Abstract
Serum osteocalcin (Oc) concentration is a highly specific measure of bone turnover, but its circulating proteoform(s) have not been well defined. Based on immunological methods, the major forms are thought to be the intact polypeptide and a large N-terminal-mid molecule fragment for which there is no consensus on the precise sequence. Vitamin K-dependent gamma (γ)-carboxylated variants of Oc are also found in circulation but there have been no methods that can define how many of the three potential γ-carboxyglutamic acid (Gla) residues are γ-carboxylated or provide their relative abundances. Recent reports that uncarboxylated and partially γ-carboxylated Oc forms have hormonal function underscore the need for precise evaluation of Oc at all three potential γ-carboxylation sites. Herein, mass spectrometric immunoassay (MSIA) was used to provide qualitative and semiquantitative (relative percent abundance) information on Oc molecular variants as they exist in individual plasma and serum samples. Following verification that observable Oc proteoforms were accurately assigned and not simply ex vivo artifacts, MALDI-MSIA and ESI-MSIA were used to assess the relative abundance of Oc truncation and γ-carboxylation, respectively, in plasma from 130 patients enrolled in vitamin K supplementation trials. Human Oc was found to circulate in over a dozen truncated forms with each of these displaying anywhere from 0–3 Gla residues. The relative abundance of truncated forms was consistent and unaffected by vitamin K supplementation. In contrast, when compared with placebo, vitamin K supplementation dramatically increased the fractional abundance of Oc with three Gla residues, corresponding to a decrease in the fractional abundance of Oc with zero Gla residues. These findings unequivocally document that increased vitamin K intake reduces the uncarboxylated form of Oc. Several reports of a positive effect of vitamin K intake on insulin sensitivity in humans have shown that un- or undercarboxylation of Oc, unlike in mice, is not associated with insulin resistance. Analyses similar to those described here will be useful to understand the functional significance of Oc γ-carboxylation in human health and disease.
Osteocalcin (Oc)1 is a member of the family of vitamin K-dependent gamma (γ)-carboxylated proteins. The formation of γ-carboxyglutamic acid (Gla) occurs via the carboxylation of three specific glutamic acid residues in the mid-molecular region of Oc (E17, E21, and E24) and results in the binding of Oc to hydroxyapatite in bone (1). In circulation Oc is a highly specific bone marker that has been used for assessing relative degrees of bone turnover in clinical studies (2). Based on immunological methods, a general notion has been that the major forms of circulating Oc are the intact molecule and a large N-terminal-mid molecule fragment encompassing residues 1–43, thought to be the result of trypsin-like activity in serum or poor sample handling (3). However, the precise sequence has never been clearly defined and considerable inconsistency is evident when comparing values from different laboratories or commercial kits because of differences in antibody specificity.
Carboxylated variants of Oc are also found both in human bone and in circulation. Initial observational studies that reported an association between poor vitamin K status and bone loss attributed the finding to an increased proportion of Oc in circulation that was not carboxylated, reflecting a nonfunctional protein in bone (4–6). However, assays for total Oc are indifferent to carboxylation status, and methods used to measure the carboxylation state of circulating Oc do not distinguish among the fully, partially, or uncarboxylated forms (7, 8). Therefore, even though the amount of undercarboxylated Oc relative to the total in circulation (%ucOC) is a biomarker of vitamin K status in bone, there is no consensus on the precise amount in circulation or how many of the three potential Gla residues are carboxylated.
Recently, mouse models have indicated that circulating Oc also serves as an endocrine hormone with a positive role in glucose metabolism (9). Paradoxically, the active form is un- or undercarboxylated, whereas the carboxylated form is inactive (10). A growing number of human studies have examined associations between total Oc and baseline or changing levels of fasting glucose, insulin, or HOMA-IR (11). However, few have directly measured the putative active form of the protein or taken into account that the carboxylation of Oc is very sensitive to daily fluctuations in intakes of vitamin K provided in such food sources as plant oils such as olive, canola and soybean, and green vegetables, such as broccoli, spinach, and kale (12, 13).
Based on results provided by mass spectrometric immunoassay (MSIA), we herein report new qualitative and semiquantitative (relative percent abundance) information on Oc molecular variants as they exist in individual blood plasma and serum samples. We further present molecular details on the responses of specific carboxylated forms and fragments of Oc in plasma of free-living older adults who received different amounts of vitamin K under controlled conditions. Such determinations of Oc γ-carboxylation in individual serum samples will ultimately be necessary to translate the functional significance of fluctuating levels of Oc in human health and disease.
EXPERIMENTAL PROCEDURES
Materials
Carboxy-dextran functionalized mass spectrometric immunoassay (MSIA) pipette tips were purchased from Molecular BioProducts (Tempe, AZ), a division of ThermoFisher Scientific (Cat. No. 990CUS01, Waltham, MA). An anti-human Oc monoclonal antibody (clone 2H9F11F8) that recognizes the γ-carboxylated and uncarboxylated forms of human Oc in the C-mid-molecular region from residues 24–32 (14) was from AbD Serotec (Kidlington, United Kingdom). Additional antibodies tested during development work, included: a polyclonal rabbit anti-human Oc antibody raised against C-terminal residues 37–49, and two monoclonals (clone numbers 8H12F9H10 and 6F9G4E10), but their affinities were too low and therefore they were unable to provide adequate detection limits for this assay relative to circulating concentrations of human Oc. Rabbit anti-human albumin and anti-human vitamin-D binding protein IgG was purchased from DAKO. Native carboxylated Oc was extracted and purified from cow bone as previously described (15). Decarboxylation of the protein was achieved by heating the dry acidified protein at 110 °C for 280 min in vacuo (16). Peptide captraps for the LC-MS system were purchased from Michrom (now a part of Bruker, Billerica, MA). Other chemicals were of the highest purity available and were purchased from Fisher Scientific or Sigma-Aldrich.
