Abstract
Background
Dystonias (Dys) represent the third most common movement disorder after essential tremor (ET) and Parkinson's disease (PD). While some pathogenetic mechanisms and genetic causes of Dys have been identified, little is known about their neuropathologic features. Previous neuropathologic studies have reported generically defined neuronal loss in various cerebral regions of Dys brains, mostly in the basal ganglia (BG), and specifically in the substantia nigra (SN). Enlarged pigmented neurons in the SN of Dys patients with and without specific genetic mutations (e.g., GAG deletions in DYT1 dystonia) have also been described. Whether or not Dys brains are associated with decreased numbers or other morphometric changes of specific neuronal types is unknown and has never been addressed with quantitative methodologies.
Methods
Quantitative immunohistochemistry protocols were used to estimate neuronal counts and volumes of nigral pigmented neurons in 13 SN of Dys patients and 13 SN of age-matched control subjects (C).
Results
We observed a significant reduction (∼20%) of pigmented neurons in the SN of Dys compared to C (p<0.01). Neither significant volumetric changes nor evident neurodegenerative signs were observed in the remaining pool of nigral pigmented neurons in Dys brains. These novel quantitative findings were confirmed after exclusion of possible co-occurring SN pathologies including Lewy pathology, tau-neurofibrillary tangles, β-amyloid deposits, ubiquitin (ubiq), and phosphorylated-TAR DNA-binding protein 43 (pTDP43)-positive inclusions.
Discussion
A reduced number of nigral pigmented neurons in the absence of evident neurodegenerative signs in Dys brains could indicate previously unconsidered pathogenetic mechanisms of Dys such as neurodevelopmental defects in the SN.
Keywords: Substantia nigra, pigmented neurons, neuronal reduction, neuronal loss, neurodevelopmental disorder, neurodegenerative disorder
Introduction
Dystonias (Dys), which manifest either as an isolated abnormal motor phenomenon or in combination with other motor and nonmotor signs of more complex neuropsychiatric syndromes, represent a relatively frequent movement disorder.1,2 Dys, in fact, represent the third most frequent movement disorder after essential tremor (ET) and Parkinson's disease (PD).3
A number of efforts have led to various classification systems of Dys,4–8 attempts to characterize their biological bases,9–13 and multiple proposals of clinical guidelines for their treatment.14–16 However, although important progress has been achieved in understanding some of the neurological bases of Dys, especially in terms of molecular mechanisms17 and genetic causes,18 the distinct characteristic neuropathologic features of Dys remain poorly defined19 with some very rare exceptions.20,21
There have been few clinicopathologic correlation studies of postmortem brain tissues from Dys patients, with most analyses described in sporadic case-reports.22–27 These neuropathologic studies have proven to be mostly inconclusive due to the clinical heterogeneity of Dys cases examined, the different classification systems used, and more importantly, difficulty in obtaining brains from clinically well-characterized Dys patients.28 However, a few neuropathologic studies have begun to shed light on possible neuropathologic and morphometric aspects of specific types of Dys, such as DYT1-dystonia (DYT1),29 cervical dystonia (CD)30, and dopa-responsive dystonia (DRD).31 Rostasy and colleagues assessed brains from five patients with DYT1 (GAG-deletions) and three Dys patients without GAG-deletions and reported larger and more compacted pigmented neurons in the substantia nigra (SN) of both types of Dys compared to control subjects (C).29 In contrast, Göttle et al. described smaller SN neurons in Lesch-Nyhan disease.32 In general, these lines of evidence support the hypothesis that the SN is indeed involved in Dys pathogenesis.33–35
The primary aim of this study was to investigate morphometric aspects of neuromelanin-containing (pigmented) neurons in the SN of Dys patients by performing rigorous neuropathologic quantitative analyses. We aimed to estimate nigral pigmented neuronal counts and cell body, nuclear, and nucleolar volumes using established quantification methods and the largest number of publicly available Dys autopsy brains to date.
We observed a significant decrease in the number of pigmented neurons in the SN of Dys brains versus C. This reduction was present in the SN of patients with both adulthood- and childhood-onset Dys. Interestingly, fewer pigmented neurons in the SN were not associated with significant changes in cellular, nuclear, or nucleolar volumes of the remaining pool of nigral pigmented neurons, or with other major neuropathologic and neurodegenerative signs. These findings might indicate that a reduction in SN pigmented neurons in Dys patients could be due to a neurodevelopmental defect rather than a neurodegenerative process.
A nigral pigmented neuronal reduction in the absence of cellular (cell body) and subcellular (nuclear/nucleolar) volumetric changes in the SN of Dys brains was confirmed by excluding other possible confounding or co-occurring nigral pathologies with immunohistochemistry protocols. In this study, the Dys and C groups did not differ in terms of mean age at death, further minimizing the influence of the important confounding factor of age.36
Methods
A total of 13 SN from patients (9 females and 4 males) with various Dys subtypes were available for this study. The mean age at death was 68.8±15.5 years (range: 44–95). These individuals had a history of childhood- or adulthood-onset Dys with focal, segmental, or generalized distribution. Dys cases did not show signs of any other major neurological, psychiatric, or medical disorders except for one patient with diagnosis of possible dementia with Lewy bodies (possDLB), and another with cardiovascular disease. Cases of DYT1, the most frequent genetic form of Dys, were excluded from this investigation to avoid performing measurements on neurons from the brains of patients with Dys attributable to specific genetic causes. With this exclusion, we aimed to obtain general but specific quantitative findings on non-DYT1 or idiopathic Dys only.
The 13 SN from Dys patients were compared to 13 SN from age-matched control subjects (C, 9 females and 4 males). The mean age at death was 64.3±13.8 years (range: 44–88 years). C did not show clinical or pathologic evidence of any major neurological, psychiatric, or medical disorders except for heart disease in some cases.
In addition to the standard microscopic neuropathological assessments, all SNs were immunohistochemically assessed for the presence of: α-synuclein-positive Lewy bodies (LB) and Lewy neurites (LN), hyperphosphorylated-tau neurofibrillary tangles (tau-NFT), hyperphosphorylated-tau threads (tau-th), ubiquitin (ubiq), intraneuronal cytoplasmic inclusions of phosphorylated-TAR-DNA binding protein-43 (pTDP-43), and extracellular deposits of insoluble 1–42 β-amyloid (diffuse-Aβ and Aβ-neuritic plaques). Subjects with neuropathologic findings indicative of any other neurodegenerative disorder or severe cerebrovascular disease were excluded from the study.
All Dys and C brains were collected after obtaining written consent from the next-of-kin or legal representative and after approval from each institution where the case was examined. Tissues were obtained from the University of Maryland Brain and Tissue Bank (UMBTB). The use of these tissues was approved by UMBTB and the local Institutional Research Board. Each hemibrain was fixed in 10% buffered formalin for at least 2 weeks and then grossly and microscopically examined in coronal sections for a general neuropathologic assessment at UMBTB.
Neuropathology and quantitative methods
Each hemi-SN block was randomly cut at different anatomical levels along the rostro-caudal anatomical axis of the structure. This anatomically random cutting minimized possible anatomical selection biases. We were unable to obtain the entire SN for each subject, but we were confident that at least three quarters of the entire structure was actually sampled for each case. This conclusion was based on our samples measurements (see below) in comparison to previous anatomical studies that assessed the entire length of SN.37,38
Tissue cutting and procedures for morphometric-quantitative analyses
The total length of each block of formalin-fixed SN received ranged from 8–10 mm. Tissues were processed at the Neuropathology Research labs at the Biomedical Research Institute of New Jersey (BRInj). Tissue blocks were processed with an automated tissue-processor (Tissue-Tek V.I.P. 1000 Vacuum Infiltration Processor, Ames Division, Miles Laboratories, Inc., Elkhart, IN, USA) using standard protocols. Tissue blocks were then embedded in paraffin, oriented with the rostral area facing the bottom of the paraffin mold, and serially cut along the rostro-caudal direction using a semi-automatic microtome (Leica RM2255, Leica Biosystems, Nussloch, Germany). Each block was cut for its entire length in series of 40 µm-thick consecutive sections, alternating to a series of 16 consecutive 10 µm-thick sections. These serial, consecutive, and alternating sectioning procedures guaranteed the constancy of the established sampling rate for this investigation (one every five 40 µm-thick sections) and simultaneously allowed the use of 10 µm-thick consecutive sections anatomically adjacent to the 40 µm-thick sections for further immunohistochemistry assessments and volumetric measurements.39
The mean length of the sampled SN did not differ between Dys and C brains (mean lengths of 9,392.31±1,518.99 µm and 10,015.38±1,573.2 µm, respectively). The total number of consecutive sections (including 40 µm- and 10 µm-thick sections) obtained for Dys and C were 792.9±129.3 and 845.4±132.3, respectively. No statistical differences in terms of total length of sampled tissue and number of sections obtained were present between the Dys and C groups. Means of 48.6±7.5 and 51.6±7.5 40 µm-thick consecutive sections were respectively obtained for Dys and C.
Each set of 40 µm-thick sections was stained with a 1.0% cresyl violet (CV) solution for neuronal counting.40 Each set of 40 µm-thick sections was separately and randomly recoded by an investigator blinded to the clinical and pathologic diagnoses (M.G.E.). Quantitative measurements using well-established stereological probes (the Optical Fractionator and Nucleator, see below) were performed by a second investigator (D.I.). Slides codes were opened, and statistical analyses were performed only after all measurements were completed.
A single 10 µm-thick tissue section was randomly chosen, stained with hematoxylin and eosin (H&E), and microscopically inspected at low (2.5×) and high (20×) magnifications to assess for possible micro-ischemic vascular pathologies, microhemorrhages, tissue rarefaction, and other possible histologic abnormalities not visible on gross examination.
Procedures for SN neuropathologic quantitative measurements
Each section was stereoscopically inspected (2×) across the entire sectional area to localize SN anatomical borders. The pars compacta and pars reticulata were identified and marked as based on previous detailed anatomical descriptions.41
For neuronal counting, the following histologic criteria were applied:
-
1)
Use of 40 µm-thick sections;
-
2)
Inclusion of any neuron, of any size, containing neuromelanin pigment;
-
3)
Exclusion of nonneuronal cells containing neuromelanin pigment, such as macrophages.
