Abstract
Acrocomia aculeata, popularly known as “bocaiuva,” is widely acknowledged in culinary and traditional medicines to treat cardiovascular diseases, a combined effect with diuretics that are also used for hypertension. However, there are no scientific data published to support its use as functional food and its ethnopharmacological use. This study intended to determine the composition of fatty acids of the pulp oil and evaluate the diuretic action and anti-inflammatory activity of the in natura and microencapsulated oil orally administrated on rats. The obtained results confirm the prevalence of monounsaturated fatty acids (68.51%), especially oleic acid (65.68%±1.05%), in the oil from the bocaiuva pulp. The in natura A. aculeata oil has diuretic (P<.01) and anti-inflammatory potential, which promoted a marked inhibition on the hind paw edema induced by carrageenan (67%±7% after 2 h) (P<.01). In addition, results show that the oral administration of the bocaiuva oil at 300 (P<.05) and 700 (P<.05) mg/kg doses significantly inhibited the leukocyte migration induced by carrageenan to the pleural cavity in rats. The inhibitions equaled 91%±3% and 81%±16%, respectively. The microencapsulated oil also showed antiedematogenic (P<.01) as well as diuretic activities (P<.01). The microencapsulation by complex coacervation was shown to be a technique that favors the bioavailability and preservation of bioactive components of the bocaiuva oil.
Key Words: : bioactivity, fatty acids, microcapsule
Introduction
Acrocomia aculeata (Jacq.) Lodd. is popularly known as bocaiuva, macaúba, coco-babão, bacaúva, mocajuba, and macaíba, and it is found in tropical regions, and it grows abundantly in the Brazilian Savana.1–3 Studies on its nutritional composition show that bocaiuva is rich in lipids, carbohydrates, and fibers.4,5 In addition, it contributes as a good source of carotenoids, among which β-carotene is highlighted.5
In popular medicine, the roots of A. aculeata have been used for a long time by indigenous people in the states of Yucatán and Tamaulipas and in Mexico for treatment of diabetes.6 The decoction of the A. aculeata leafs is used to treat hypertension.7 However, there are no scientific data published to support their use as functional food and for pharmacological use.
Naturally occurring chemical substances derived from plants have been used to treat human diseases and, historically, plant derivatives have been invaluable as a source of nutraceutical, supplement, and therapeutic agents. The study of oil from bocaiuva fruits makes possible the scientific knowledge of the pharmacological properties of the plant part unexplored. In addition to this, there is wide acceptance of bocaiuva among the population of the Cerrado.8 Together with this idea, recent technological advances, such as microencapsulated technique, help to address these issues and renewed interest in natural products in drug discovery.
Having that in mind, the objective of this article was to evaluate the diuretic and anti-inflammatory action of the oil from the pulp of the fruit from the A. aculeata palm both in natura and microencapsulated in rats, as well as its chemical components. Considering that several of the bioactive compounds are susceptible to degradation and, consequently, to the loss of interesting properties and biological activities, the microencapsulation technique may favor the preservation of these compounds and facilitate their application in food, pharmacological, cosmetic, and other products.
Materials and Methods
Plant material
The ripe fruits of A. aculeata (Jacq.) Lodd. were collected at Foundation MS in the district of Maracaju–Mato Grosso do Sul—latitude 21°36'52'' and longitude 55°10'06'', 384 m of altitude—during the period from December 2011 to January 2012, and they were transported to the Laboratory of Food Technology from the Federal University of Grande Dourados (UFGD), in Dourados-MS. The fruits were selected to obtain a uniform batch, and they were washed and sanitized with a sodium dichloroisocyanurate, dehydrate solution 0.66% (active chlorine content 3%). Then, the fruits were peeled and pulped. The pulp was stored at −5°C up to the time when it was used.