Human Blood Plasma Samples
It should be noted that serum collected in serum separator tubes containing a gel plug for automatic separation of serum and red cells upon centrifugation contains interferents that render it incompatible with the analytical procedures described below. Study A: Matched EDTA plasma and serum samples from nonoverweight, nonsmoking healthy volunteers ages 21–40 (two female and two male; n = 4 plasma and n = 4 serum samples) were collected at Arizona State University. Plasma samples were processed at room temperature, aliquoted and (with the exception of samples analyzed immediately) placed in a −80 °C freezer within 30 min of collection; serum samples were placed at −80 °C within 90 min of collection. Samples that were not analyzed immediately were analyzed within 3 months of collection. Study B: In a 3-year, double-blind, controlled trial, study participants (60–80 years) came to the research site at Tufts University every 6–12 months for measurements of bone mineral density (BMD), biochemical assays, and other measurements, as described elsewhere (17). Participants were randomized to either the treatment or nontreatment group, with stratification according to sex. The subjects were advised to maintain their usual diets and to avoid taking dietary supplements, including calcium, vitamin D, or vitamin K, throughout the study. The treatment group (n = 30) received 500 μg phylloquinone as part of a daily effervescent multivitamin formulation (one tablet), whereas the nontreatment group (n = 29) received the multivitamin formulation without phylloquinone (one tablet). All blood samples were drawn between 7 and 10 h after a minimum 10 h fast, processed within 30 min of collection, and dedicated aliquots of plasma and serum were stored immediately at −80 °C and protected from light until the time of analysis. For the purpose of this study, archived aliquots of EDTA plasma collected at 24 months of supplementation that had been stored for approximately four years were used. The total concentration of Oc in these samples (as the sum of all molecular forms) was determined by RIA according to the method of Gundberg (18). Study C: In a nonrandomized, nonmasked study conducted at Tufts University, 21 younger (18–40 years) and 21 older (55–80 years) men and women consumed a baseline diet (200 μg phylloquinone/day) for 5 days, followed by a phylloquinone-restricted diet (10 μg phylloquinone/day) for 28 days and then a phylloquinone supplementation diet (500 μg phylloquinone/day) for 28 days, as described elsewhere (19). All blood samples were drawn between 7 and 10 am after a 12 h fast and processed within 30 min of collection. Dedicated aliquots were stored immediately after processing at −80 °C and protected from light until the time of analysis. For the purpose of this study, archived citrated plasma samples collected following 7 days of vitamin K depletion (d13; n = 35) and 7 days of vitamin K supplementation (d41; n = 35) from the same study participants and that had been stored for less than two years were used. All three studies were approved by their respective Institutional Review Boards (Study A- Arizona State University and Studies B and C at Tufts University. Studies B and C were registered with ClinicalTrials.gov (NCT00183001 - Study B; NCT0036232 - Study C).
Mass Spectrometric Immunoassay
Anti-human Oc was immobilized to MSIA pipette tips via 1,1′−carbonyldiimidazole coupling chemistry as previously described (20). The derivatized pipette tips were mounted onto a Beckman Multimek 96-channel automated pipetting robot and prerinsed (400 μl/well; 150 μl aspirate and dispense cycles; 10 cycles) with HEPES buffered saline (HBS). Affinity purification of human Oc from plasma samples was performed at room temperature (750 μl of plasma diluted with 750 μL of HBS; 150 μl aspirate and dispense cycles; 1500 cycles). MSIA pipette tips were subsequently rinsed (in situ) with eight cycles of 200 μl of 100 mm Tris, pH 7.6, another eight cycles of 200 μl of fresh 100 mm Tris, pH 7.6 (aspirate and dispense to waste, ADW), eight cycles of 200 μl distilled water (ADW), eight cycles 200μl of 2 m ammonium acetate/acetonitrile (3:1 v/v) (ADW), and eight cycles of 200μl of distilled water (ADW). Elution of samples for analysis by MALDI-MS was accomplished by briefly air-drying the pipette frits then aspirating 4 μl of a solution of MALDI matrix solution (33% acetonitrile in 0.4% trifluoroacetic acid (TFA) saturated with α-cyano-4-hydroxycinnamic acid), mixing the solution over the affinity capture frit for 30 s, and dispensing onto a 96-well MALDI target. Elution of samples for analysis by ESI-MS analysis was accomplished by briefly air-drying the pipette frits then aspirating 12 μl of a solution of 1 μm anti-human serum albumin IgG (to serve as an antiadsorption agent) and 1 mm methionine-serine dipeptide (to serve as an antioxidant) in 0.4% TFA, mixing over the pipette affinity capture frit for 30 s, and dispensing into a 96-conical well polypropylene autosampler tray.
MALDI-TOF Mass Spectrometry
Linear MALDI-TOF mass spectrometry (MS) was carried out on a Bruker Autoflex MALDI-TOF instrument operating in positive-ion mode with 50 ns delayed-extraction. Ion source 1 was set at 20.00 kV, ion source 2 at 18.55 kV, and the focusing lens at 8.50 kV. To ensure good ion-counting statistics at least five thousand laser shots were averaged per sample. Spectra were externally calibrated with a mixture of four proteins supplied by Bruker Daltonics (Billerica, MA) (Protein Standard I; Cat. No. 208241), ranging from m/z 5734.52 (bovine insulin [M+H]+) to m/z 12,360.97 (cytochrome C [M+H]+). Individual mass spectra were baseline subtracted (Tophat algorithm) and smoothed (SavitzkyGolay algorithm; width = 0.2 m/z; cycles = 1) before peak integration by use of Zebra 1.0 (Beavis Informatics (Winnipeg, Canada)).
ESI-TOF Mass Spectrometry
The reversed phase liquid chromatography of human Oc was performed on an Eksigent nanoLC*1D LC system in a simple trap-and-elute format using a polymeric peptide captrap. Pre-isolated human Oc samples were injected onto the peptide captrap (polymeric/reversed phase sorbent) under isocratic conditions of 80% water, 20% acetronitrile, and 0.1% formic acid (Loading Solvent) at a flow rate of 10 μl per minute. After 2 min, the 6-port divert valve position was automatically toggled and flow rate over the captrap cartridge was changed to 1 μl/min of 80% Solvent A (98/2 water/acetonitrile with 0.1% formic acid) and 20% Solvent B (100% acetonitrile). The mobile phase composition was then ramped linearly over 7.2 min from 20% to 90% Solvent B. The captrap eluate was directed to a Bruker MicrOTOF-Q (Q-TOF) mass spectrometer operating in positive ion, TOF-only mode, acquiring spectra in the m/z range of 50 to 3000 Th. ESI settings for the Agilent G1385A capillary nebulizer ion source were as follows: End Plate Offset −500 V, Capillary −4500 V, Nebulizer nitrogen 2 Bar, Dry Gas nitrogen 3.0 L/min at 225 °C. No in-source collision-induced dissociation (CID) energy was employed and the minimum amount of energy necessary to maintain effective ion transmission was added at the quadrupole (5 V) and the collision cell (10 V). At these settings, there was no significant loss of Gla during analysis of intact Oc by ESI-MS. This statement is based on the observation of only minor quantities (< 10% relative MS abundance) of uncarboxylated, singly carboxylated, and doubly carboxylated Oc when the ESI-MSIA assay was applied to bovine plasma – which apparently is almost completely γ-carboxylated with three Gla residues on nearly all Oc protein molecules (supplemental Fig. S1). Data were acquired in profile mode at a digitizer sampling rate of 2 GHz. Spectra rate control was by summation at 1 Hz. Approximately 1 min of recorded spectra were averaged across the chromatographic peak apex of Oc elution. The ESI charge-state envelope was deconvoluted with Bruker DataAnalysis v3.4 software to a mass range of 1000 Da on either side of any identified peak. Deconvoluted spectra were baseline subtracted and all peaks were integrated. Tabulated mass spectral peak areas were exported to a spreadsheet for further calculation and determination of the peak areas of interest that provided an estimate of fractional abundance compared with all other variants of Oc present in the mass spectrum.