For neuronal volumetric measurements, the following histologic criteria were applied:
-
1)
Use of 10 µm-thick sections;
-
2)
Individuation of a well-defined nonneuromelanin-covered nucleolus for each randomly sampled pigmented neuron;
-
3)
Establishing the nucleolus as the only point of reference for all volumetric measurements in each randomly sampled pigmented neuron.
Measurements were performed using the Stereo-Investigator system, Version 10.0 (MBF Bioscience, Williston, VT, USA), equipped with a digital camera (AxioCam MRm, and MRc, Zeiss, Oberkochen, Germany) and multiple objective head (2.5–100× oil-immersion). The sampling grid area was 500×500 µm, the counting frame was 40×40 µm, and the disector height was 25 µm with guard zones of ±2 µm. The total number of SN pigmented neurons was estimated using the Optical Fractionator probe.42,43
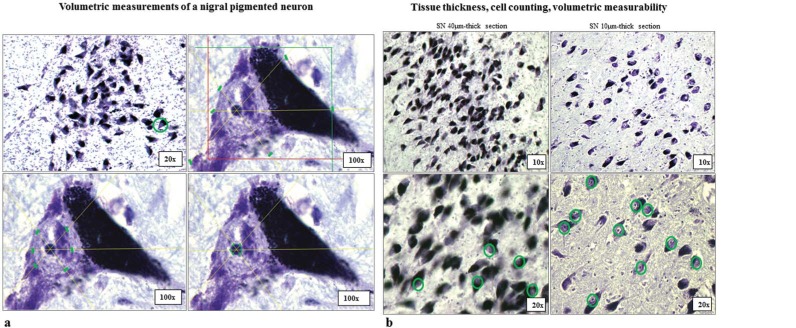
Series of 10 µm-thick consecutive sections adjacent to each previously analyzed 40 µm-thick section were stained with CV to measure the cellular, nuclear, and nucleolar volumes of pigmented neurons. Volumetric measurements were performed using the Nucleator probe.38 Pigmented neuron volumes were measured by placing six rays that automatically centered on the nucleolus and randomly intersecting the cell membrane (Figure 1A). Pigmented neurons were measured if their nucleolus was inside the counting frame intersecting or touching the green inclusion line; they were excluded if their nucleolus intersected or touched the red exclusion line (Figure 1A, upper right quadrant). This second-run of volumetric measurements on separate sets of 10 µm-thick consecutive sections was chosen to increase the number of available pigmented neurons showing a well-defined nucleolus, that is, the established point of spatial reference for volumetric measurements in this study. In fact, a consistent number of pigmented neurons that could be clearly counted on 40 µm-thick sections could not be volumetrically measured due to the presence of neuromelanin covering, partially or totally, the nucleolus (Figure 1B).
Figure 1A/1B. Quantification of SN Pigmented Neurons. The quadrants in the figure show how a randomly sampled pigmented neuron (first upper-right quadrant, 20× objective), and 6 randomly projected rays on that pigmented neuron intersect its cell body, nucleus, and nucleolus (respectively, upper-right, lower-left, and lower-right quadrant). A 100× oil immersion objective was used for all volumetric measurements and for each pigmented neuron. The green markers in each quadrant indicate the point of measurement from which the areas of the cell body, nucleus, and nucleolus were calculated. The colored square upper-right quadrant represents the “counting frame” used for the Optical Fractionator probe. The Stereo-investigator software automatically calculates the volumes of each single neuron computing various parameters such as the thickness of the tissue, the disector height, and guard zones. Figure 1B. Tissue thickness, cell counting, volumetric measurability of nigral pigmented neurons. SNc from a C brain (case#16), cut at different levels of thicknesses (40 µm and 10 µm), stained with CV, and inspected at two different magnifications (10× and 20× objectives). The green circles in the inferior parts of the figure indicate the number of pigmented neurons measurable when pigmented neurons were sampled using 40 µm-thick and 10 µm-thick tissue sections, respectively, with a 20× objective. Notably, the number of measurable pigmented neurons in SN cut at 10 µm of thickness (right of figure) is markedly higher due to the increased number of clear visible CV-stained nucleoli. The nucleolus was the established point of spatial reference on this study. All measurements were performed using a 100× oil-immersion, NA 1.30, neofluor ∞/0.17 objective. Abbreviations: C, Control; CV, Cresyl Violet; SN, Substantia Nigra; SNc, Pars Compacta of Substantia Nigra.
Immunohistochemistry
Each Dys and C case underwent an extensive immunohistochemical assessment. For each case, we examined 16 10-µm thick sections adjacent to the median 40 µm-thick set of sections. The 10 µm-thick tissue sections were deparaffinized, hydrated, and treated to block endogenous peroxidase activity (3% hydrogen peroxide in water). They were then rinsed in buffer (Tris with 0.05% Triton-X), microwaved with antigen retrieval solution (sodium citrate, pH 6.0) for 10 minutes, and cooled. Protein block (1.5% horse serum) was applied for 30 minutes. The following antibodies were used: mouse anti-β-amyloid, 17–24 (dilution 1:500, 4G8; SIG-39220, Covance, Princeton, NJ, USA) with overnight incubation at 4°C (sections were pre-treated with 90% formic acid, 5 minutes); mouse anti-PHF-tau (dilution 1:500, MN1020; Thermo Fisher Scientific, Waltham, MA, USA); mouse anti-α-synuclein (dilution 1:500, Ab27766; Abcam, Cambridge, UK) rabbit anti-ubiq (dilution 1:250, ab7780, Abcam); rabbit anti phospho-TDP-43 (dilution 1:2,000, TIP-PTD-P02; Cosmo Bio Co. LTD, Carlsbad, CA, USA). With the exception of the anti-β-amyloid antibody, sections were incubated overnight at 4°C, rinsed with buffer, incubated with biotinylated horse anti-mouse or anti-rabbit secondary antibodies (30 minutes), Vector Kit reagents (Vector Labs, Inc., Burlingame, CA, USA), then with a 3, 3'-diaminobenzidine substrate system (D3939; Sigma, St. Louis, MO, USA) for 5 minutes. Slides were counterstained with hematoxylin, dehydrated in xylene, and coverslipped.
Statistical analyses
Statistical analyses for quantification and volumes were performed to compare Dys and C, adulthood-onset Dys and C, childhood-onset Dys and C, childhood-onset Dys and adulthood-onset Dys, and generalized and segmental/focal Dys. All comparisons were computed before and after excluding co-occurring pathology (LB, LN, tau-NFT, tau-th, ubiq, and pTDP43 inclusions, β-amyloid deposits). The childhood-onset Dys group included subjects with early adulthood-onset diagnoses and the only subject with posttraumatic Dys (Dys#10, onset at age 12).
Neuronal counting results revealed a Gaussian distribution, and a t-test was initially performed to compare Dys and C and Dys and C without copathologies. Furthermore, we performed analyses of variance (ANOVAs) with Tukey's posthoc tests across all comparisons. The Tukey's test compares every mean with every other mean and takes into account multiple comparisons and adjusted p-values for each comparison. Statistical significance was established at p<0.01 (adjusted for all five types of comparisons).
The preliminary analyses for the obtained neuronal volumetric measurements did not show a Gaussian curve, so nonparametric Mann-Whitney tests were performed across all comparisons for those measurements. Data refer to the values estimated for the bilateral SN. All computations were performed using GraphPad Prism software, version 5 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
The demographic, medical, and genetic data for both the Dys and C groups are summarized in Table 1. Table 1 shows diagnoses of Dys as received from UMBTB records, while Table 2 classifies each Dys patient based on a review of supplemental medical records according to the newest Dys classification system.4 This most recent classification system was not available for any of the subjects in this cohort at the time of their clinical diagnosis. Table 2 also summarizes all available data in terms of age at onset, body distribution, temporal pattern, variability, associated features, occurrence of other neurological and systemic manifestations, and etiologies (i.e., following the Axis I and II of the newer classification).4 In terms of temporal patterns, 3 out of 13 Dys cases were classified as adulthood onset, 3 as early adulthood onset, 6 as childhood onset, and 1 case of childhood-onset posttraumatic Dys. For body distribution, 6 out of 13 Dys cases were classified as generalized, 6 as segmental/focal, and 1 segmental/focal posttraumatic Dys.
Table 1. Demographic, Diagnostic, Genetic, and Medical Data.