Oil extraction and gas chromatography–mass spectrometry analysis
The pulp was previously dehydrated in dryer (NG Científica) with airflow of 0.5 m/s at 40°C for 72 h. The oil extract of A. aculeata from the dried pulp was made by cold pressing on an expeller-type press model MPE-40P (Ecirtec) with a yield of 15.30%±0.19% per 100 g of dry pulp. After the extraction, the oils were centrifuged at 1308×g for 15 min to remove the residues, and they were transferred to an amber glass container and stored under refrigeration (7°C) up to the time they were used. The characterization of the fatty acids in the oil was conducted at Food Technology Institute (Campinas, ITAL). Transmethylation was conducted according to the method by Hartman and Lago,9 using an ammonium chloride solution and sulfuric acid in methanol as the esterifying agent. The treated samples were analyzed on a gas chromatographer (HP-6890), equipped with an automated sampler (HP-7683); split injector, 75:1 ratio; capillary column CP-SIL 88 (100 m×0.25 mm i.d., 0.20 mm of film); and flame ionization detector (FID). The chromatographic conditions were as follows: initial temperature at 120°C/2 min, heating at 120–220°C on a scale of 2.2°C/min, and 220–235°C; carrier gas, hydrogen (flow of 1 mL/min); make-up gas, nitrogen at 30 mL/min; injector's temperature, 270°C; detector's temperature, 310°C; and injection volume, 1 mL. The identification of the fatty acids was conducted by comparing the retention time of the fatty acids from the sample and the standards. The quantification was conducted by area normalization, and the results were expressed in g/100 g of the sample.
Animals
The experiments were conducted using male rats from the Wistar lineage (200–300 g) that were housed under 12 h light–dark cycle, controlled humidity (60–80%), and temperature (22°C±1°C) conditions. Food and water were freely available to the rats. The animals were acclimatized to the experimentation room for at least 2 h before testing and were used only once throughout the experiments. The studies reported in this article were carried out in accordance with the current guidelines for the care of laboratory animals and following the guidelines set by the U.S. National Institutes of Health10 and were approved by the ethics committee for research on laboratory animal use of the institution (Nbr. 016/2012).
Microcapsules of the A. aculeata oil
After preliminary tests and through other studies described in the literature, the microcapsules were produced using the complex coacervation technique according to the method described by Alvim and Grosso,11 employing the full-factorial design. The formulations (n= 3) that showed better results in terms of process, encapsulation efficiency of the oil, and smaller size were used for the in vivo tests: microcapsule 1 with 5 g of oil (M1), microcapsule 2 with 7.5 g of oil (M2), and microcapsule 3 with 10 g of oil (M3).
Acute diuretic activity
The diuretic activity was previously determined,12 with small changes. Male rats were divided into five groups with five animals each, in laboratory cages, for the acute effects (single dose) of the study. The rats fast during the night with free access to water. Before the treatment, all the animals received an oral dose of 5 mL/100 g of body mass of sterile saline (NaCl 0.9%) to impose a uniform load of water and salt.13 Subsequently, the control group received a tween solution, another group received furosemide (Sanofi-Aventis; 10 mg/kg), and the three last groups of rats were orally administered 100, 300, or 700 mg/kg of the A. aculeata oil in natura and microencapsulated. Immediately after the administration, the rats were placed in metabolic cages. The urine was collected and measured after 1, 2, 4, 6, and 8 h. The cumulative urinary excretion was calculated in relation to the body mass and expressed in g/100 mL.
Pleurisy
Different groups of rats were orally treated (1 h before pleurisy) with doses of 100, 300, or 700 mg/kg with in natura oil and vehicle (sterile 0.9% saline+tween). In the positive control group, dexamethasone was administered (Sigma, St. Louis, MO, USA) at a dose of 1 mg/kg subcutaneously (60 min before pleurisy). Pleurisy was induced by injecting 0.25 mL of a carrageenan suspension (200 μg) in the intrapleural cavity.14 A group named naive was treated only with the sterile 0.9% saline and was not given intrapleural carrageenan (Cg). Cg was diluted in phosphate-buffered saline solution (pH 7.4) and injected after 1 h of the treatments with oil and dexamethasone. Four hours after pleurisy was induced, the animals underwent euthanasia using ketamine and xylazine, and after anesthesia with decapitation by guillotine, the inflammatory exudates were collected. The volume of exudates was measured, and a 50 μL portion was diluted in Turk's solution (1:20 v/v) and used to determine the total number of leucocytes in a Neubauer chamber. In addition, the dosage of total proteins was conducted on the exudates using Bradford's methods.15
Paw edema
Different groups of rats from the Wistar lineage were treated orally with the in natura oil and the microencapsulated oil at a dose of 300 mg/kg and with vehicle and dexamethasone (Dex, 1 mg/kg) 1 h before the edema. After 1 h, the animals were given an intraplantar injection on the right paw (i.pl) with 100 μL of sterile saline (vehicle) containing carrageenan (Cg, 300 μg). The left paw was given the same volume of the vehicle. After 30, 60, 120, and 240 min, after the injection of Cg, both paws were measured with the help of a digital micrometer (Digimess), and the edema was measured by the difference between both paws.
