi: 10.1016/j.autrev.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Zhang B, Asadi S, Weng Z, Sismanopoulos N, Theoharides TC. Stimulated human mast cells secrete mitochondrial components that have autocrine and paracrine inflammatory actions. PloS One. 2012;7(12):e49767. doi: 10.1371/journal.pone.0049767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lauritzen KH, Moldestad O, Eide L, et al. Mitochondrial DNA toxicity in forebrain neurons causes apoptosis, neurodegeneration, and impaired behavior. Mol Cell Biol. 2010;30(6):1357–1367. doi: 10.1128/MCB.01149-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelly D, Mulder IE. Microbiome and immunological interactions. Nutr Rev. 2012;70(Suppl 1):S18–S30. doi: 10.1111/j.1753-4887.2012.00498.x. [DOI] [PubMed] [Google Scholar]
- 39.Lopez CA, Kingsbury DD, Velazquez EM, Baumler AJ. Collateral damage: microbiota-derived metabolites and immune function in the antibiotic era. Cell Host Microbe. 2014;16(2):156–163. doi: 10.1016/j.chom.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hadhazy A. Think Twice: How the Gut's "Second Brain" Influences Mood and Well-Being. Scientific American. 2010 http://www.scientificamerican.com/article/gut-second-brain/. Ref Type: Magazine Article. [Google Scholar]
- 41.Theoharides TC, Zhang B, Conti P. Decreased mitochondrial function and increased brain inflammation in bipolar disorder and other neuropsychiatric diseases. J Clin Psychopharmacol. 2011;31(6):685–687. doi: 10.1097/JCP.0b013e318239c190. [DOI] [PubMed] [Google Scholar]
- 42.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Severance EG, Alaedini A, Yang S, et al. Gastrointestinal inflammation and associated immune activation in schizophrenia. Schizophr Res. 2012;138(1):48–53. doi: 10.1016/j.schres.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ozdemir V, Jamal MM, Osapay K, et al. Cosegregation of gastrointestinal ulcers and schizophrenia in a large national inpatient discharge database: revisiting the "brain-gut axis" hypothesis in ulcer pathogenesis. J Investig Med. 2007;55(6):315–320. doi: 10.2310/6650.2007.00014. [DOI] [PubMed] [Google Scholar]
- 45.Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74(10):720–726. doi: 10.1016/j.biopsych.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 46.Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008;43(2):164–174. doi: 10.1016/j.jpsychires.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 47.Hsiao EY, McBride SW, Hsien S, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155(7):1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bravo JA, Forsythe P, Chew MV, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108(38):16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.it-Belgnaoui A, Durand H, Cartier C, et al. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology. 2012;37(11):1885–1895. doi: 10.1016/j.psyneuen.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 50.Bercik P, Park AJ, Sinclair D, et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil. 2011;23(12):1132–1139. doi: 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.rseneault-Breard J, Rondeau I, Gilbert K, et al. Combination of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 reduces post-myocardial infarction depression symptoms and restores intestinal permeability in a rat model. Br J Nutr. 2012;107(12):1793–1799. doi: 10.1017/S0007114511005137. [DOI] [PubMed] [Google Scholar]
- 52.Lyte M, Li W, Opitz N, Gaykema RP, Goehler LE. Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium. Physiol Behav. 2006;89(3):350–357. doi: 10.1016/j.physbeh.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 53.Sudo N, Chida Y, Aiba Y, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558(Pt 1):263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kotan Z, Sarandol E, Kirhan E, Ozkaya G, Kirli S. Serum brain-derived neurotrophic factor, vascular endothelial growth factor and leptin levels in patients with a diagnosis of severe major depressive disorder with melancholic features. Ther Adv Psychopharmacol. 2012;2(2):65–74. doi: 10.1177/2045125312436572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Theoharides TC, Athanassiou M, Panagiotidou S, Doyle R. Dysregulated brain immunity and neurotrophin signaling in Rett syndrome and autism spectrum disorders. J Neuroimmunol. 2015 doi: 10.1016/j.jneuroim.2014.12.003. in press. [DOI] [PubMed] [Google Scholar]