The detection limits of the ESI-MSIA assay for Oc in terms of the minimum EDTA plasma concentration of Oc needed to determine the relative degree of Oc γ-carboxylation was assessed via a plot of the signal/noise (S/N) ratio of the mass spectral base peak (most intense peak) versus total Oc concentration in plasma (supplemental Fig. S2). Total Oc concentration in plasma was determined by RIA using the method of Gundberg (18). A minimum base peak S/N ratio of 15 was designated as acceptable in that, in general, it allowed the major forms of Oc contributing to each γ-carboxylation state to be detected with a S/N ratio of greater than three. Given this requirement the limit of quantification for determination of the degree of Oc γ-carboxylation in terms of total Oc concentration was 1 ng/ml (supplemental Fig. S2). A similar estimation of the limit of quantification of the MALDI-MSIA assay was not carried out because it was clearly well below the Oc concentration of all 130 plasma and serum samples analyzed in Studies A–C.
RESULTS
Molecular Heterogeneity of Oc in Circulation
Oc was analyzed by MSIA in a total of 130 samples from Studies A–C. Circulating Oc was found in differentially truncated and differentially γ-carboxylated forms. Most of the truncated forms had not previously been identified (Table I), and the molecular delineation of differentially γ-carboxylated forms of Oc within individual human plasma samples was also without precedent. In order to capture the full spectrum of Oc molecular heterogeneity, samples were analyzed by both MALDI-MSIA and ESI-MSIA. MALDI-MS has the advantage of better sensitivity, which facilitates the identification of low abundance Oc fragments. But MALDI-MS is blind to γ-carboxylation because γ-carboxyl groups are instantly lost as CO2 when the laser strikes the sample (21–23). Oc γ-carboxyl groups are retained during ESI-MS, allowing for relative quantification of Oc with 0, 1, 2, or 3 γ-carboxyl groups.
Table I. Circulating human Oc fragments observed by MSIA in 130 samples from studies A–C.
| Truncated Peptide (Descending MW) | Designation in Fig. 1 | aMALDI Obs. MH+Avg | Calc. MH+Avg | aESI Obs. MH+Mono | Calc. MH+Mono | bFractional Abundance (S.D.) |
|---|---|---|---|---|---|---|
| Y[1–49]V | A | 5798.4 | 5798.5 | 5794.65 | 5794.75 | 48.9 (9.9) |
| Y[1–48]P | B | 5699.7 | 5699.3 | 5695.48 | 5695.68 | 4.6 (1.1) |
| L[2–49]V | C | 5635.4 | 5635.3 | 5631.50 | 5631.68 | 3.6 (0.9) |
| Y[3–49]V | D | 5522.5 | 5522.1 | 5518.46 | 5518.60 | 17.1 (4.2) |
| Y[3–48]P | E | 5423.8 | 5423 | 5419.46 | 5419.53 | 4.7 (1.5) |
| cpE[4–49]V | F | 5342.4 | 5342 | 5338.53 | 5338.52 | 11.4 (3.9) |
| pE[4–48]P | G | 5242.3 | 5242.8 | 5239.35 | 5239.47 | 1.1 (0.5) |
| d W[5–49]V | H | 5230.6 | 5230.8 | 5227.48 | 5227.48 | 1.6 (0.7) |
| W[5–48]P | I | 5131.4 | 5131.7 | 5128.36 | 5128.41 | 1.7 (0.8) |
| G[7–49]V | J | 4930.6 | 4931.5 | eN.O. | 4928.31 | 1.2 (1.0) |
| Y[1–42]Y | K | 4921.4 | 4922.4 | N.O. | 4919.27 | 0.3 (0.5) |
| fA[8–49]V | L | 4873.5 | 4874.4 | N.O. | 4871.29 | 2.3 (2.4) |
| Y[1–41]A or L[2–42]Y | M | 4758.1 | 4759.3 | N.O. | 4756.21 | 1.5 (1.3) |
a For MALDI spectra MH+ designates the intact, protonated gas phase ion analyzed by the mass spectrometer. For ESI spectra it represents the charge state-deconvoluted mass of the protonated molecule. Since protein isotopes are not resolved by MALDI-MS, MH+Avg refers to the average molecular mass (in Da). Since protein isotopes are resolved by ESI-MS, MH+mono refers to the monoisotopic molecular mass (in Da). Observed mass values were taken from representative individual spectra. Multiple γ-carboxylated forms of each species were also observed by ESI.
b Fractional abundance as determined by MALDI-MSIA (which includes, but does not discriminate between all Gla-containing proteoforms); expressed as average percentage ± S.D. for all samples in Supplemental Fig. 5b (n = 130).
c pE = Pyroglutamic acid, formed spontaneously when Q was at the N-terminus.
d Previously observed as a product of MMP -1, -2, and -8 digestion of human Oc (41).
e N.O. indicates fragment not observed.
Oc Truncation
Human Oc was found to circulate in over a dozen differentially truncated forms (Fig. 1). Immunoaffinity captured Oc fragments were identified on the basis of both MALDI- and ESI-based mass mapping analysis to within 2 and 0.2 Da, respectively (Table I, supplemental Fig. S3). Negative control MALDI-MSIA spectra obtained from the analysis of plasma and serum using a MSIA tip derivatized with an antibody against human vitamin D binding protein confirmed the Oc-specificity of the mass mapping assignments (supplemental Fig. S4). MALDI peaks less than m/z 4750 (not shown) were of low relative abundance (< 10% peak intensity of intact Oc) and could not be confirmed as Oc because they were also present in negative control MALDI-MSIA spectra. Vitamin K supplementation did not have a significant impact on Oc truncation (Table I and supplemental Fig. S5). Using repeated measures ANOVA with Tukey HSD post-hoc testing, no significant differences in any of the 13 truncated Oc proteoforms were noted between: (1) the placebo and vitamin K treated groups in Study B, (2) the vitamin K restricted versus vitamin K fortified stages in Study C, or (3) any combination of the four groups from these two separate studies. Several studies have suggested that proteolysis of Oc occurs during sample handling. To control for this possibility, and to determine if there are differences in Oc truncation between plasma and serum, a series of experiments were conducted on blood collected from healthy volunteers (two male and two female from Study A). Samples were immediately processed after venipuncture, Oc extracted from matched EDTA plasma and serum and analyzed by MALDI-MSIA. A few minor, but statistically significant increases in low abundant fragments were evident in serum compared with plasma, resulting in a concomitant 2–3% decrease in intact Oc in serum (supplemental Fig. S6).