| Subject#/UMBTB Code | Age at Death | Sex | Diagnosis (As Received) | Genetics | Other Relevant Medical Data |
|---|---|---|---|---|---|
| 1 (Dys)/1458 | 62 | M | Dystonia | Neg for GAG del in DYT1 | None |
| 2 (Dys)/1484 | 81 | F | Dystonia (started as blepharospasm) | Not tested for DYT1 mutations | None |
| 3 (Dys)/1850 | 50 | F | Dystonia (started at 42 years old: adulthood onset, focal and segmental) | Not tested for DYT1 mutations | Mother with PD, multiple drugs |
| 4 (Dys)/4897 | 49 | F | Dystonia (generalized) | Neg for GAG del in DYT1 | None |
| 5 (Dys)/4880 | 44 | F | Dystonia (generalized dystonia with involvement of trunk, limbs, neck, and flexion contractures in the right leg; severe episodes of jerking [head back and forth] with generalized body contortions; possible seizures generalized) | Neg for GAG del in DYT1 | Brain aneurysm, intracerebral hemorrhage, multiple drugs |
| 6 (Dys)/5145 | 84 | F | Dystonia (cervical dystonia for >4 decades, head begun to turn 43 years before death with pain 20 years later, mouth involvement 22 years later) | Not tested for DYT1 mutations | Father with tremor (unspecified) |
| 7 (Dys)/5414 | 95 | F | Dystonia (tremor since the 6th grade, myoclonic jerks and terminal intentional tremor, marked paraspinal muscles spasms with lordosis, spastic dysphonia) | Not tested for DYT1 mutations | None |
| 8 (Dys)/5421 | 81 | F | Dystonia | Not tested for DYT1 mutations | None |
| 9 (Dys)/1457 | 72 | M | Dystonia | Neg for GAG del in DYT1 | None |
| 10 (Dys)/1353 | 78 | M | Dystonia (severe bike-automobile accident at 12 years old caused a fractured skull, tremor and posttraumatic dystonia followed) | Neg for GAG del in DYT1 | None |
| 11 (Dys)/4635 | 76 | M | Dystonia (generalized in combination with cervical myelopathy, initial cervical dystonia at 27 years old) | Neg for GAG del in DYT1 | History of familial dystonia symptoms (father and sister), possDLB |
| 12 (Dys)/1481 | 64 | F | Dystonia | Neg for GAG del in DYT1 | Diabetes, congestive heart failure, angina, angioplasty, daughter with dystonia |
| 13 (Dys)/1554 | 59 | F | Dystonia | Neg for GAG del in DYT1 | Small cell carcinoma, pneumonia, history of dystonia preceding lung carcinoma |
| 14 (C)/4263 | 61 | M | Control | Not tested | Ischemic cardiomyopathy, heart transplant, left ventricular dysfunction, depression, chronic renal failure, diabetes mellitus |
| 15 (C)/4921 | 73 | F | Control | Not tested | Gallbladder dysfunction, high blood pressure |
| 16 (C)/5357 | 51 | F | Control | Not tested | Drug overdose, suicidal attempt |
| 17 (C)/4788 | 48 | F | Control | Not tested | Multiple injuries resulting from a motorcycle accident |
| 18 (C)/4228 | 44 | F | Control | Not tested | None |
| 19 (C)/5219 | 76 | F | Control | Not tested | None |
| 20 (C)/M3642M | 88 | F | Control | Not tested | Congestive heart disease |
| 21 (C)/5274 | 64 | F | Control | Not tested | Hypertension, heart disease, skin cancer |
| 22 (C)/1818 | 76 | M | Control | Not tested | None |
| 23 (C)/5171 | 79 | M | Control | Not tested | COPD |
| 24 (C)/M3903M | 71 | M | Control | Not tested | Coronary artery disease, coronary angioplasty (twice), COPD |
| 25 (C)/1503 | 53 | F | Control | Not tested | Knee surgery after falling |
| 26 (C)/1379 | 53 | F | Control | Not tested | High blood pressure, asthma |
Abbreviations: C, Control; Cauc, Caucasian; COPD, Chronic Obstructive Pulmonary Disease; del, Deletion; Dys, Dystonia; DYT1, Dystonia Gene; F, Female; GAG, Three-nucleotide Deletion; M, Male; Neg, Negative; PD, Parkinson's Disease; possDLB, Possible Dementia with Lewy Bodies; UMBTB, University of Maryland Brain and Tissue Bank.
Table 2. Dys Patients Classified Following the Newer Classification System of Dystonia. The table shows the specific diagnosis of Dys for each patient in study based on the Axis I (clinical features) and Axis II (etiologies) of the newer Dys classification system.4 .
| Subject# | Diagnosis (As Received) | Dystonia Diagnosis based on the Newer Classification | Dystonia Diagnosis based on the Newer Classification |
|---|---|---|---|
|
| |||
| Axis I | Axis II | ||
|
| |||
| (Clinical Features) | (Etiology) | ||
| 1 (Dys) | Dystonia | Age at onset: not reported (probably childhood-onset as for medical history) | Nervous system pathology: no evidence of degeneration or structural lesions |
| Body distribution: not reported (probably generalized as for medical history) | Inherited: possible | ||
| Temporal pattern: not reported | Acquired: no | ||
| Variability: not reported | Idiopathic: yes | ||
| Associated features: unknown/not clinically significant | |||
| Occurrence of other neurological/systemic manifestations: unknown/not clinically significant | |||
| 2 (Dys) | Dystonia (started as blepharospasm in 1983, 22 years of dystonia history until death) | Age at onset: late adulthood | Nervous system pathology: no evidence of degeneration or structural lesions |
| Body distribution: initially focal, then segmental (oromandibular dystonia with jaw closure and lip pursing, facial grimacing) | Inherited: no (aunt, mother's sister, with resting tremor) | ||
| Temporal pattern: static | Acquired: no | ||
| Variability: diurnal | Idiopathic: yes | ||
| Associated features: headache (with temporal and orbital pain) | |||
| Occurrence of other neurological/systemic manifestations: tardive dyskinesia secondary to adverse effects to metoclopramide, moderate bradykinesia, rigidity, and rest tremor; possible side effects of various drugs used for the treatment of the blepharospasm | |||
| 3 (Dys) | Dystonia (started at 42 years old: adulthood onset, focal and segmental) | Age at onset: late adulthood | Nervous system pathology: evidence of degeneration (SN) |
| Body distribution: focal, segmental (face, neck, shoulders), dysphagia | Inherited: no (mother with PD) | ||
| Temporal pattern: static | Acquired: no | ||
| Variability: diurnal | Idiopathic: yes | ||
| Associated features: unknown/not clinically significant | |||
| Occurrence of other neurological/systemic manifestations: unknown/not clinically significant | |||
| 4 (Dys) | Dystonia (generalized) | Age at onset: childhood | Nervous system pathology: no evidence of degeneration or structural lesion |
| Body distribution: generalized (with leg involvement) | Inherited: no | ||
| Temporal pattern: progressive | Acquired: no | ||
| Variability: diurnal | Idiopathic: yes | ||
| Associated features: headache (not clinically significant) | |||
| Occurrence of other neurological/systemic manifestations: unknown/not clinically significant | |||
| 5 (Dys) | Dystonia (generalized) | Age at onset: childhood | Nervous system pathology: cerebellar cortical degeneration |
| Body distribution: multifocal neck, trunk, limbs), segmental (leg involvement) | Inherited: no | ||
| Temporal pattern: progressive | Acquired: no | ||
| Variability: paroxysmal | Idiopathic: yes | ||
| Associated features: pain, headache | |||
| Occurrence of other neurological/systemic manifestations: frontal lobe intracerebral hemorrhage (after many years of generalized dystonia) | |||
| 6 (Dys) | Dystonia (cervical dystonia for >4 decades) | Age at onset: early adulthood | Nervous system pathology: no evidence of degeneration or structural lesion |
| Body distribution: segmental (started as cervical dystonia, then mouth and lingual involvement) | Inherited: unknown (father with head tremor) | ||
| Temporal pattern: progressive (mount involvement, paraspinal muscles spasm, spastic dysphonia) | Acquired: no | ||
| Variability: diurnal | Idiopathic: yes | ||
| Associated features: facial pain | |||
| Occurrence of other neurological/systemic manifestations: resting hand tremor (later, after many years cervical dystonia appearance) | |||
| 7 (Dys) | Dystonia | Age at onset: childhood | Nervous system pathology: no evidence of degeneration or structural lesion |
| Body distribution: segmental (truncal, paraspinal spams with lordosis) | Inherited: no | ||
| Temporal pattern: diurnal | Acquired: no | ||
| Variability: diurnal | Idiopathic: yes | ||
| Associated features: spastic dysphonia, tremor started in 6th grade | |||
| Occurrence of other neurological/systemic manifestations: unknown | |||
| 8 (Dys) | Dystonia | Age at onset: late adulthood | Nervous system pathology: evidence of degeneration (SN), LB pathology |
| Body distribution: segmental (started as blepharospasm then spasmodic dysphonia; lip pursing, grimacing, and jaw opening) | Inherited: no | ||
| Temporal pattern: static | Acquired: no | ||
| Variability: diurnal (getting worse with action); not action-specific but with associated | Idiopathic: yes | ||
| features of late-onset tardive dyskinesia (oromandibular, pharyngeal, and facial dystonia) due to possible side effects of neuroleptic drugs (perphenazine/amitriptyline) | |||
| Occurrence of other neurological/systemic manifestations: unknown/not clinically significant | |||
| 9 (Dys) | Dystonia | Age at onset: childhood (probably) | Nervous system pathology: cerebellar atrophy (moderate) |
| Body distribution: generalized | Inherited: no | ||
| Temporal pattern: progressive (probably) | Acquired: no | ||
| Variability: diurnal | Idiopathic: yes | ||
| Associated features: unknown/not clinically significant | |||
| Occurrence of other neurological/systemic manifestations: unknown/not clinically significant | |||
| 10 (Dys) | Dystonia (posttraumatic) | Age at onset: childhood | Nervous system pathology: cerebellar atrophy, LBs in the SN and LC |
| Body distribution: focal (right hand) | Inherited: no | ||
| Temporal pattern: static | Acquired: yes, (posttraumatic) | ||
| Variability: persistent | Idiopathic: no | ||
| Associated features: tremor (right hand) | |||
| Occurrence of other neurological/systemic manifestations: unknown/not clinically significant | |||
| 11 (Dys) | Dystonia (generalized in combination with cervical myelopathy; initial cervical dystonia at 27 years old) | Age at onset: early adulthood | Nervous system pathology: diffuse LB pathology (brainstem, cortex), cerebellar heterotaxia (white matter) |
| Body distribution: generalized | Inherited: possible (reported familiarity for movement disorders) | ||
| Temporal pattern: progressive (started as cervical dystonia/torticollis then progressed with arms and legs) | Acquired: no | ||
| Variability: diurnal | Idiopathic: yes | ||
| Associated features: marked diffuse tremor | |||
| Occurrence of other neurological/systemic manifestations: possible dementia | |||
| 12 (Dys) | Dystonia | Age at onset: early adulthood | Nervous system pathology: no evidence of degeneration or structural lesion |
| Body distribution: generalized | Inherited: possible (daughter with dystonia) | ||
| Temporal pattern: progressive | Acquired: no | ||
| Variability: diurnal | Idiopathic: yes | ||
| Associated features: unknown/not clinically significant | |||
| Occurrence of other neurological/systemic manifestations: mastectomy for breast cancer | |||
| 13 (Dys) | Dystonia | Age at onset: childhood (probably) | Nervous system pathology: no evidence of degeneration or structural lesion (metastatic cells in cerebrum, cerebellum, brainstem) |
| Body distribution: generalized | Inherited: no | ||
| Temporal pattern: progressive | Acquired: no | ||
| Variability: diurnal | Idiopathic: yes | ||
| Associated features: unknown/not clinically significant | |||
| Occurrence of other neurological/systemic manifestations: lung cancer | |||
Abbreviations: C, Control; Dys, Dystonia; LB, Lewy Body; LC, Locus Coeruleus; PD, Parkinson's Disease; SN, Substantia Nigra.