- 56.Winston JH, Sarna SK. Developmental origins of functional dyspepsia-like gastric hypersensitivity in rats. Gastroenterology. 2013;144(3):570–579. doi: 10.1053/j.gastro.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schab DW, Trinh NH. Do artificial food colors promote hyperactivity in children with hyperactive syndromes? A meta-analysis of double-blind placebo-controlled trials. J Dev Behav Pediatr. 2004;25(6):423–434. doi: 10.1097/00004703-200412000-00007. [DOI] [PubMed] [Google Scholar]
- 58.Chang HY, Seo JH, Kim HY, et al. Allergic diseases in preschoolers are associated with psychological and behavioural problems. Allergy Asthma Immunol Res. 2013;5(5):315–321. doi: 10.4168/aair.2013.5.5.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Genuneit J, Braig S, Brandt S, et al. Infant atopic eczema and subsequent attention-deficit/hyperactivity disorder - A prospective birth cohort study. Pediatr Allergy Immunol. 2014;25(1):51–56. doi: 10.1111/pai.12152. [DOI] [PubMed] [Google Scholar]
- 60.Tsai JD, Chang SN, Mou CH, Sung FC, Lue KH. Association between atopic diseases and attention-deficit/hyperactivity disorder in childhood: a population-based case-control study. Ann Epidemiol. 2013;23(4):185–188. doi: 10.1016/j.annepidem.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 61.Jedrychowski W, Maugeri U, Perera F, et al. Cognitive function of 6-year old children exposed to mold-contaminated homes in early postnatal period. Prospective birth cohort study in Poland. Physiol Behav. 2011;104(5):989–995. doi: 10.1016/j.physbeh.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scahill L, Graft-Johnson A. Food allergies, asthma, and attention deficit hyperactivity disorder. J Child Adolesc Psychiatr Nurs. 1997;10(2):36–40. doi: 10.1111/j.1744-6171.1997.tb00279.x. [DOI] [PubMed] [Google Scholar]
- 63.Schmitt J, Romanos M, Schmitt NM, Meurer M, Kirch W. Atopic eczema and attention-deficit/hyperactivity disorder in a population-based sample of children and adolescents. JAMA. 2009;301(7):724–726. doi: 10.1001/jama.2009.136. [DOI] [PubMed] [Google Scholar]
- 64.Abrahamsson TR, Wu RY, Jenmalm MC. Gut microbiota and allergy: the importance of the pregnancy period. Pediatr Res. 2015;77(1–2):214–219. doi: 10.1038/pr.2014.165. [DOI] [PubMed] [Google Scholar]
- 65.Kim BJ, Lee SY, Kim HB, Lee E, Hong SJ. Environmental changes, microbiota, and allergic diseases. Allergy Asthma Immunol Res. 2014;6(5):389–400. doi: 10.4168/aair.2014.6.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cao S, Feehley TJ, Nagler CR. The role of commensal bacteria in the regulation of sensitization to food allergens. FEBS Lett. 2014;588(22):4258–4266. doi: 10.1016/j.febslet.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nigg JT, Lewis K, Edinger T, Falk M. Meta-analysis of attention-deficit/hyperactivity disorder or attention-deficit/hyperactivity disorder symptoms, restriction diet, and synthetic food color additives. J Am Acad Child Adolesc Psychiatry. 2012;51(1):86–97. doi: 10.1016/j.jaac.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Murphy EF, Cotter PD, Healy S, et al. Composition and energy harvesting capacity of the gut microbiota: relationship to diet, obesity and time in mouse models. Gut. 2010;59(12):1635–1642. doi: 10.1136/gut.2010.215665. [DOI] [PubMed] [Google Scholar]
- 69.Gillies D, Sinn JK, Lad SS, Leach MJ, Ross MJ. Polyunsaturated fatty acids (PUFA) for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev. 2012;7:CD007986. doi: 10.1002/14651858.CD007986.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Theije CG, Bavelaar BM, Lopes da SS, et al. Food allergy and food-based therapies in neurodevelopmental disorders. Pediatr Allergy Immunol. 2014;25(3):218–226. doi: 10.1111/pai.12149. [DOI] [PubMed] [Google Scholar]
- 71.Finegold SM. State of the art; microbiology in health and disease. Intestinal bacterial flora in autism. Anaerobe. 2011;17(6):367–368. doi: 10.1016/j.anaerobe.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 72.Horvath K, Papadimitriou JC, Rabsztyn A, Drachenberg C, Tildon JT. Gastrointestinal abnormalities in children with autistic disorder. J Pediatr. 1999;135(5):559–563. doi: 10.1016/s0022-3476(99)70052-1. [DOI] [PubMed] [Google Scholar]