Statistical analysis
The data were presented as the mean±standard error of the mean (SEM). The statistical significance between the groups was assessed by one-way analysis of variance (ANOVA) followed by a post hoc Newman-Keuls test. Statistical significance was set at P<.05; values<.01 were regarded as significant and P values <.001 as extremely significant. All tests were carried out using GraphPad Software 6.0 (San Diego, CA, USA).
Results and Discussion
Characterization of the oil
The fatty acids found in the oil from the pulp of bocaiuva are shown in Table 1. The oil from the pulp of bocaiuva was constituted by saturated fatty acids (25.01%) and unsaturated fatty acids (74.99%), from which 68.51% were monounsaturated and 6.48% polyunsaturated.
Table 1.
Composition of the Fatty Acids of the Oil from the Pulp of Acrocomia aculeata (Jacq.) Lodd
| Fatty acids (%) | Oil extracted from the fruit pulp by pressing |
|---|---|
| Caproic acid (C 6:0) | 0.13±0.01 |
| Caprylic acid (C 8:0) | 0.14±0.00 |
| Capric acid (C 10:0) | 0.10±0.01 |
| Lauric acid (C 12:0) | 0.59±0.00 |
| Myristic acid (C 14:0) | 0.39±0.01 |
| Pentadecanoic acid (C 15:0) | 0.03±0.00 |
| Palmitic acid (C 16:0) | 20.11±0.26 |
| Palmitoleic acid (C 16:1) | 2.56±0.01 |
| Margaric acid (C 17:0) | 0.08±0.01 |
| Heptadecenoic acid (C 17:1) | 0.11±0.01 |
| Stearic acid (C 18:0) | 3.09±0.34 |
| Oleic acid (C 18:1) | 65.68±1.05 |
| Linoleic acid (C 18:2) | 5.46±0.35 |
| Alpha-linolenic acid (C 18:3) | 1.02±0.07 |
| Arachidic acid (C 20:0) | 0.21±0.00 |
| Gadoleic acid (C 20:1) | 0.16±0.01 |
| Behenic acid (C 22:0) | 0.06±0.01 |
| Lignoceric acid (C 24:0) | 0.08±0.02 |
| Saturated fatty acids (%) | 25.01 |
| Monounsaturated fatty acids (%) | 68.51 |
| Polyunsaturated fatty acids (%) | 6.48 |
The results represent the mean±standard deviation of the analysis performed in triplicate.
The unsaturated fatty acids perform important functions, such as the maintenance of the immune system in inflammatory processes,16,17 and they have an antimicrobial action.18 The values found in the bocaiuva oil suggest an effective action for such pathologies.
In Table 1, it can be observed that oleic acid, palmitic acid, and linoleic acid prevail. The prevailing polyunsaturated fatty acids in the pulp were the linoleic acid (5.46%) and the linolenic acid (1.02%). These data corroborated with study performed by Hiane et al.,19 with bocaiuva, and by Coimbra and Jorge.20 In both studies, the fruit pulp was rich in monounsaturated fatty acids. Medicinal properties have been attributed to the Cocos nuciferas (Arecaceae) oil, such as antibacterial, antifungal, antiviral, antiparasitic, antioxidant, hypoglycemic, immunostimulant, and hepatoprotective attributes, correlated to fatty acids.18
Fatty acids have generated great interest due to their involvement in human health. For example, oleic acid has been associated with a reduction in blood pressure.21 The docosahexaenoic acid and eicosapentaenoic acid have also been associated with the prevention of cardiovascular diseases and cancer,22 while gamma-linolenic acid is known to possess anti-inflammatory properties.23
Based in this context and that A. aculeata oil is rich in fatty acid, specially oleic acid, maybe this oil could be useful for the treatment of inflammatory diseases. The fatty acids can also modify the repair of tissues,24 changing the balance of metaloproteinases, which are key molecules in the process of healing and tissue remodeling, and may influence the inflammation, lymphocyte function, and cell-mediated immunity.25 The unsaturated fatty acid omega-3 can affect the expression of proinflammatory cytokine genes, changing the fluidity of cell membrane, cell signaling, cell mobility, the interaction of receptors with its agonist, the function of the membrane, and formation of secondary signals.24
Diuretic and anti-inflammatory activities of the microencapsulated and in natura oil
The traditional use of medicinal plants, such as bocaiuva, to treat kidney, cardiovascular, and inflammatory diseases in Brazil is usually common as an alternative in popular medicine. However, plants are generally used without taking into consideration the pharmacological aspects,26 therefore, and experimental validation is necessary.