All main neuropathologic data, postmortem delay (PMD, time between death and autopsy) values, and causes of death are shown in Table 3. The mean PMD was 11.7±6.4 hours, with no significant differences between the Dys (13±6.7 hours) and C (10.4±6.0 hours) groups. The majority of Dys and C did not show signs of major recent or remote ischemic, hemorrhagic lesions, metastatic infiltration, or arteriovenous or congenital malformations. The exception was case#5 (Dys who showed microhemorrhages, probably due to the prolonged periagonal status and history of remote hemorrhage from an aneurysmatic rupture. Intima thickening was observed in most of the arteries and arterioles in case #3, #12, #13, and #23, which was compatible with arteriosclerotic disease (see Table 4).
Table 3. Causes of Death, Autopsy Data, and Main Neuropathologic Findings.
| Subjects# | BW (Grams) | PMD (Hours) | Cause of Death | Main Neuropathologic Findings |
|---|---|---|---|---|
| 1 (Dys) | 1,455 | 12 | Natural | No significant neuropathologic findings |
| 2 (Dys) | N/A | 16 | Natural | Remote infarct in right parieto-occipital region; moderate cerebral artery atherosclerosis |
| 3 (Dys) | N/A | 3 | Brain ischemic injury | Acute hypoxic-ischemic encephalopathy, idiopathic SN degeneration |
| 4 (Dys) | N/A | 16 | Complications of the disorder (multiple falls) | No significant neuropathologic findings |
| 5 (Dys) | N/A | 20 | Complications of the disorder | Encephalomalacia involving frontal lobes with subarachnoid hematoma due to ruptured berry aneurysm and surgical clip placement, cerebellar cortical degeneration |
| 6 (Dys) | N/A | 20 | Complications of the disorder | Generalized mild atrophy, arteriosclerotic cerebrovascular disease |
| 7 (Dys) | N/A | 16 | Complications of the disorder | Neocortical gyral atrophy, arteriosclerosis with leukomalacia |
| 8 (Dys) | N/A | 8 | Complications of the disorder | Diffuse brain atrophy, arteriosclerotic cerebrovascular disease, SN degeneration with LBs in the SN and limbic cortex, AD-type lesions (amyloid neuritic plaques and neurofibrillary tangles) in the hippocampus |
| 9 (Dys) | N/A | 12 | Natural | Microscopic remote infarct in the left caudate, moderate cerebellar atrophy |
| 10 (Dys) | N/A | 5 | Septic shock, metabolic acidosis, respiratory failure | Mild cerebral hemispheric atrophy, rare LBs in the SN and LC, mild cerebellar atrophy |
| 11 (Dys) | N/A | 9 | Complications of the disorder | Amyloid neuritic plaques in the cortex; diffuse LBs in the SN, limbic structures, and cortical regions; incidental cerebellar white matter heterotopia |
| 12 (Dys) | N/A | 26 | Natural | No significant neuropathologic findings |
| 13 (Dys) | N/A | 6 | Lung cancer | Metastatic poorly differentiated carcinoma in the cerebrum, cerebellum, and brainstem |
| 14 (C) | 1,309 | 6 | Cardiac arrest | Small cystic infarcts in the left parieto-occipital and frontal lobes |
| 15 (C) | N/A | 13 | Peritonitis | No significant neuropathologic findings |
| 16 (C) | N/A | 8 | Atherosclerosis-Cardiovascular disease | No significant neuropathologic findings |
| 17 (C) | N/A | 17 | Multiple traumatic injuries | No significant neuropathologic findings |
| 18 (C) | 1,260 | 16 | Coronary artery atherosclerosis and thrombosis | Acute hypoxic-ischemic encephalopathy in hippocampus |
| 19 (C) | N/A | 3 | Complications of cancer | No significant neuropathologic findings |
| 20 (C) | N/A | 8 | Congestive heart failure | No significant neuropathologic findings |
| 21 (C) | N/A | 20 | Acute cerebrovascular disease | No significant neuropathologic findings |
| 22 (C) | N/A | 3 | Acute cerebrovascular disease | Mild to moderate atheromatosis of the circle of Willis, arteriosclerosis of the middle- and small-sized intraparenchymal arteries, microinfarct in the right Sommer's sector (cornu ammonis 1 of the hippocampus) |
| 23 (C) | N/A | 5 | COPD, peripheral vascular disease | No significant neuropathologic findings |
| 24 (C) | N/A | 16 | Cardiac arrest | Brain edema |
| 25 (C) | N/A | 5 | Pulmonary thromboembolism | No significant neuropathologic findings |
| 26 (C) | N/A | 15 | Respiratory distress | No significant neuropathologic findings |
Abbreviations: AD, Alzheimer's Disease; BW, Brain Weight; C, Control; COPD, Chronic Obstructive Pulmonary Disease; Dys, Dystonia; LB, Lewy Body; LC, Locus Coeruleus; N/A, Not Applicable; PMD, Postmortem Delay; SN, Substantia Nigra.
Table 4. H&E and Immunohistochemistry Assessment of other SN Pathologies. The table shows the types and frequencies of co-occurring brain pathologies in the SN of Dys and C subjects. Co-occurring brain pathologies were assessed by specific immunohistochemistry protocols for the following antigens: 1–42 β-amyloid, hyperphosphorylated-tau, α-synuclein, ubiq, and phosphorylated-TDP43. The lesions considered were 1–42 β-amyloid diffuse and neuritic plaques, hyperphosphorylated-tau positive NFTs, tau threads, and ubiq- and phosphorylated-TDP43-positive intraneuronal cytoplasmic inclusions.
| Case# | 1–42 β-amyloid | Hyperphosphorylated-tau | α-synuclein | Ubiq-cytopl. incl./MB | Phosphorylated-TDP43 | H&E |
|---|---|---|---|---|---|---|
| 1 (Dys) | Neg | Neg | Neg | Neg/Neg | Neg | Normal |
| 2 (Dys) | Neg | Neg | Neg | Neg/Pos | Neg | Normal |
| 3 (Dys) | Neg | Neg | Neg | Neg/Pos | Neg | Intimal thickening |
| 4 (Dys) | Neg | Neg | Neg | Neg/Neg | Neg | Normal |
| 5 (Dys) | Neg | Neg | Neg | Neg/Neg | Neg | Microhemorrhages |
| 6 (Dys) | Neg | NFT and Th (sparse), tau-glia | Neg | Neg/Pos | Neg | Normal |
| 7 (Dys) | Neg | NFT and Th (moderate) | Neg | Neg/Pos | Neg | Normal |
| 8 (Dys) | Neg | Th (moderate) | Pos (sparse) | Neg/Neg | Neg | Normal |
| 9 (Dys) | Neg | Neg | Neg | Neg/Neg | Neg | Normal |
| 10 (Dys) | Neg | NFT (rare) | Pos (rare) | Neg/Pos | Neg | Normal |
| 11 (Dys) | Neg | Th (sparse), pre-NFT (rare) | Pos (rare) | Neg/Neg | Neg | Normal |
| 12 (Dys) | Neg | Neg | Neg | Neg/Neg | Neg | Intimal thickening |
| 13 (Dys) | Neg | Neg | Neg | Neg/Pos | Neg | Intimal thickening |
| 14 (C) | Neg | Neg | Neg | Neg/Neg | Neg | Normal |
| 15 (C) | Neg | Th (sparse) | Neg | Neg/Neg | Neg | Normal |
| 16 (C) | Neg | Neg | Neg | Neg/Neg | Neg | Normal |
| 17 (C) | Neg | Neg | Neg | Neg/Neg | Neg | Normal |
| 18 (C) | Neg | Neg | Neg | Neg/Neg | Neg | Normal |
| 19 (C) | Neg | Th (rare) | Neg | Neg/Neg | Neg | Normal |
| 20 (C) | Neg | NFT (rare) | Neg | Neg/Neg | Neg | Hyperemia |
| 21 (C) | Neg | Th (rare) | Neg | Neg/Neg | Neg | Normal |
| 22 (C) | Neg | Neg | Neg | Neg/Pos | Neg | Normal |
| 23 (C) | Neg | Neg | Neg | Neg/Neg | Neg | Intimal thickening |
| 24 (C) | Neg | Th (sparse) | Neg | Neg/Neg | Neg | Normal |
| 25 (C) | Neg | Neg | Neg | Neg/Neg | Neg | Normal |
| 26 (C) | Neg | Neg | Neg | Neg/Neg | Neg | Normal |
Abbreviations: C, Control; Dys, Dystonia; H&E, Hematoxylin and Eosin; Neg, Negative; NFT, Neurofibrillary Tangle; Pos, Positive; SN, Substantia Nigra; TDP43, TAR DNA-binding Protein 43; Th, Thread
Immunohistochemistry findings
Overall, 5/13 Dys and 5/13 C were positive for tau lesions. In the majority of cases, tau lesions were sparse or rare (tau-NFT or tau-th), and only two Dys cases (cases #7 and #8) had moderate levels of tau lesions, mostly tau-th.