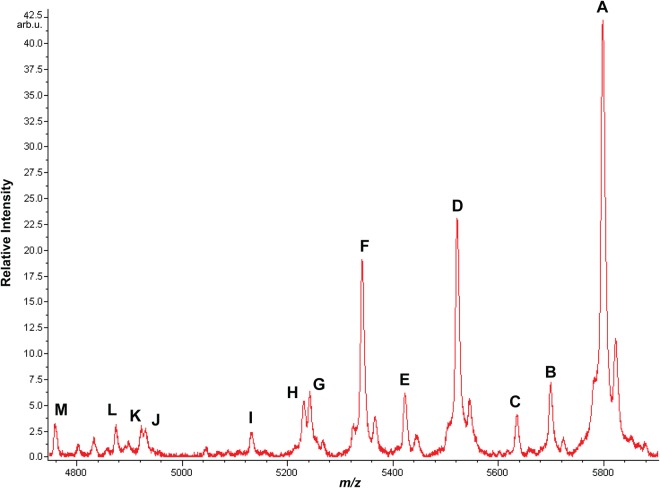
Fig. 1.
Oc from a single human blood plasma sample analyzed by MALDI-MSIA. The intact, full length protein is the most abundant form, but several previously undocumented N- and C-terminally truncated forms are also evident (Table I). Evidence described in the text indicates that these did not appear to form artifactually ex vivo. Because of prompt fragmentation induced by the laser (21–23), γ-carboxyl post-translational modifications could not be observed by MALDI-MS. The spectrum shown is a single representative of spectra from 130 specimens analyzed (supplemental Fig. S5). Lower mass shoulder peaks as observed on peaks A, D, and F correspond to in-source neutral loss of water or ammonia. Higher mass shoulder peaks correspond to sodium adducts [M + Na]+.
To determine whether sample handling at room temperature might affect the relative abundance of the different truncated forms of Oc, once-frozen aliquots of matched plasma and serum samples (from Study A) were thawed and allowed to sit overnight at room temperature then re-analyzed. Statistically significant changes of over 1% relative abundance were observed for variants Y[1–49]V (intact), pyroglutamate pE[4–49]V, and Y[1–41]A or L[2–42]Y (supplemental Fig. S7). Pyroglutamate (pE) forms from N-terminal Gln4, which spontaneously cyclizes to pyroglutamic acid with concurrent loss of ammonia – a well-documented spontaneous phenomenon in proteomics work (24, 25). These changes were not significantly different in plasma versus serum in the former two variants but the latter variant was increased by a statistically significant 6.7 ± 1.0% in serum versus 1.3 ± 0.54% in plasma (p = 0.0032).
To further search for evidence that ex vivo proteolytic activity might have been responsible for the fragments of Oc evident in plasma and serum, full length bovine Oc (which has 92% sequence homology to human Oc and is identical from residues 20–49) was fortified into freshly collected, never-frozen human plasma and serum from Study A and allowed to incubate at room temperature for 4 h. Both fully γ-carboxylated and uncarboxylated forms of bovine Oc were tested separately by MALDI-MSIA. Minimal cleavage of bovine Oc was observed in both instances (Fig. 2); the only significant change was an increase in the relative abundance of L[2–49]V, likely a product of a serum aminopeptidase. For both γ-carboxylated and uncarboxylated bovine Oc, this fragment increased from less than 1% to between 1 and 2% relative abundance.
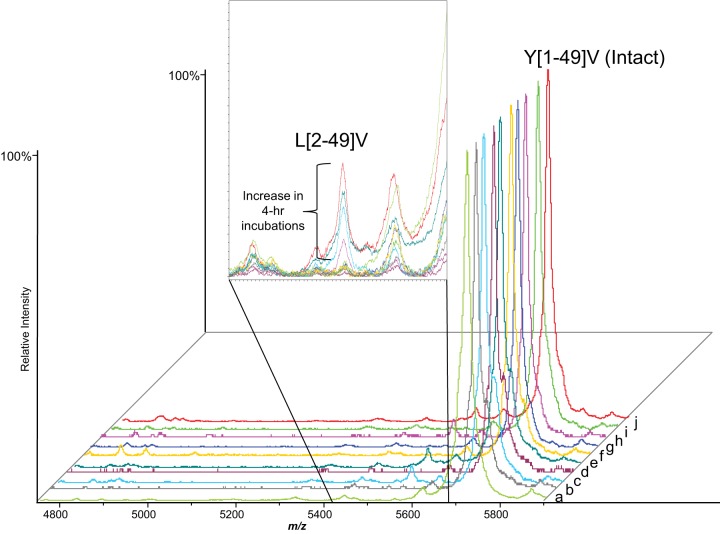
Fig. 2.
Carboxylated (COOH) and uncarboxylated (unCOOH) bovine Oc fortified into human serum and plasma at 120 ng/ml and incubated at 25 °C for up to 4 h followed by analysis by MALDI-MSIA. Cleavage of bovine Oc was minimal; the most striking change was a slight increase in L[2–49]V after 4 h incubation in serum or plasma as shown in the inset. The adjacent small peak at m/z 5620 is Y[1–48]P and did not increase in relative abundance over time. a) Pure COOH Oc standard, b) COOH Oc fortified into plasma then immediately extracted, c) COOH Oc fortified into plasma and incubated for 4 h, d) COOH Oc fortified into serum then immediately extracted, e) COOH Oc fortified into serum and incubated for 4 h, f) Pure unCOOH Oc standard, g) unCOOH Oc fortified into plasma then immediately extracted, h) unCOOH Oc fortified into plasma and incubated for 4 h, i) unCOOH Oc fortified into serum then immediately extracted, and j) unCOOH Oc fortified into serum and incubated for 4 h.
Differential γ-Carboxylation
When individual plasma and serum samples were analyzed by ESI-MSIA, human Oc was found not only in differentially truncated forms, but each fragment was also differentially γ-carboxylated as well (Fig. 3). Within a given sample the percentage of Oc that contains 3 Gla, 2 Gla, 1 Gla, and 0 Gla residues does not vary significantly across the different truncated Oc fragments observed by ESI-MSIA. Following confirmation of quantitative reproducibility (supplemental Table S1), we assessed the differences in γ-carboxylation between freshly drawn plasma and serum in two healthy males and two healthy females (Study A). No significant differences between plasma and serum were observed for all four γ-carboxylation states (supplemental Fig. S8). The approximate limit of detection of this assay for the relative quantification of Oc molecules with 0, 1, 2, or 3 Gla residues was found to be 1 ng/ml total Oc (supplemental Fig. S2).