- 73.White JF. Intestinal pathophysiology in autism. Exp Biol Med (Maywood) 2003;228(6):639–649. doi: 10.1177/153537020322800601. [DOI] [PubMed] [Google Scholar]
- 74.Buie T, Campbell DB, Fuchs GJ, III, et al. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: a consensus report. Pediatrics. 2010;125(Suppl 1):S1–S18. doi: 10.1542/peds.2009-1878C. [DOI] [PubMed] [Google Scholar]
- 75.Theoharides TC, Asadi S, Patel A. Focal brain inflammation and autism. J Neuroinflammation. 2013;10(46) doi: 10.1186/1742-2094-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhan Y, Paolicelli RC, Sforazzini F, et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci. 2014;17(3):400–406. doi: 10.1038/nn.3641. [DOI] [PubMed] [Google Scholar]
- 77.Gupta S, Ellis SE, Ashar FN, et al. Transcriptome analysis reveals dysregulation of innate immune response genes and neuronal activity-dependent genes in autism. Nat Commun. 2014;5:5748. doi: 10.1038/ncomms6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wills S, Cabanlit M, Bennett J, Ashwood P, Amaral DG, Van de Water J. Detection of autoantibodies to neural cells of the cerebellum in the plasma of subjects with autism spectrum disorders. Brain Behav Immun. 2008;23:64–74. doi: 10.1016/j.bbi.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mostafa GA, Al-Ayadhi LY. The possible relationship between allergic manifestations and elevated serum levels of brain specific auto-antibodies in autistic children. J Neuroimmunol. 2013;261(1–2):77–81. doi: 10.1016/j.jneuroim.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 80.Tsilioni I, Dodman N, Petra AI, et al. Elevated serum neurotensin and CRH levels in children with autistic spectrum disorders and tail-chasing bull terriers with a phenotype similar to autism. Transl Psychiatry. 2014;4:e466. doi: 10.1038/tp.2014.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang B, Angelidou A, Alysandratos KD, et al. Mitochondrial DNA and anti-mitochondrial antibodies in serum of autistic children. J Neuroinflammation. 2010;7(1):80. doi: 10.1186/1742-2094-7-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Asadi S, Theoharides TC. Corticotropin-releasing hormone and extracellular mitochondria augment IgE-stimulated human mast-cell vascular endothelial growth factor release, which is inhibited by luteolin. J Neuroinflam. 2012;9(1):85. doi: 10.1186/1742-2094-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen S, Zongchang L, He Y, et al. Elevated mitochondrial DNA copy number in peripheral blood cells is associated with childhood autism. BMC Psychiatry. 2015;15(50) doi: 10.1186/s12888-015-0432-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Theoharides TC, Asadi S, Panagiotidou S, Weng Z. The "missing link" in autoimmunity and autism: Extracellular mitochondrial components secreted from activated live mast cells. Autoimmun Rev. 2013;12(12):1136–1142. doi: 10.1016/j.autrev.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 85.Hranilovic D, Bujas-Petkovic Z, Tomicic M, Bordukalo-Niksic T, Blazevic S, Cicin-Sain L. Hyperserotonemia in autism: activity of 5HT-associated platelet proteins. J Neural Transm. 2009;116(4):493–501. doi: 10.1007/s00702-009-0192-2. [DOI] [PubMed] [Google Scholar]
- 86.Veenstra-Vanderweele J, Jessen TN, Thompson BJ, et al. Modeling rare gene variation to gain insight into the oldest biomarker in autism: construction of the serotonin transporter Gly56Ala knock-in mouse. J Neurodev Disord. 2009;1(2):158–171. doi: 10.1007/s11689-009-9020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Platten M, Ho PP, Youssef S, et al. Treatment of autoimmune neuroinflammation with a synthetic tryptophan metabolite. Science. 2005;310(5749):850–855. doi: 10.1126/science.1117634. [DOI] [PubMed] [Google Scholar]