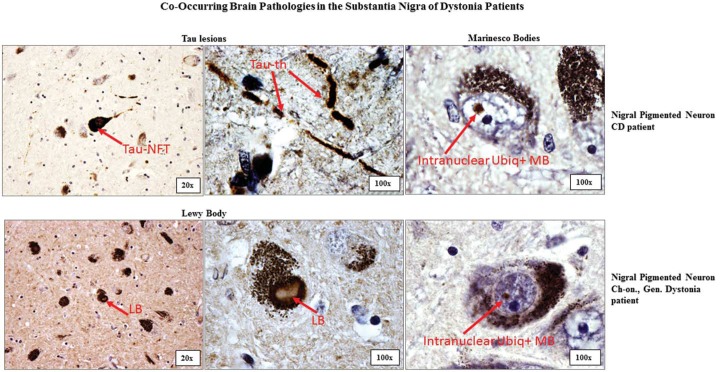
No C cases had α-synuclein-positive lesions (LBs or LNs), whereas three Dys cases (#8, #10, and #11) did. These three cases were classified as possible Lewy Body disease (possLBD) of the brainstem.40 None of the SN samples were positive for 1–42 β-amyloid (diffuse amyloid or neuritic plaques), ubiq, or pTDP43-positive cytoplasmic intraneuronal inclusions. Ubiq-positive intranuclear inclusions (Marinesco bodies [MB])44 were observed in some cases, and more often in Dys than C. As a reminder, MB are eosinophilic intranuclear neuronal inclusions observed in human brains that have an uncertain origin and pathologic meaning. This intriguing finding has been reported previously; 30 however, larger studies are necessary to confirm the higher frequency of MB in Dys in general or in a specific Dys subtype. Table 4 summarizes the immunohistochemistry findings in both groups.
Quantitative findings: neuronal counting
A total number of 496,601±130,010 and 648,926±162,475 pigmented neurons were estimated in the SNs of the Dys and C groups, respectively. The estimated nigral neuronal population in C did not differ proportionally from previous stereologic studies that assessed the entire SN length in control subjects.45,46
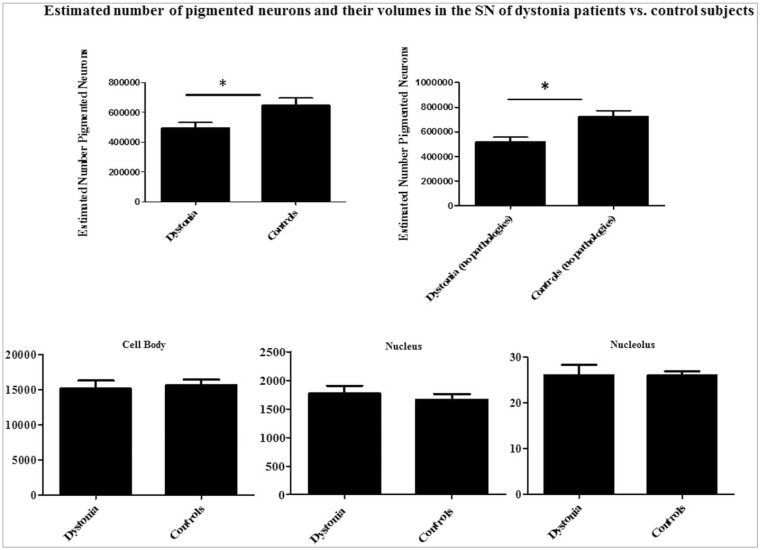
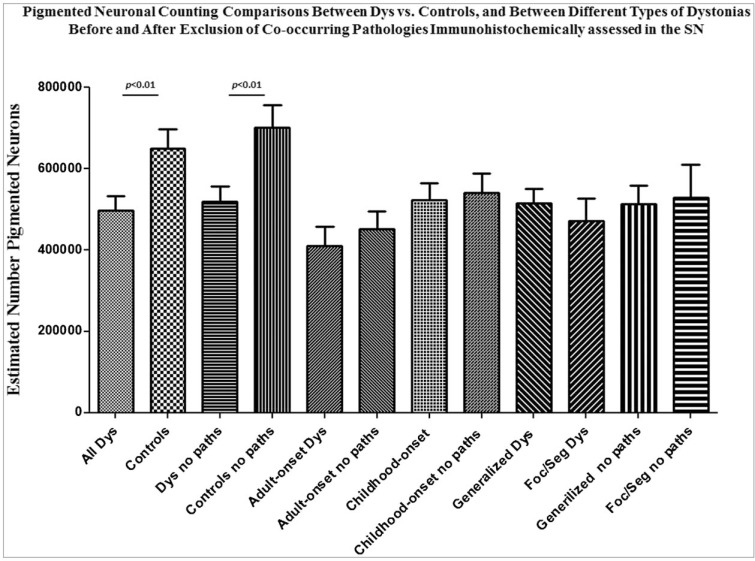
Histograms depicting the estimated nigral pigmented neuronal counts and volumes are shown in Figure 2. The estimated nigral pigmented neuronal population was calculated using neuronal numbers weighted for each section thickness per each subject. A significant reduction of pigmented neurons in SN of Dys versus C was found (p = 0.01). A separate computation after exclusion of all positive LB/LN and/or tau-NFT/th Dys (#6, #7, #8, #10, and #11) and C (#15, #19, #20, #21, and #24) cases was also performed. This second narrower computation confirmed a significant reduction of pigmented neurons in the SN of Dys (n = 8) versus C (n = 7) (p = 0.01). No significant differences in terms of neuronal counts were observed between childhood-onset Dys and adulthood-onset Dys cases regardless of whether cases with co-occurring nigral pathologies were excluded. Table 5 summarizes all comparisons for neuronal counting between Dys and C with their numerical values and statistical significance. See also Figure 4.
Figure 2. Histograms of the Estimated Mean Number of SN Pigmented Neurons in 13 Dys Patients and 13 C Subjects. The histograms on the left side include all Dys and C brains, while those on the right show Dys and C data after exclusion of all brains with immunohistochemical evidence of co-occurring pathologies. The lower part of the figure shows histograms of cell body, nuclear, and nucleolar mean volumes of pigmented neurons in the SN. The y-axis indicates mean volumetric values expressed in µm3. *p<0.01. Abbreviations: C, Control; Dys, Dystonia; SN, Substantia Nigra.
Table 5. Estimated Number of Pigmented Neuron Population and their Cellular and Subcellular Volumes in the SN of Dys and C Subjects. The table shows the estimated number and cell body, nuclear, and nucleolar volumes of pigmented neurons (mean±SD) in the SN of Dys and C across all considered subtypes. The significance of p-values is indicated for each type of comparison. The significance was established when p<0.01.
| Comparisons | Estimated Number of Pigmented Neurons (±SD) | Cell Body | Nuclear | Nucleolar |
|---|---|---|---|---|
|
| ||||
| Volume (µm3) | Volume (µm3) | Volume (µm3) | ||
| Dys (n = 13) vs. C (n = 13) | 496,601.6±130,009.4 vs. 648,925.7±169,700.2 (p = 0.0186) | 15,136.1±4,122.2 vs. 15,632.1±2,718.2 (ns) | 1,768.1±505.4 vs. 1,662.2±347.0 (ns) | 26.0±8.2 vs. 25.9±3.1 (ns) |
| *Dys (n = 8) vs. *C (n = 8) | 518,991.3± 139,210.6 vs. 725,432.2±16,531.8 (p = 0.0045) | 14,340.1±5,097.2 vs. 15,936.1±2,992.1 (ns) | 1,627.1±560.1 vs. 1,767.2±399.2 (ns) | 25.8±8.9 vs. 26.0±3.8 (ns) |
| Adulthood-onset Dys (n = 3) vs. C (n = 13) | 408,748.1±85,747.9 vs. 648,925.7±169,700.2 (p = 0.0366) | 13,669.1±3,557.2 vs. 15,936.1±2,992.1 (ns) | 1,661.1±491.9 vs. 1,662.2±347.0 (ns) | 24.0±6.8 vs. 25.9±3.1 (ns) |
| *Adulthood-onset Dys (n = 2) vs. *C (n = 8) | 408,748.1±85,747.9 vs. 725,432.2±16,531.8 (p = 0.0366) | 11,709.5±1,510.5 vs. 15,222.7±766.4 (ns) | 1,491.1±556.6 vs. 1,796.1±372.0 (ns) | 24.1±9.7 vs. 26.0±5.2 (ns) |
| Childhood-onset Dys (n = 10) vs. C (n = 13) | 503,648.9±124,734.1 vs. 648,925.7±169,700.2 (p = 0.0436) | 15,764.1±4,569.2 vs. 15,632.1±2,718.2 (ns) | 1,764±533.1 vs. 1,662±347.0 (ns) | 27.1±9.1 vs. 25.9±3.1(ns) |
| *Childhood-onset Dys (n = 6) vs. *C (n = 8) | 541,369.8±112,651.7 vs. 725,432.2±16,531.8 (p = 0.0192) | 15,216.7±1,373.2 vs. 15,222.7±766.4 (ns) | 1,671.7±400.9 vs. 1,796.1±372.0 (ns) | 25.6±5.2 vs. 26.0±5.2 (ns) |
| Childhood-onset Dys (n = 10) vs. Adulthood-onset Dys (n = 3) | 504,647.0±371,697.8 vs. 463,247.9±97,180.9 (ns) | 15,764.1±4,569.2 vs. 13,669.1±3,557.2 (ns) | 1,764±533.1 vs. 1,662.2±347.0 (ns) | 27.1±9.1 vs. 25.9±3.1 (ns) |
| *Childhood-onset Dys (n = 6) vs. *Adulthood-onset Dys (n = 2) | 535,589.0±371,697.8 vs. 512,103.1±67,582.0 (ns) | 15,216.7±1,373.2 vs. 11,709.5±1,510.5 (ns) | 1,671.7±400.9 vs. 1,796.1±372.0 (ns) | 25.6±5.2 vs. 26.0±5.2 (ns) |
| Generalized Dys (n = 6) vs. segmental/focal Dys (n = 7) | 514,023.1±98,491.31 vs. 481,668.9± 163,184.3 (ns) | 14,379.1±1,004.9 vs. 15,784.8±3,587.4 (ns) | 1,767.6±409.5 vs. 15,784.8±3,587.4 (ns) | 28.9±3.5 vs. 23.4±4.2 (ns) |
| *Generalized Dys (n = 5) vs. *segmental/focal Dys (n = 3) | 513,204.1±139,210.5 vs. 528,636.7±139,513.19 (ns) | 13,984.5±1,373.2 vs. 14,932.3±5,683.2 (ns) | 1,655.5±400.9 vs. 1,982.4±312.7 (ns) | 27.2±1.0 vs. 32.1±5.5 (ns) |
Abbreviations: C, Control; Dys, Dystonia; ns, not significant; SD, Standard Deviations; SN, Substantia Nigra.