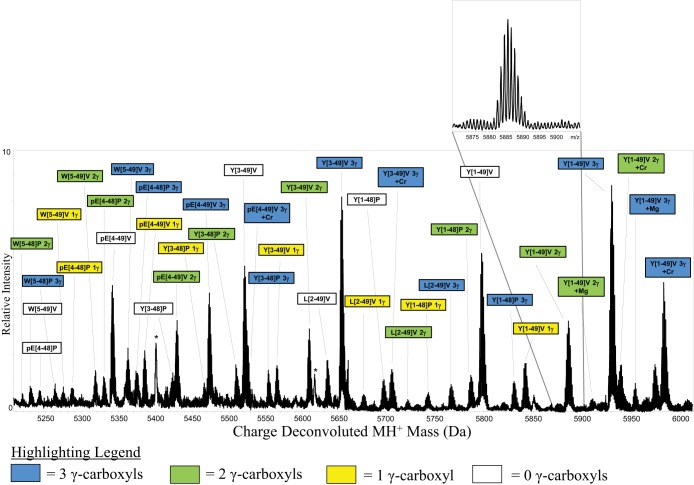
Fig. 3.
ESI-MSIA mass spectrum of Oc extracted from human blood plasma donated by a 24-yr old female. This sample was purchased from a commercial biobank for the purposes of initial assay development and was not part of Studies A-C described in the text. γ-carboxyl post-translational modifications were readily detected and mass mapped – revealing the precise number of γ-carboxyl groups present on each protein molecule. Nine differentially truncated forms of Oc were detected by ESI-MSIA (Table I). Magnesium and chromium adducts (which have previously been documented (39, 40) are likely derived from the metal electrospray needle. All mass spectral peak area integrals were calculated and used to determine the relative percent abundance of Oc with 0, 1, 2, and 3 γ-carboxyl groups. Peaks appear as solid black because protein ions are monoisotopically resolved (inset). * Indicates nonspecific detection of a multiply charged form of apolipoprotein A–I. Qualitatively, the lack of spacing between the isotopes indicates that the peak does not arise from Oc.
Effect of Vitamin K Supplementation on the Molecular Heterogeneity of Oc
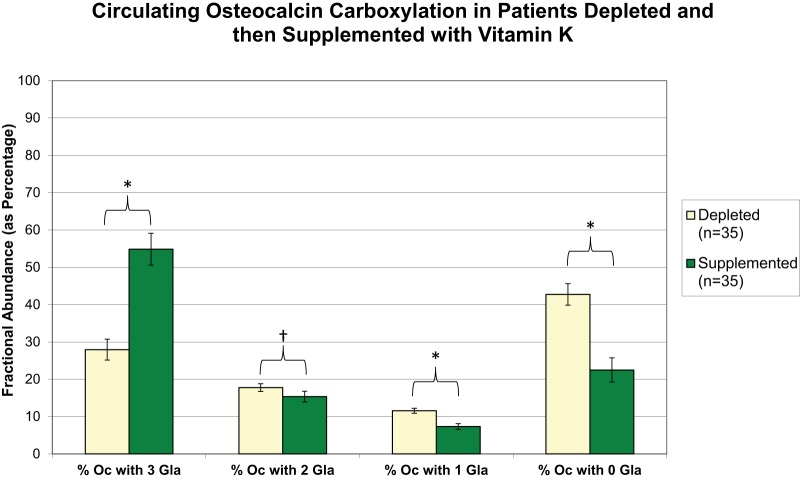
To gain a detailed molecular view of the effects of vitamin K supplementation on human Oc γ-carboxylation, the ESI-MSIA assay was applied to human plasma samples that were collected as part of Studies B and C. Specimens were analyzed by MALDI-MSIA to reveal any potential effects of vitamin K supplementation on circulating Oc truncation, and by ESI-MSIA to reveal the effects of vitamin K supplementation on Oc γ-carboxylation. Neither vitamin K supplementation nor depletion altered the degree to which circulating Oc was truncated (supplemental Fig. S5). As expected, however, vitamin K supplementation dramatically increased the fractional abundance of Oc with three Gla residues and decreased the fractional abundance of Oc with zero Gla residues relative to nontreatment (Fig. 4). Less drastic, but statistically significant decreases were also noted in the fractional abundance of Oc with one and two Gla residues. Likewise, vitamin K supplementation for 7 days following a 28-day period of deficiency resulted in dramatic increases in the fractional abundance of Oc with three Gla residues (Fig. 5). This shift was accompanied by a proportionate decrease in completely uncarboxylated Oc and less pronounced decreases in Oc with one and two Gla residues.
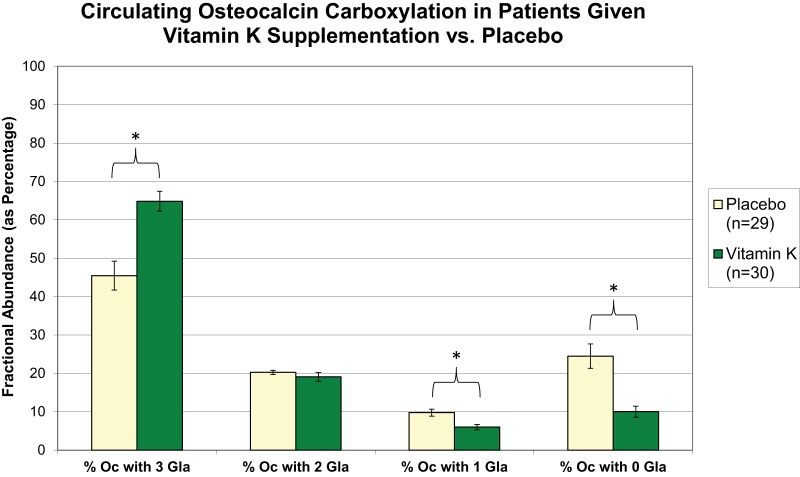
Fig. 4.
Detailed molecular comparison provided by ESI-MSIA of circulating Oc γ-carboxylation in volunteers receiving a vitamin K supplement or placebo (Study B). The relative percent abundance of circulating Oc molecules bearing 0, 1, 2, and 3 Gla residues is shown for each group. Samples were collected after 24 months of treatment. Error bars represent 95% confidence intervals. * Indicates statistical significance as determined by a t test (p < 0.001).
Fig. 5.