- 88.Kerr TM, Muller CL, Miah M, et al. Genetic background modulates phenotypes of serotonin transporter Ala56 knock-in mice. Mol Autism. 2013;4(1):35. doi: 10.1186/2040-2392-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Banks WA, Erickson MA. The blood-brain barrier and immune function and dysfunction. Neurobiol Dis. 2010;37(1):26–32. doi: 10.1016/j.nbd.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 90.Finegold SM, Molitoris D, Song Y, et al. Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis. 2002;35(Suppl 1):S6–S16. doi: 10.1086/341914. [DOI] [PubMed] [Google Scholar]
- 91.Adams JB, Johansen LJ, Powell LD, Quig D, Rubin RA. Gastrointestinal flora and gastrointestinal status in children with autism--comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011;11:22. doi: 10.1186/1471-230X-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sandler RH, Finegold SM, Bolte ER, et al. Short-term benefit from oral vancomycin treatment of regressive-onset autism. J Child Neurol. 2000;15(7):429–435. doi: 10.1177/088307380001500701. [DOI] [PubMed] [Google Scholar]
- 93.de Theije CG, Wu J, da Silva SL, et al. Pathways underlying the gut-to-brain connection in autism spectrum disorders as future targets for disease management. Eur J Pharmacol. 2011;668(Suppl 1):S70–S80. doi: 10.1016/j.ejphar.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 94.Hemmer B, Kerschensteiner M, Korn T. Role of the innate and adaptive immune responses in the course of multiple sclerosis. Lancet Neurol. 2015;14(4):406–419. doi: 10.1016/S1474-4422(14)70305-9. [DOI] [PubMed] [Google Scholar]
- 95.Mahad DH, Trapp BD, Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015;14(2):183–193. doi: 10.1016/S1474-4422(14)70256-X. [DOI] [PubMed] [Google Scholar]
- 96.Berer K, Mues M, Koutrolos M, et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479(7374):538–541. doi: 10.1038/nature10554. [DOI] [PubMed] [Google Scholar]
- 97.Berer K, Mues M, Koutrolos M, et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479(7374):538–541. doi: 10.1038/nature10554. [DOI] [PubMed] [Google Scholar]
- 98.Ochoa-Reparaz J, Mielcarz DW, Ditrio LE, et al. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J Immunol. 2009;183(10):6041–6050. doi: 10.4049/jimmunol.0900747. [DOI] [PubMed] [Google Scholar]
- 99.Piccio L, Stark JL, Cross AH. Chronic calorie restriction attenuates experimental autoimmune encephalomyelitis. J Leukoc Biol. 2008;84(4):940–948. doi: 10.1189/jlb.0208133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Banati M, Csecsei P, Koszegi E, et al. Antibody response against gastrointestinal antigens in demyelinating diseases of the central nervous system. Eur J Neurol. 2013;20(11):1492–1495. doi: 10.1111/ene.12072. [DOI] [PubMed] [Google Scholar]
- 101.Varrin-Doyer M, Spencer CM, Schulze-Topphoff U, et al. Aquaporin 4-specific T cells in neuromyelitis optica exhibit a Th17 bias and recognize Clostridium ABC transporter. Ann Neurol. 2012;72(1):53–64. doi: 10.1002/ana.23651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Theoharides TC, Enakuaa S, Sismanopoulos N, et al. Contribution of stress to asthma worsening through mast cell activation. Ann Allergy Asthma Immunol. 2012;109(1):14–19. doi: 10.1016/j.anai.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 103.Chandler N, Jacobson S, Connolly R, Esposito P, Theoharides TC. Acute stress shortens the time of onset of experimental allergic encephalomyelitis (EAE) in SJL/J mice. Brain Behav Immun. 2002;16:757–763. doi: 10.1016/s0889-1591(02)00028-4. [DOI] [PubMed] [Google Scholar]
- 104.Tache Y, Martinez V, Million M, Wang L. Stress and the gastrointestinal tract III. Stress-related alterations of gut motor function: role of brain corticotropin-releasing factor receptors. Am J Physiol Gastrointest Liver Physiol. 2001;280(2):G173–G177. doi: 10.1152/ajpgi.2001.280.2.G173. [DOI] [PubMed] [Google Scholar]
- 105.Gur TL, Worly BL, Bailey MT. Stress and the commensal microbiota: importance in parturition and infant neurodevelopment. Front Psychiatry. 2015;6:5. doi: 10.3389/fpsyt.2015.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Aguilar-Valles A, Inoue W, Rummel C, Luheshi GN. Obesity, adipokines and neuroinflammation. Neuropharmacology. 2015 doi: 10.1016/j.neuropharm.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 107.Byrne ML, O'Brien-Simpson NM, Mitchell SA, Allen NB. Adolescent-Onset Depression: Are Obesity and Inflammation Developmental Mechanisms or Outcomes? Child Psychiatry Hum Dev. 2015 doi: 10.1007/s10578-014-0524-9. [DOI] [PubMed] [Google Scholar]