Figure 3. Co-occurring Pathologies in the SN of Dys Patients. Abbreviations: CD, Cervical Dystonia (subject Dys#6); Ch-on, Childhood-onset; Gen., Generalized (Subject Dys#13); LB, α-synuclein-positive Lewy Body; MB, Marinesco Body (intranuclear eosinophilic body); Tau-NFT, Tau-positive Neurofibrillary Tangle; Tau-th, Tau-positive Thread; Ubiq+, Ubiquitin Positivity.
The mean for each type of volumetric measurement (cell body, nucleus, and nucleolus) performed on nigral pigmented neurons of Dys and C are shown in Table 5. A mean of 179.7±56.3 nigral pigmented neurons were volumetrically measured (range: 97–297) across all cases. No difference was measured between the mean numbers of pigmented neurons volumetrically measured in Dys (157.5±40.0) and C (201.9±56.3). Importantly, no significant differences in mean cellular, nuclear, or nucleolar volumes were found across all types of comparisons between the Dys and C groups (Table 5). Table 6 shows the coefficient error (CE) values for each performed measurement (counting and volumes) for each subject in the study.
Table 6. Estimated Number of Nigral Pigmented Neuronal Populations and Volumetric Measurements with Corresponding Values of Gunderson's Coefficient Errors in the Dys and C Groups. The table shows single values obtained in each Dys and C subject in terms of estimated number of pigmented nigral neurons counted. Each mean neuronal counting estimation has a corresponding mean cell body, nuclear, and nucleolar volume for all examined subjects. CE is an indicator of precision for the performed estimations and is generally acceptable if <0.10.
| Subject# | Mean Pigmented Neuronal Count | CE | Mean Cellular Volume | CE | Mean Nuclear Volume | CE | Mean Nucleolar Volume | CE |
| 1 (Dys) | 530,990.7 | 0.05 | 18,457.9 | 0.006 | 2,332.3 | 0.006 | 38.1 | 0.007 |
| 2 (Dys) | 409,690.0 | 0.07 | 12,777.6 | 0.003 | 1,884.7 | 0.003 | 31.0 | 0.004 |
| 3 (Dys) | 494,021.3 | 0.06 | 10,641.4 | 0.005 | 1,097.5 | 0.006 | 17.2 | 0.022 |
| 4 (Dys) | 604,631.9 | 0.06 | 12,589.7 | 0.004 | 1,730.0 | 0.005 | 27.3 | 0.005 |
| 5 (Dys) | 682,198.8 | 0.05 | 21,377.8 | 0.005 | 1,752.9 | 0.005 | 24.4 | 0.006 |
| 6 (Dys) | 491,138.2 | 0.05 | 17,297.2 | 0.007 | 1,510.4 | 0.007 | 24.8 | 0.006 |
| 7 (Dys) | 275,364.9 | 0.08 | 16,926.6 | 0.009 | 1,840.5 | 0.009 | 21.6 | 0.011 |
| 8 (Dys) | 322,533.2 | 0.07 | 17,586.4 | 0.007 | 2,002.1 | 0.007 | 23.7 | 0.062 |
| 9 (Dys) | 405,516.6 | 0.06 | 5,273.8 | 0.006 | 581.8 | 0.009 | 8.0 | 0.008 |
| 10 (Dys) | 696,735.9 | 0.05 | 13,886.4 | 0.004 | 2,286.8 | 0.005 | 21.3 | 0.049 |
| 11 (Dys) | 518,118.1 | 0.06 | 16,352.0 | 0.006 | 2,328.5 | 0.007 | 37.4 | 0.007 |
| 12 (Dys) | 414,003.9 | 0.06 | 17,771.6 | 0.006 | 1,533.1 | 0.006 | 30.6 | 0.079 |
| 13 (Dys) | 610,877.4 | 0.05 | 15,829.5 | 0.005 | 2,100.2 | 0.005 | 32.0 | 0.005 |
| 14 (C) | 787,705.8 | 0.06 | 13,858.9 | 0.004 | 1,115.2 | 0.005 | 22.0 | 0.016 |
| 15 (C) | 648,925.7 | 0.06 | 11,253.3 | 0.007 | 1,390.6 | 0.008 | 23.6 | 0.045 |
| 16 (C) | 351,255.9 | 0.05 | 15,631.9 | 0.003 | 1,663.0 | 0.003 | 25.8 | 0.004 |
| 17 (C) | 856,559.1 | 0.06 | 11,864.0 | 0.003 | 1,600.5 | 0.003 | 24.0 | 0.004 |
| 18 (C) | 621,172.8 | 0.07 | 15,532.5 | 0.007 | 2,084.9 | 0.008 | 29.0 | 0.053 |
| 19 (C) | 370,591.1 | 0.06 | 20,711.7 | 0.010 | 1,585.1 | 0.009 | 21.1 | 0.010 |
| 20 (C) | 571,960.5 | 0.06 | 18,168.9 | 0.003 | 1,704.5 | 0.004 | 27.3 | 0.048 |
| 21 (C) | 696,553.0 | 0.06 | 15,519.3 | 0.003 | 1,365.8 | 0.003 | 26.8 | 0.034 |
| 22 (C) | 488,401.4 | 0.05 | 14,754.4 | 0.004 | 1,327.2 | 0.005 | 25.1 | 0.057 |
| 23 (C) | 800,241.8 | 0.05 | 18,947.8 | 0.004 | 1,660.0 | 0.004 | 28.4 | 0.004 |
| 24 (C) | 718,722.8 | 0.04 | 15,375.8 | 0.004 | 2,007.7 | 0.003 | 29.7 | 0.004 |
| 25 (C) | 750,282.5 | 0.05 | 16,342.6 | 0.004 | 1,788.9 | 0.005 | 23.0 | 0.059 |
| 26 (C) | 773,662.1 | 0.05 | 15,258.7 | 0.003 | 2,315.1 | 0.003 | 30.5 | 0.004 |
Abbreviations: C, Control; CE, Coefficient of Error (of Gunderson); Dys, Dystonia.
Discussion
To our knowledge, this is the first investigation to apply rigorous neuropathologic quantitative methods to estimate the numbers and volumes of nigral pigmented neurons in a consistent series of Dys and an equivalent number of age-matched control brains that were systematically assessed by specific immunohistochemistry protocols for the individuation and exclusion of possible co-occurring pathologies affecting the SN. Importantly, this is the largest quantitative analyses of Dys brain tissue to date. Our results show that both adulthood- and childhood-onset Dys patients exhibited significant reductions of pigmented SN neurons compared to the C groups. Furthermore, these quantitative analyses showed no significant differences between Dys and C in terms of nigral pigmented neuronal volumes. These findings would seem to exclude phenomena of atrophy or hypertrophy for this type of pigmented neuron in non-DYT1 idiopathic Dys brains. Previous studies describing semiquantitative or pathologic findings on Dys brains mainly focused on genetic Dys, specifically DYT1 cases.20,29 Therefore, most of the findings from those studies do not seem to be directly applicable to the Dys cohort examined here.
The previous report of larger pigmented neurons in the SN of subjects with Dys (with and without GAG deletions)29 was not confirmed in our non-DYT1 idiopathic Dys cohort. However, our findings do not exclude the possibility that certain genetic forms of Dys, such as DYT1 characterized by TorsinA protein dysfunction, could induce specific cellular adaptation phenomena during brain development. Indeed, it could be hypothesized that congenital reductions or enlargements of specific neuronal types are present in some specific sensory-motor cortical or subcortical areas47 as adaptive or compensatory neuronal mechanisms against the subjacent initial pathology. Phenomena of neuroplasticity48 in Dys patients illustrated by some recent functional neuroimaging studies49 might support a neurodevelopmental hypothesis of idiopathic Dys.
Limitations and strengths
Although the quantitative methods used in this study were very similar to those used in unbiased stereology investigations, and we tried to minimize confounding factors, it is important to consider our results in the context of some important limitations:
-
1)
The obtained quantitative estimations were not based on the entire length of the SN. Although we used well-established and reliable stereology tools (such as the Optical Fractionator and Nucleator probe) and precise histological, neuropathologic, and clinical approaches (i.e., serial sectioning, anatomical randomization, exclusion of co-occurring pathologies, same mean age at death, etc.), this investigation cannot be defined as an unbiased stereology study mainly due to the impossibility of obtaining the entire structure of interest (SN). Our preliminary novel findings need to be confirmed by unbiased stereological investigations that include the entire anatomical extension of SN.
-
2)
We assessed a low number of cases for each specific type of Dys. It is highly probable that different types of Dys have different causes and unique pathologic features. For example, it is highly probable that adulthood- and childhood-onset forms of Dys represent two different pathogenetic pathways. However, it cannot be totally excluded that they share some common clinical and pathologic features. Then, we cannot be certain that future larger neuropathologic quantitative stereologic studies focusing on a single type of Dys could confirm our findings only on some specific type or group of Dys.
This investigation, however, also has several strengths:
-
1)
To the best of our knowledge, this is the first clinicopathologic correlative study performed on a relatively large number of autopsy-Dys brains (n = 13) and an equivalent amount of autopsy-confirmed age-matched control brains applying rigorous neuropathologic quantitative methods to describe possible specific characteristics (e.g., a reduced number of pigmented neurons in the SN).
-
2)
Extensive histopathologic analyses were performed on each single Dys and C case, including possible co-occurring pathologies affecting the SN. In our opinion, this type of immunohistochemistry assessment is essential to decrease the possibility that a lower number of nigral pigmented neurons could be due to the simultaneous presence of nigral degenerative pathologies. These could indeed represent important confounders that are not directly or necessarily related to the pathogenesis of idiopathic forms of Dys. However, our findings do not exclude the possibility that nigral or extranigral neurodegenerative phenomena (e.g., during aging), could contribute to the progression or initial disease manifestations.