Circulating Oc γ-carboxylation in volunteers from Study C after vitamin K depletion (d13) and vitamin K supplementation (d41). The relative percent abundance of circulating Oc molecules bearing 0, 1, 2, and 3 Gla residues is shown for each group. Specimens analyzed were collected 7 days after initiation of each treatment. Error bars represent 95% confidence intervals. * Indicates statistical significance as determined by a paired t test (* for p < 0.001; † for p < 0.01).
DISCUSSION
The evidence provided here shows that circulating human Oc is present in over a dozen N- and/or C-terminally truncated forms. Notably, the relative abundances of the circulating Oc variants were found to be consistent in 130 samples analyzed (Table I and supplemental Fig. S5).
Herein we have provided precise molecular definitions for the circulating forms of Oc in human serum, the majority of which were previously undocumented. The antibody employed here recognizes a region encompassing approximately amino acids 24–32 (14). Any larger species that includes this region would be identified. Although the anticipated trypsin-like fragment Y[1–43]R was not detected in either the plasma or serum samples analyzed Y[1–42]Y (Fragment K) was identified by MALDI-MSIA, albeit at very low abundance. Notably, however, an increase in pE[4–49]V (Fragment F) was the most significant change in Oc after samples were left out at room temperature overnight (supplemental Fig. S7), suggesting at least a partial role for circulating proteases in the production of this fragment. Sequential exopeptidase activity may also be responsible for the production of other observed fragments.
Because Y[1–43]R was not detected in freshly obtained plasma and serum (or in plasma and serum samples allow to sit overnight at room temperature), it most likely is not produced in any significant quantity either in vivo or ex vivo. This notion is in conflict with previous studies (3), but, as pointed out in the Introduction, the precise sequence has never been clearly defined. Although intact Oc derives from osteoblastic synthesis, there are data that suggest that smaller fragments come from bone resorption and can be measured in urine (26). However, these are smaller than those we find here. The larger fragments we detect encompass amino acids 8–42 and are likely the result of proteolysis of the intact form in the serum (in vivo). All of these could contain 0–3 Gla residues. An assay that measures these mid-molecular forms, along with the intact, would be the most clinically relevant. However, definitive identification of the origin of such fragments could be gained from studies such as ours investigating potential changes in circulating fragments before and after antiresorptive therapy. Hence, this study underscores the value of the unparalleled molecular specificity provided by targeted mass spectrometric analysis. Nevertheless, our data confirm that an assay capable of measuring the intact and large mid-molecular forms of the protein would provide the most clinically relevant information. That stated, the stability data indicate that care must be taken to avoid ex vivo proteolysis if intact Oc is to be accurately quantified.
Because of the specificity of the antibody used, we cannot rule out the possibility of smaller fragments encompassing the first 23 residues. However, this seems unlikely because we do not observe any mid-molecular species that would result from such cleavages, such as one that would be derived from trypsin-like activity or plasmin activity at residue 19. Other antibodies, specific for the C- or N terminus, and capable of providing sufficient mass spectral signal, may provide additional insights into the nature of circulating human Oc beyond what we were able to show here.
In contrast to other species, human dietary intake of vitamin K is suboptimal and, as a consequence, both bone and serum Oc are undercarboxylated (27). As such, the assessment of the degree of circulating undercarboxylated Oc has been shown to be a sensitive measure of vitamin K nutrition in humans. The importance of vitamin K to the function of Oc in bone led to an interest in the potential that vitamin K could have a protective effect against age-related bone loss (28). However, several controlled studies suggest that vitamin K supplementation is not effective in preventing bone loss and vitamin K does not add to the beneficial effects of those who are also receiving calcium and vitamin D supplementation (17, 29–31). Nevertheless, the evaluation of efficacy of supplementation relied on methods that only approximated the degree of Oc carboxylation, and the amount of vitamin K required to maximally carboxylate the protein could not reliably be evaluated. Although we show in this study that vitamin K supplementation at daily doses of 500 μg, which is approximately five times higher than the current dietary recommendations for U.S. adults (12) reduced the degree of uncarboxylated Oc, complete γ-carboxylation was not achieved. Perhaps a more important question is whether complete γ-carboxylation of Oc is necessary for optimal function in human bone. The work described here provides a solid foundation to begin to address this critical gap in our understanding of this bone-derived protein.
The recent findings that Oc acts as a hormone to affect glucose homeostasis (9) suggested that not only the fully uncarboxylated form was active, but decarboxylation of just one Gla residue during bone resorption would activate the protein (10). Although evidence does not support the notion that bone resorption is associated with glucose metabolism in humans (32), direct measurement of the carboxylation status of osteocalcin before and after antiresorption therapy could provide more definitive data. Furthermore, in recent years many studies have examined associations between total Oc and glucose metabolism, but because of the inherent difficulty in the methods to measure them, few have quantified the un- or undercarboxylated osteocalcin forms (8, 11). These observations underscored the need for a precise evaluation of Oc at all three potential γ-carboxylation sites. Herein, we have shown the capacity to routinely carry out such evaluations of the γ-carboxylation status of human Oc within individual samples with unprecedented molecular detail.
Our studies revealed that relative to the general population, individuals supplemented with vitamin K experienced a shift wherein about 20% of their circulating Oc transitions from a completely uncarboxylated state to a fully carboxylated state rendering that fraction of the protein inactive as a putative bone-derived hormone – in effect reducing the concentration of the hormone by ∼50% (Figs. 4–5). This occurred with significant but smaller changes in the relative abundance of Oc carrying one or two Gla residues, which would both remain active. This finding can be explained by the fact that carboxylation of Oc is an ordered process with Glu-24 being carboxylated first, followed by Glu21, then Glu17 in humans (27). As more vitamin K becomes available, the major effect is the elimination of a portion of the uncarboxylated forms and the formation of a fully carboxylated protein. In mice, only fully carboxylated osteocalcin has no hormonal activity. Insulin sensitivity is affected by less than a 5% change of either un- or under-carboxylated osteocalcin in these animals (10, 33), considerably less than the fluctuation that would be observed in humans during daily intakes of green vegetables.
We have previously shown that vitamin K supplementation (from study B) reduced insulin resistance in older men, the opposite of what is proposed in the mouse model (34). In observational studies of free-living adults, we, and others, have also reported an inverse relationship between vitamin K intake and risk of insulin resistance and diabetes (35–37). Our findings here unequivocally document that increased vitamin K intake reduces the uncarboxylated form of Oc. These data lend further support to our earlier findings from Study B that in older adults, circulating uncarboxylated Oc was not associated with insulin resistance (38).