- 108.Musso G, Gambino R, Cassader M. Obesity, diabetes, and gut microbiota: the hygiene hypothesis expanded? Diabetes Care. 2010;33(10):2277–2284. doi: 10.2337/dc10-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Manco M, Putignani L, Bottazzo GF. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr Rev. 2010;31(6):817–844. doi: 10.1210/er.2009-0030. [DOI] [PubMed] [Google Scholar]
- 110.Amar J, Burcelin R, Ruidavets JB, et al. Energy intake is associated with endotoxemia in apparently healthy men. Am J Clin Nutr. 2008;87(5):1219–1223. doi: 10.1093/ajcn/87.5.1219. [DOI] [PubMed] [Google Scholar]
- 111.Cani PD, Neyrinck AM, Fava F, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50(11):2374–2383. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 112.Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 113.Creely SJ, McTernan PG, Kusminski CM, et al. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;292(3):E740–E747. doi: 10.1152/ajpendo.00302.2006. [DOI] [PubMed] [Google Scholar]
- 114.Mehta NN, McGillicuddy FC, Anderson PD, et al. Experimental endotoxemia induces adipose inflammation and insulin resistance in humans. Diabetes. 2010;59(1):172–181. doi: 10.2337/db09-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102(31):11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Backhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101(44):15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lyra A, Lahtinen S, Tiihonen K, Ouwehand AC. Intestinal microbiota and overweight. Benef Microbes. 2010;1(4):407–421. doi: 10.3920/BM2010.0030. [DOI] [PubMed] [Google Scholar]
- 118.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 119.Schwiertz A, Taras D, Schafer K, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 2010;18(1):190–195. doi: 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- 120.Thomas RH, Meeking MM, Mepham JR, et al. The enteric bacterial metabolite propionic acid alters brain and plasma phospholipid molecular species: further development of a rodent model of autism spectrum disorders. J Neuroinflammation. 2012;9:153. doi: 10.1186/1742-2094-9-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Erejuwa OO, Sulaiman SA, Ab Wahab MS. Modulation of gut microbiota in the management of metabolic disorders: the prospects and challenges. Int J Mol Sci. 2014;15(3):4158–4188. doi: 10.3390/ijms15034158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ianiro G, Bibbo S, Gasbarrini A, Cammarota G. Therapeutic modulation of gut microbiota: current clinical applications and future perspectives. Curr Drug Targets. 2014;15(8):762–770. doi: 10.2174/1389450115666140606111402. [DOI] [PubMed] [Google Scholar]
- 123.Langlands SJ, Hopkins MJ, Coleman N, Cummings JH. Prebiotic carbohydrates modify the mucosa associated microflora of the human large bowel. Gut. 2004;53(11):1610–1616. doi: 10.1136/gut.2003.037580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ramakrishna BS. Probiotic-induced changes in the intestinal epithelium: implications in gastrointestinal disease. Trop Gastroenterol. 2009;30(2):76–85. [PubMed] [Google Scholar]
- 125.Bercik P, Collins SM, Verdu EF. Microbes and the gut-brain axis. Neurogastroenterol Motil. 2012;24(5):405–413. doi: 10.1111/j.1365-2982.2012.01906.x. [DOI] [PubMed] [Google Scholar]
- 126.Clarke G, Cryan JF, Dinan TG, Quigley EM. Review article: probiotics for the treatment of irritable bowel syndrome--focus on lactic acid bacteria. Aliment Pharmacol Ther. 2012;35(4):403–413. doi: 10.1111/j.1365-2036.2011.04965.x. [DOI] [PubMed] [Google Scholar]
- 127.McKernan DP, Fitzgerald P, Dinan TG, Cryan JF. The probiotic Bifidobacterium infantis 35624 displays visceral antinociceptive effects in the rat. Neurogastroenterol Motil. 2010;22(9):1029–1035. e268. doi: 10.1111/j.1365-2982.2010.01520.x. [DOI] [PubMed] [Google Scholar]
- 128.Rousseaux C, Thuru X, Gelot A, et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med. 2007;13(1):35–37. doi: 10.1038/nm1521. [DOI] [PubMed] [Google Scholar]