-
3)
There was a remarkable effort in reclassifying most of the non-DYT1 Dys autopsy cases available at UMBTB following the most recent classification system changes.4 The reclassification was based on the careful re-examination of all available medical records. This effort was an important attempt to describe new neuropathologic findings using precious brain tissues previously donated and already available to the scientific community in consideration of newer clinical views and modern neuropathologic quantitative and immunohistochemistry techniques.
Our neuropathologic morphometric-quantitative findings, which are similar to the results of other recent pathologic studies,31,32 seem to confirm the direct involvement of the SN in the pathomechanisms of Dys. Importantly, the involvement of the SN in Dys with regard to a reduced number of pigmented neurons (mainly dopaminergic cells) is not necessarily or directly related to dopaminergic dysfunction per se (i.e., dopaminergic function loss), or to a specific clinical phenomenology of Dys. In fact, in a hypothetical and more global context of a neurodevelopmental disorder, it is possible that other nondopaminergic circuits and neurotrasmettitorial abnormalities (i.e., gamma-aminobutyric acid, acetylcholine)50,51 could play important or even major roles. Moreover, results from two clinical and functional neuroimaging series52,53 support a more global view on Dys phenomenology where the motor and nonmotor abnormalities observed in Dys patients are associated with a broad spectrum of abnormalities in multiple cerebral regions, some of which are nondopaminergic. Furthermore, at a higher speculative level, a neurodevelopmental hypothesis for idiopathic Dys (or some specific form of it) that would not primarily involve dopaminergic dysfunction (i.e., dopaminergic function loss) could help to explain the constant and frustrating inefficacy of dopaminergic therapies in most Dys patients, with the remarkable exception of DRD.
Larger studies are needed to confirm our novel findings on idiopathic Dys. However, these preliminary results seem to suggest the presence of a specific neuropathologic substrate in idiopathic Dys in the form of a reduced number of nigral pigmented neurons that is not due to a neurodegenerative process.
Some very important questions remain:
-
1)
In Dys brains, is the nigral pigmented neuronal reduction a loss of neurons (neurodegenerative hypothesis), or does it represent, as we hypothesize, a possible neurodevelopmental defect (neurodevelopmental hypothesis)?54 In support of the latter hypothesis is the observation that volumetric measurements of nigral pigmented neurons in Dys cases were not significantly different from those in C brains as would be expected if atrophic-neurodegenerative processes were actually involved. These morphometric findings are especially relevant considering that the reduction of nigral pigmented neurons in Dys versus C remained significant even after immunohistochemical exclusion of some copathologies frequently associated with SN neuronal degeneration.
In addition, the absence of a difference in the number of nigral pigmented neurons between the only available posttraumatic dystonia case (#10) and C brains seems to support a neurodevelopmental hypothesis for idiopathic Dys. In the future, larger studies of posttraumatic and nonposttraumatic/idiopathic Dys could provide pathologic findings that support or exclude a neurodevelopmental defect of the SN in idiopathic Dys.
-
2)
Does the nigral pigmented neuronal reduction primarily or exclusively affect dopaminergic neurons? Does it specifically impact the pars compacta or pars reticulata of the SN? It is important to recall that the SN (and basal ganglia [BG] in general) contains a considerable level of somatotopic neuronal organization and neurotrasmettitorial complexity. It is not possible to exclude then that fewer nigral pigmented neurons could mainly affect specific subregions of the SN, thus influencing specific cellular and pathogenetic mechanisms and causing various clinical phenotypes and motor and nonmotor manifestations of Dys.
-
3)
Does the nigral neuronal reduction (or loss) represent a cause or an effect of other pathologic (or neurodevelopmental) phenomena?
Our preliminary quantitative findings, if confirmed, would offer novel insight into specific neuronal alterations in idiopathic Dys. However, these new findings need to take into account very complex pathophysiologic mechanisms and possible pathologic differences across the various forms of Dys.55–58 Nonetheless, partial overlapping or common mechanisms among groups or subgroups of Dys with similar clinical phenotypes remain possible.59
Future major efforts should include unbiased stereological analyses in larger groups of different genetic and nongenetic forms of Dys to confirm our observation of fewer pigmented neurons in the SN, and if so, clarify whether this decrease is “congenital” or neurodegenerative in nature. Another important issue is whether this cellular abnormality is unique to the SN or specific forms of Dys.
Figure 4. Histograms of Estimated Mean Numbers of Pigmented Neurons. We quantified neurons in the substantia nigra (SN) of dystonia (Dys) vs. age-matched control subjects (C) and compared counts in types of dystonia (adulthood-onset, childhood-onset, generalized, focal/segmental [foc/seg] dystonias). For each measurement there are two histograms showing the obtained estimated number of neurons before and after exclusion of all nigral co-occurring pathologies (β-amyloid, tau, Lewy bodies, and ubiquitin). All co-occurring nigral pathologies were assessed using immunohistochemistry protocols.
Acknowledgements
We are grateful to all dystonia patients, control subjects, and families for their generous gifts. Human tissue was obtained from the NIH NeuroBioBank at the University of Maryland, Baltimore, MD. We are also thankful to all personnel of the University of Maryland Brain and Tissue Bank (UMBTB), and especially to John Cottrell, M.S., P.A., A.S.C.P. for his technical support.
Footnotes
Funding: The Parkinson's & Movement Disorder Foundation (PDMD), Fountain Valley, CA, Grant 2012; Biomedical Research Institute of New Jersey, BRInj, NJ, “Dystonia Grant” 2013.
Financial disclosures: D.I., M.G.E., H.P. have no disclosures to report. M.L.R. has received honoraria from Teva and Allergan and grants from Ipsen; R.K. reports grants from the NIH, Astra-Zeneca, Psyadon, Rhythm, and Kyowa and honoraria from Teva.
Conflict of interests: The authors report no conflict of interest.
References
- 1.Defazio G, Abbruzzese G, Livrea P, Berardelli A. Epidemiology of primary dystonia. Lancet Neurol. 2004;3:673–678. doi: 10.1016/S1474-4422(04)00907-X. [DOI] [PubMed] [Google Scholar]
- 2.Steeves TD, Day L, Dykeman J, Jette N, Pringsheim T. The prevalence of primary dystonia: a systematic review and meta-analysis. Mov Disord. 2012;27:1789–1796. doi: 10.1002/mds.25244. [DOI] [PubMed] [Google Scholar]
- 3.Defazio G, Jankovic J, Giel JL, Papapetropoulos S. Descriptive epidemiology of cervical dystonia. Tremor Other Hyperkinet Mov (N Y) 2013;3 doi: 10.7916/D80C4TGJ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albanese A, Bhatia K, Bressman SB, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord. 2013;28:863–873. doi: 10.1002/mds.25475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marsden CD. Dystonia: the spectrum of the disease. Res Publ Assoc Res Nerv Ment Dis. 1976;55:351–367. [PubMed] [Google Scholar]
- 6.Fahn S, Eldridge R. Definition of dystonia and classification of the dystonic states. Adv Neurol. 1976;14:1–5. [PubMed] [Google Scholar]
- 7.Fahn S. Concept and classification of dystonia. Adv Neurol. 1988;50:1–8. [PubMed] [Google Scholar]
- 8.Bressman SB. Dystonia genotypes, phenotypes, and classification. Adv Neurol. 2004;94:101–107. [PubMed] [Google Scholar]
- 9.Ozelius LJ, Hewett JW, Page CE, et al. The early-onset torsion dystonia gene (DYT1) encodes an ATP-binding protein. Nat Genet. 1997;17:40–48. doi: 10.1038/ng0997-40. [DOI] [PubMed] [Google Scholar]
- 10.Kaji R, Goto S, Tamiya G, Ando S, Makino S, Lee LV. Molecular dissection and anatomical basis of dystonia: X-linked recessive dystonia-parkinsonism (DYT3) J Med Invest. 2005;52(Suppl:280–283) doi: 10.2152/jmi.52.280. [DOI] [PubMed] [Google Scholar]
- 11.Chen P, Burdette AJ, Porter JC, et al. The early-onset torsion dystonia-associated protein, torsinA, is a homeostatic regulator of endoplasmic reticulum stress response. Hum Mol Genet. 2010;19:3502–3515. doi: 10.1093/hmg/ddq266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bragg DC, Armata IA, Nery FC, Breakefield XO, Sharma N. Molecular pathways in dystonia. Neurobiol Dis. 2011;42:136–147. doi: 10.1016/j.nbd.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hettich J, Ryan SD, de Souza ON, et al. Biochemical and cellular analysis of human variants of the DYT1 dystonia protein, TorsinA. Hum Mutat. 2014;35:1101–1113. doi: 10.1002/humu.22602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jankovic J. Treatment of dystonia. Lancet Neurol. 2006;5:864–872. doi: 10.1016/S1474-4422(06)70574-9. [DOI] [PubMed] [Google Scholar]
- 15.Swope D, Barbano R. Treatment recommendations and practical applications of botulinum toxin treatment of cervical dystonia. Neurol Clin. 2008;26(Suppl 1):54–65. doi: 10.1016/s0733-8619(08)80005-9. [DOI] [PubMed] [Google Scholar]
- 16.Albanese A, Asmus F, Bhatia KP, et al. EFNS guidelines on diagnosis and treatment of primary dystonias. Eur J Neurol. 2011;18:5–18. doi: 10.1111/j.1468-1331.2010.03042.x. [DOI] [PubMed] [Google Scholar]