In conclusion, we present herein the identities and relative abundances of previously uncharacterized circulating Oc fragments – verified in over 100 individual human plasma samples. We also show the capacity to routinely quantify the relative abundance of circulating Oc molecules with 0, 1, 2, or 3 Gla residues. These results open up a new avenue by which to explore the functional properties of the varied molecular forms of human Oc and emphasize the need to control vitamin K intake when studying metabolic changes.
Supplementary Material
Acknowledgments
We thank Randall Nelson, Ph.D. for project support and the use of instrumentation.
Footnotes
Author contributions: D.S.R., C.M.G., S.L.B., and C.R.B. designed research; D.S.R. performed research; C.M.G. contributed new reagents or analytic tools; D.S.R. and C.R.B. analyzed data; D.S.R., C.M.G., S.L.B., and C.R.B. wrote the paper.
* This work was supported by: The National Institutes of Health DK082542 and DK090958 (DR, CB), AR38460 (CG), AG14759 (SB) and DK69341 (SB), and the USDA, Agricultural Research Service under Cooperative Agreement No. 58-1950-7-707).
 This article contains supplemental Figs. S1 to S8 and Table S1.
This article contains supplemental Figs. S1 to S8 and Table S1.
1 The abbreviations used are:
- Oc
- Osteocalcin
- BMD
- bone mineral density
- Gia
- γ-carboxyglutamic acid
- MSIA
- mass spectrometric immunoassay
- HBS
- HEPES-buffered saline
- ADW
- aspirate and dispense to waste
- pE
- pyroglutamic acid.
REFERENCES
- 1. Hauschka P. V., Lian J. B., Cole D. E., Gundberg C. M. (1989) Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol. Rev. 69, 990–1047 [DOI] [PubMed] [Google Scholar]
- 2. Eastell R., Hannon R. A. (2008) Biomarkers of bone health and osteoporosis risk. Proc. Nutr. Soc. 67, 157–162 [DOI] [PubMed] [Google Scholar]
- 3. Garnero P., Grimaux M., Demiaux B., Preaudat C., Seguin P., Delmas P. D. (1992) Measurement of serum osteocalcin with a human-specific two-site immunoradiometric assay. J. Bone Miner Res. 7, 1389–1398 [DOI] [PubMed] [Google Scholar]
- 4. Booth S. L., Martini L., Peterson J. W., Saltzman E., Dallal G. E., Wood R. J. (2003) Dietary phylloquinone depletion and repletion in older women. J. Nutr. 133, 2565–2569 [DOI] [PubMed] [Google Scholar]
- 5. Booth S. L., Al Rajabi A. (2008) Determinants of vitamin K status in humans. Vitam. Horm. 78, 1–22 [DOI] [PubMed] [Google Scholar]
- 6. Binkley N. C., Krueger D. C., Engelke J. A., Foley A. L., Suttie J. W. (2000) Vitamin K supplementation reduces serum concentrations of under-gamma-carboxylated osteocalcin in healthy young and elderly adults. Am. J. Clin. Nutr. 72, 1523–1528 [DOI] [PubMed] [Google Scholar]
- 7. Gundberg C. M., Lian J. B., Booth S. L. (2012) Vitamin K-dependent carboxylation of osteocalcin: friend or foe? Adv. Nutr. 3, 149–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gundberg C. M., Nieman S. D., Abrams S., Rosen H. (1998) Vitamin K status and bone health: an analysis of methods for determination of undercarboxylated osteocalcin. J. Clin. Endocrinol. Metab. 83, 3258–3266 [DOI] [PubMed] [Google Scholar]
- 9. Lee N. K., Sowa H., Hinoi E., Ferron M., Ahn J. D., Confavreux C., Dacquin R., Mee P. J., McKee M. D., Jung D. Y., Zhang Z., Kim J. K., Mauvais-Jarvis F., Ducy P., Karsenty G. (2007) Endocrine regulation of energy metabolism by the skeleton. Cell 130, 456–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferron M., Wei J., Yoshizawa T., Del Fattore A., DePinho R. A., Teti A., Ducy P., Karsenty G. (2010) Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell 142, 296–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Booth S. L., Centi A., Smith S. R., Gundberg C. (2013) The role of osteocalcin in human glucose metabolism: marker or mediator? Nat. Rev. Endocrinol. 9, 43–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Institute of Medicine (U.S.). Panel on Micronutrients. (2001) DRI : dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc : a report of the Panel on Micronutrients and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine, National Academy Press, Washington, D.C. [Google Scholar]
- 13. Shearer M. J., Fu X., Booth S. L. (2012) Vitamin K nutrition, metabolism, and requirements: current concepts and future research. Adv. Nutr. 3, 182–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hellman J., Kakonen S. M., Matikainen M. T., Karp M., Lovgren T., Vaananen H. K., Pettersson K. (1996) Epitope mapping of nine monoclonal antibodies against osteocalcin: combinations into two-site assays affect both assay specificity and sample stability. J. Bone Miner Res. 11, 1165–1175 [DOI] [PubMed] [Google Scholar]
- 15. Gundberg C. M., Hauschka P. V., Lian J. B., Gallop P. M. (1984) Osteocalcin: isolation, characterization, and detection. Methods Enzymol. 107, 516–544 [DOI] [PubMed] [Google Scholar]
- 16. Lian J. B., Gundberg C. M., Hauschka P. V., Gallop P. M. (1985) gamma-Carboxyglutamic acid. Methods Enzymol. 113, 133–146 [DOI] [PubMed] [Google Scholar]
- 17. Booth S. L., Dallal G., Shea M. K., Gundberg C., Peterson J. W., Dawson-Hughes B. (2008) Effect of vitamin K supplementation on bone loss in elderly men and women. J. Clin. Endocrinol. Metab. 93, 1217–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gundberg C. M. (1998) Biology, physiology, and clinical chemistry of osteocalcin. J. Clin. Ligand Assay 21, 128–138 [Google Scholar]
- 19. Truong J. T., Fu X. Y., Saltzman E., Al Rajabi A., Dallal G. E., Gundberg C. M., Booth S. L. (2012) Age group and sex do not influence responses of vitamin K biomarkers to changes in dietary vitamin K. J. Nutr. 142, 936–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rehder D. S., Borges C. R. (2010) Possibilities and pitfalls in quantifying the extent of cysteine sulfenic acid modification of specific proteins within complex biofluids. BMC Biochem. 11, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Prorok M., Warder S. E., Blandl T., Castellino F. J. (1996) Calcium binding properties of synthetic gamma-carboxyglutamic acid-containing marine cone snail “sleeper” peptides, conantokin-G and conantokin-T. Biochemistry-Us 35, 16528–16534 [DOI] [PubMed] [Google Scholar]