- 129.it-Belgnaoui A, Han W, Lamine F, et al. Lactobacillus farciminis treatment suppresses stress induced visceral hypersensitivity: a possible action through interaction with epithelial cell cytoskeleton contraction. Gut. 2006;55(8):1090–1094. doi: 10.1136/gut.2005.084194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Aroniadis OC, Brandt LJ. Fecal microbiota transplantation: past, present and future. Curr Opin Gastroenterol. 2013;29(1):79–84. doi: 10.1097/MOG.0b013e32835a4b3e. [DOI] [PubMed] [Google Scholar]
- 131.Whorwell PJ, Altringer L, Morel J, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101(7):1581–1590. doi: 10.1111/j.1572-0241.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- 132.Guglielmetti S, Mora D, Gschwender M, Popp K. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life--a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33(10):1123–1132. doi: 10.1111/j.1365-2036.2011.04633.x. [DOI] [PubMed] [Google Scholar]
- 133.Enck P, Zimmermann K, Menke G, Muller-Lissner S, Martens U, Klosterhalfen S. A mixture of Escherichia coli (DSM 17252) and Enterococcus faecalis (DSM 16440) for treatment of the irritable bowel syndrome--a randomized controlled trial with primary care physicians. Neurogastroenterol Motil. 2008;20(10):1103–1109. doi: 10.1111/j.1365-2982.2008.01156.x. [DOI] [PubMed] [Google Scholar]
- 134.Kajander K, Hatakka K, Poussa T, Farkkila M, Korpela R. A probiotic mixture alleviates symptoms in irritable bowel syndrome patients: a controlled 6-month intervention. Aliment Pharmacol Ther. 2005;22(5):387–394. doi: 10.1111/j.1365-2036.2005.02579.x. [DOI] [PubMed] [Google Scholar]
- 135.Kajander K, Krogius-Kurikka L, Rinttila T, Karjalainen H, Palva A, Korpela R. Effects of multispecies probiotic supplementation on intestinal microbiota in irritable bowel syndrome. Aliment Pharmacol Ther. 2007;26(3):463–473. doi: 10.1111/j.1365-2036.2007.03391.x. [DOI] [PubMed] [Google Scholar]
- 136.Sondergaard B, Olsson J, Ohlson K, Svensson U, Bytzer P, Ekesbo R. Effects of probiotic fermented milk on symptoms and intestinal flora in patients with irritable bowel syndrome: a randomized, placebo-controlled trial. Scand J Gastroenterol. 2011;46(6):663–672. doi: 10.3109/00365521.2011.565066. [DOI] [PubMed] [Google Scholar]
- 137.Simren M, Ohman L, Olsson J, et al. Clinical trial: the effects of a fermented milk containing three probiotic bacteria in patients with irritable bowel syndrome - a randomized, double-blind, controlled study. Aliment Pharmacol Ther. 2010;31(2):218–227. doi: 10.1111/j.1365-2036.2009.04183.x. [DOI] [PubMed] [Google Scholar]
- 138.Ligaarden SC, Axelsson L, Naterstad K, Lydersen S, Farup PG. A candidate probiotic with unfavourable effects in subjects with irritable bowel syndrome: a randomised controlled trial. BMC Gastroenterol. 2010;10:16. doi: 10.1186/1471-230X-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pimentel M, Lembo A, Chey WD, et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364(1):22–32. doi: 10.1056/NEJMoa1004409. [DOI] [PubMed] [Google Scholar]
- 140.Gee JM, Johnson IT. Polyphenolic compounds: interactions with the gut and implications for human health. Curr Med Chem. 2001;8(11):1245–1255. doi: 10.2174/0929867013372256. [DOI] [PubMed] [Google Scholar]
- 141.Mascaraque C, Aranda C, Ocon B, et al. Rutin has intestinal antiinflammatory effects in the CD4+ CD62L+ T cell transfer model of colitis. Pharmacol Res. 2014;90:48–57. doi: 10.1016/j.phrs.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 142.Weng Z, Patel A, Panagiotidou S, Theoharides TC. The novel flavone tetramethoxyluteolin is a potent inhibitor of human mast cells. J Allergy Clin Immunol. 2014;14:01574–01577. doi: 10.1016/j.jaci.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Aroniadis OC, Brandt LJ. Intestinal microbiota and the efficacy of fecal microbiota transplantation in gastrointestinal disease. Gastroenterol Hepatol (N Y) 2014;10(4):230–237. [PMC free article] [PubMed] [Google Scholar]
- 144.van NE, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368(5):407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 145.Colman RJ, Rubin DT. Fecal microbiota transplantation as therapy for inflammatory bowel disease: A systematic review and meta-analysis. J Crohns Colitis. 2014;8(12):1569–1581. doi: 10.1016/j.crohns.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]