- 17.Tanabe LM, Kim CE, Alagem N, Dauer WT. Primary dystonia: molecules and mechanisms. Nat Rev Neurol. 2009;5:598–609. doi: 10.1038/nrneurol.2009.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao J, Vemula SR, LeDoux MS. Recent advances in the genetics of dystonia. Curr Neurol Neurosci Rep. 2014;14:462. doi: 10.1007/s11910-014-0462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Standaert DG. Update on the pathology of dystonia. Neurobiol Dis. 2011;42:148–151. doi: 10.1016/j.nbd.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNaught KS, Kapustin A, Jackson T, et al. Brainstem pathology in DYT1 primary torsion dystonia. Ann Neurol. 2004;56:540–547. doi: 10.1002/ana.20225. [DOI] [PubMed] [Google Scholar]
- 21.Paudel R, Kiely A, Li A, et al. Neuropathological features of genetically confirmed DYT1 dystonia: investigating disease-specific inclusions. Acta Neuropathol Commun. 2014;2:159. doi: 10.1186/s40478-014-0159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altrocchi PH, Forno LS. Spontaneous oral-facial dyskinesia: neuropathology of a case. Neurology. 1983;33:802–805. doi: 10.1212/wnl.33.6.802. [DOI] [PubMed] [Google Scholar]
- 23.Gibb WR, Lees AJ, Marsden CD. Pathological report of four patients presenting with cranial dystonias. Mov Disord. 1988;3:211–221. doi: 10.1002/mds.870030305. [DOI] [PubMed] [Google Scholar]
- 24.Kulisevsky J, Marti MJ, Ferrer I, Tolosa E. Meige syndrome: neuropathology of a case. Mov Disord. 1988;3:170–175. doi: 10.1002/mds.870030209. [DOI] [PubMed] [Google Scholar]
- 25.Zweig RM, Hedreen JC, Jankel WR, Casanova MF, Whitehouse PJ, Price DL. Pathology in brainstem regions of individuals with primary dystonia. Neurology. 1988;38:702–706. doi: 10.1212/wnl.38.5.702. [DOI] [PubMed] [Google Scholar]
- 26.Zweig RM, Hedreen JC. Brain stem pathology in cranial dystonia. Adv Neurol. 1988;49:395–407. [PubMed] [Google Scholar]
- 27.Bhatia K, Daniel SE, Marsden CD. Orofacial dystonia and rest tremor in a patient with normal brain pathology. Mov Disord. 1993;8:361–362. doi: 10.1002/mds.870080320. [DOI] [PubMed] [Google Scholar]
- 28.Holton JL, Schneider SA, Ganesharajah T, et al. Neuropathology of primary adult-onset dystonia. Neurology. 2008;70:695–699. doi: 10.1212/01.wnl.0000302175.76229.f0. [DOI] [PubMed] [Google Scholar]
- 29.Rostasy K, Augood SJ, Hewett JW, et al. TorsinA protein and neuropathology in early onset generalized dystonia with GAG deletion. Neurobiol Dis. 2003;12:11–24. doi: 10.1016/s0969-9961(02)00010-4. [DOI] [PubMed] [Google Scholar]
- 30.Prudente CN, Pardo CA, Xiao J, et al. Neuropathology of cervical dystonia. Exp Neurol. 2013;241:95–104. doi: 10.1016/j.expneurol.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mencacci NE, Isaias IU, Reich MM, et al. International Parkinson's Disease Genomics Consortium and UCL-exomes consortium. Parkinson's disease in GTP cyclohydrolase 1 mutation carriers. Brain. 2014;137:2480–2492. doi: 10.1093/brain/awu179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Göttle M, Prudente CN, Fu R, et al. Loss of dopamine phenotype among midbrain neurons in Lesch-Nyhan disease. Ann Neurol. 2014;76:95–107. doi: 10.1002/ana.24191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asanuma K, Carbon-Correll M, Eidelberg D. Neuroimaging in human dystonia. J Med Invest. 2005;52(Suppl:272–279) doi: 10.2152/jmi.52.272. [DOI] [PubMed] [Google Scholar]
- 34.Neychev VK, Gross RE, Lehéricy S, Hess EJ, Jinnah HA. The functional neuroanatomy of dystonia. Neurobiol Dis. 2011;42:185–201. doi: 10.1016/j.nbd.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stoessl AJ, Lehericy S, Strafella AP. Imaging insights into basal ganglia function, Parkinson's disease, and dystonia. Lancet. 2014;18 doi: 10.1016/S0140-6736(14)60041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rudow G, O′Brien R, Savonenko AV, et al. Morphometry of the human substantia nigra in ageing and Parkinson's disease. Acta Neuropathol. 2008;115:461–470. doi: 10.1007/s00401-008-0352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cabello CR, Thune JJ, Pakkenberg H, Pakkenberg B. Ageing of substantia nigra in humans: cell loss may be compensated by hypertrophy. Neuropathol Appl Neurobiol. 2002;28:283–291. doi: 10.1046/j.1365-2990.2002.00393.x. [DOI] [PubMed] [Google Scholar]
- 38.Ma SY, Röytt M, Collan Y, Rinne JO. Unbiased morphometrical measurements show loss of pigmented nigral neurones with ageing. Neuropathol Appl Neurobiol. 1999;25:394–399. doi: 10.1046/j.1365-2990.1999.00202.x. [DOI] [PubMed] [Google Scholar]
- 39.West MJ. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2012. Basic Stereology for Biologists and Neuroscientists. [Google Scholar]
- 40.Luna G. 3rd ed. New York, NY: McGraw-Hill Publications; 1968. AFIP Manual of Histologic Staining Techniques. [Google Scholar]
- 41.van Domburg PH, ten Donkelaar HJ. The human substantia nigra and ventral tegmental area. A neuroanatomical study with notes on aging and aging diseases. Adv Anat Embryol Cell Biol. 1991;121:1–132. [PubMed] [Google Scholar]
- 42.Gundersen HJ, Bagger P, Bendtsen TF, et al. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- 43.King MA, Scotty N, Klein RL, Meyer EM. Particle detection, number estimation, and feature measurement in gene transfer studies: optical fractionator stereology integrated with digital image processing and analysis. Methods. 2002;28:293–299. doi: 10.1016/s1046-2023(02)00235-9. [DOI] [PubMed] [Google Scholar]
- 44.Yuen P, Baxter DW. The morphology of Marinesco bodies (paranucleolar corpuscles) in the melanin pigmented nuclei of the brain-stem. J Neurol Neurosurg Psychiatry. 1963;26:178–183. doi: 10.1136/jnnp.26.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pakkenberg B, Møller A, Gundersen HJ, Mouritzen Dam A, Pakkenberg H. The absolute number of nerve cells in substantia nigra in normal subjects and in patients with Parkinson's disease estimated with an unbiased stereological method. J Neurol Neurosurg Psychiatry. 1991;54:30–33. doi: 10.1136/jnnp.54.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma SY, Collan Y, Röyttä M, Rinne JO, Rinne UK. Cell counts in the substantia nigra: a comparison of single section counts and disector counts in patients with Parkinson's disease and in controls. Neuropathol Appl Neurobiol. 1995;21:10–17. doi: 10.1111/j.1365-2990.1995.tb01023.x. [DOI] [PubMed] [Google Scholar]
- 47.Liang CC, Tanabe LM, Jou S, Chi F, Dauer WT. TorsinA hypofunction causes abnormal twisting movements and sensorimotor circuit neurodegeneration. J Clin Invest. 2014;12:3080–3092. doi: 10.1172/JCI72830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peterson DA, Sejnowski TJ, Poizner H. Convergent evidence for abnormal striatal synaptic plasticity in dystonia. Neurobiol Dis. 2010;37:558–573. doi: 10.1016/j.nbd.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lerner RP, Niethammer M, Eidelberg D. Understanding the anatomy of dystonia: determinants of penetrance and phenotype. Curr Neurol Neurosci Rep. 2013;13:401. doi: 10.1007/s11910-013-0401-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hallett M. Neurophysiology of dystonia: The role of inhibition. Neurobiol Dis. 2011;42:177–184. doi: 10.1016/j.nbd.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eskow Jaunarajs KL, Bonsi P, Chesselet MF, Standaert DG, Pisani A. Striatal cholinergic dysfunction as a unifying theme in the pathophysiology of dystonia. Prog Neurobiol. 2015 doi: 10.1016/j.pneurobio.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stamelou M, Edwards MJ, Hallett M, Bhatia KP. The non-motor syndrome of primary dystonia: clinical and pathophysiological implications. Brain. 2012;135:1668–1681. doi: 10.1093/brain/awr224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lehéricy S, Tijssen MA, Vidailhet M, Kaji R, Meunier S. The anatomical basis of dystonia: current view using neuroimaging. Mov Disord. 2013;28:944–957. doi: 10.1002/mds.25527. [DOI] [PubMed] [Google Scholar]
- 54.Niethammer M, Carbon M, Argyelan M, Eidelberg D. Hereditary dystonia as a neurodevelopmental circuit disorder: evidence from neuroimaging. Neurobiol Dis. 2011;42:202–209. doi: 10.1016/j.nbd.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simonyan K, Berman BD, Herscovitch P, Hallett M. Abnormal striatal dopaminergic neurotransmission during rest and task production in spasmodic dysphonia. J Neurosci. 2013;33:14705–14714. doi: 10.1523/JNEUROSCI.0407-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Herzfeld T, Nolte D, Grznarova M, Hofmann A, Schultze JL, Müller U. X-linked dystonia parkinsonism syndrome (XDP, lubag): disease-specific sequence change DSC3 in TAF1/DYT3 affects genes in vesicular transport and dopamine metabolism. Hum Mol Genet. 2013;22:941–951. doi: 10.1093/hmg/dds499. [DOI] [PubMed] [Google Scholar]
- 57.Vo A, Sako W, Niethammer M, Carbon M, Bressman SB, Uluğ AM, Eidelberg D. Thalamocortical connectivity correlates with phenotypic variability in dystonia. Cereb Cortex. 2014 May 23; doi: 10.1093/cercor/bhu104. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Edwards MJ, Huang YZ, Mir P, Rothwell JC, Bhatia KP. Abnormalities in motor cortical plasticity differentiate manifesting and nonmanifesting DYT1 carriers. Mov Disord. 2006;21:2181–2186. doi: 10.1002/mds.21160. [DOI] [PubMed] [Google Scholar]
- 59.Thompson VB, Jinnah HA, Hess EJ. Convergent mechanisms in etiologically-diverse dystonias. Expert Opin Ther Targets. 2011;15:1387–1403. doi: 10.1517/14728222.2011.641533. [DOI] [PMC free article] [PubMed] [Google Scholar]