- 22. Kalume D. E., Stenflo J., Czerwiec E., Hambe B., Furie B. C., Furie B., Roepstorff P. (2000) Structure determination of two conotoxins from Conus textile by a combination of matrix-assisted laser desorption/ionization time-of-flight and electrospray ionization mass spectrometry and biochemical methods. J. Mass Spectrom. 35, 145–156 [DOI] [PubMed] [Google Scholar]
- 23. Ivaska K. K., Hellman J., Likojarvi J., Kakonen S. M., Gerdhem P., Akesson K., Obrant K. J., Pettersson K., Vaananen H. K. (2003) Identification of novel proteolytic forms of osteocalcin in human urine. Biochem. Bioph. Res. Co. 306, 973–980 [DOI] [PubMed] [Google Scholar]
- 24. Liu Y. D., Goetze A. M., Bass R. B., Flynn G. C. (2011) N-terminal glutamate to pyroglutamate conversion in vivo for human IgG2 antibodies. J. Biol. Chem. 286, 11211–11217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kumar M., Chatterjee A., Khedkar A. P., Kusumanchi M., Adhikary L. (2013) Mass spectrometric distinction of in-source and in-solution pyroglutamate and succinimide in proteins: a case study on rhG-CSF. J. Am. Soc. Mass Spectrom. 24, 202–212 [DOI] [PubMed] [Google Scholar]
- 26. Ivaska K. K., Kakonen S. M., Gerdhem P., Obrant K. J., Pettersson K., Vaananen H. K. (2005) Urinary osteocalcin as a marker of bone metabolism. Clin. Chem. 51, 618–628 [DOI] [PubMed] [Google Scholar]
- 27. Cairns J. R., Price P. A. (1994) Direct demonstration that the Vitamin-K-dependent bone Gla protein is incompletely gamma-carboxylated in humans. J. Bone Miner Res. 9, 1989–1997 [DOI] [PubMed] [Google Scholar]
- 28. Booth S. L. (2009) Roles for vitamin K beyond coagulation. Annu. Rev. Nutr. 29, 89–110 [DOI] [PubMed] [Google Scholar]
- 29. Binkley N., Harke J., Krueger D., Engelke J., Vallarta-Ast N., Gemar D., Checovich M., Chappell R., Suttie J. (2009) Vitamin K treatment reduces undercarboxylated osteocalcin but does not alter bone turnover, density, or geometry in healthy postmenopausal north american women. J. Bone Miner Res. 24, 983–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Emaus N., Gjesdal C. G., Almas B., Christensen M., Grimsgaard A. S., Berntsen G. K., Salomonsen L., Fonnebo V. (2010) Vitamin K2 supplementation does not influence bone loss in early menopausal women: a randomised double-blind placebo-controlled trial. Osteoporos. Int. 21, 1731–1740 [DOI] [PubMed] [Google Scholar]
- 31. Bolton-Smith C., McMurdo M. E., Paterson C. R., Mole P. A., Harvey J. M., Fenton S. T., Prynne C. J., Mishra G. D., Shearer M. J. (2007) Two-year randomized controlled trial of vitamin K1 (phylloquinone) and vitamin D3 plus calcium on the bone health of older women. J. Bone Miner Res. 22, 509–519 [DOI] [PubMed] [Google Scholar]
- 32. Schwartz A. V., Schafer A. L., Grey A., Vittinghoff E., Palermo L., Lui L. Y., Wallace R. B., Cummings S. R., Black D. M., Bauer D. C., Reid I. R. (2013) Effects of antiresorptive therapies on glucose metabolism: results from the FIT, HORIZON-PFT, and FREEDOM trials. J. Bone Miner Res. 28, 1348–1354 [DOI] [PubMed] [Google Scholar]
- 33. Ferron M., Hinoi E., Karsenty G., Ducy P. (2008) Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc. Natl. Acad. Sci. U.S.A. 105, 5266–5270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yoshida M., Jacques P. F., Meigs J. B., Saltzman E., Shea M. K., Gundberg C., Dawson-Hughes B., Dallal G., Booth S. L. (2008) Effect of vitamin K supplementation on insulin resistance in older men and women. Diabetes Care 31, 2092–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yoshida M., Booth S. L., Meigs J. B., Saltzman E., Jacques P. F. (2008) Phylloquinone intake, insulin sensitivity, and glycemic status in men and women. Am. J. Clin. Nutr. 88, 210–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ibarrola-Jurado N., Salas-Salvado J., Martinez-Gonzalez M. A., Bullo M. (2012) Dietary phylloquinone intake and risk of type 2 diabetes in elderly subjects at high risk of cardiovascular disease. Am. J. Clin. Nutr. 96, 1113–1118 [DOI] [PubMed] [Google Scholar]
- 37. Beulens J. W., van der A. D., Grobbee D. E., Sluijs I., Spijkerman A. M., van der Schouw Y. T. (2010) Dietary phylloquinone and menaquinones intakes and risk of type 2 diabetes. Diabetes Care 33, 1699–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shea M. K., Gundberg C. M., Meigs J. B., Dallal G. E., Saltzman E., Yoshida M., Jacques P. F., Booth S. L. (2009) Gamma-carboxylation of osteocalcin and insulin resistance in older men and women. Am. J. Clin. Nutr. 90, 1230–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nousiainen M., Derrick P. J., Kaartinen M. T., Maenpaa P. H., Rouvinen J., Vainiotalo P. (2002) A mass spectrometric study of metal binding to osteocalcin. Chem. Biol. 9, 195–202 [DOI] [PubMed] [Google Scholar]
- 40. Niiranen H., Budnik B. A., Zubarev R. A., Auriola S., Lapinjoki S. (2002) High-performance liquid chromatography – mass spectrometry and electron-capture dissociation tandem mass spectrometry of osteocalcin. Determination of gamma-carboxyglutamic acid residues. J. Chromatogr.. A 962, 95–103 [DOI] [PubMed] [Google Scholar]
- 41. Gundberg C. M., Clough M., Mort J. S. (2002) Proteolysis of human osteocalcin by MMP's and cathepsin K. J. Bone Miner Res. 17, S406-S407 [Google Scholar]
- 42. Ivaska K. K., Hentunen T. A., Vaaraniemi J., Ylipahkala H., Pettersson K., Vaananen H. K. (2004) Release of intact and fragmented osteocalcin molecules from bone matrix during bone resorption in vitro. J. Biol. Chem. 279, 18361–18369 [DOI] [PubMed] [Google Scholar]
- 43. Baumgrass R., Williamson M. K., Price P. A. (1997) Identification of peptide fragments generated by digestion of bovine and human osteocalcin with the lysosomal proteinases cathepsin B, D, L, H, and S. J. Bone Miner Res. 12, 447–455 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.