A diuretic is a substance that increases the urinary excretion rate.27 Several diuretics that are clinically used act by reducing the sodium reabsorption rate of the tubules, which cause natriuresis (increase in the sodium excretion), which in turn leads to diuresis (increase in the water excretion).28 The most common clinical use of diuretics is to reduce the volume of the extracellular fluid, especially in diseases associated to edema and hypertension.29
As previously mentioned, bocaiuva has been popularly used to treat cardiovascular diseases.8 As far as we know, only our research group has been trying to support its ethnopharmacological use, and in the literature, only one publication of this kind has been found (Acrocomia mexicana), showing the antidiabetic properties.30
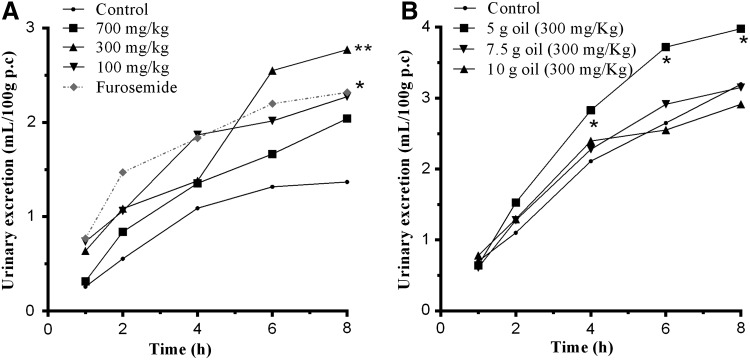
The bocaiuva oil (300 or 700 mg/kg) caused a significant increase in the urinary excretion of liquids (Fig. 1A). Similarly, an increase in the excretion of liquids was observed with the furosemide drug, which has a diuretic and natriuretic effect.31 The diuretic effects shown by the bocaiuva oil could be explained by the presence of bioactive compounds (carotenoids, tocoferol, vitamin C, and others).8,32
FIG. 1.
(A) The time and urinary excretion variation in rats treated with different doses of bocaiuva oil. (B) Time variation for urinary excretion in rats treated with different microcapsules—M1, M2, and M3 (300 mg/kg)—of bocaiuva oil. Data were expressed as mean±SEM. The asterisks denote the statistical significance levels between groups from control group followed. One-way ANOVA followed by Newman-Keuls test: *P<.05 and **P<.01.
Furosemide is a loop diuretic, and as other diuretics, it induces several effects, such as metabolic alkalosis, ototoxicity, hyperuricemia, hypomagnesemia, allergic reactions, and others.33 In that sense, the research for new molecules with less or no activity was shown, particularly in medicinal plants, because they produce less-adverse effects.34
The microencapsulated bocaiuva oil at a dose of 300 mg/kg caused a significant increase in the urinary excretion of liquids (Fig. 1B). The treatment with microcapsule 1 (M1) from the bocaiuva oil also significantly increased the urinary excretion when compared with the control group.
Inflammatory cell recruitment is important to maintain several inflammatory and noninflammatory diseases. Pharmacological therapies to treat inflammatory diseases focus on reducing the productive phase of the inflammatory response, including inhibition of leukocyte influx.35 In the first carrageenan-induced pleurisy test, doses of 100, 300, and 700 mg/kg were orally tested in rats. This test allows the evaluation of two important parameters in the inflammatory process: the migration of leukocytes and the protein extravasation. The carrageenan injection induced an acute inflammatory reaction that was verified in comparison with the control group (which was given the carrageenan injection in the pleural cavity) and naive (which was given saline in the pleural cavity). The results showed that the oil was efficient to inhibit the migration of leukocytes and plasmatic extravasation. Through a timing that involves changes in leukocyte responsiveness, chemokine and cytokine signalization, and adhesion molecule interaction, the leukocytes can migrate from blood vessel to the affected site36–39; then, the proposition of mechanism of bocaiuva oil that reduces the leukocyte migration is still unclear, only the efficacy could be evaluated in this work. The perspective of work with bocaiuva oil now describes some evidence of mechanism of action.
Commercially available anti-inflammatory drugs can inhibit leukocyte migration, like steroidal anti-inflammatory drugs; therefore, the selective COX-2 inhibitors celecoxib and rofecoxib, both by oral route at 10 mg/kg dosage, had no significant effect over inflammatory cell influx induced by carrageenan in pleurisy model.40 The dexamethasone was inserted as a positive control to verify whether the product tested exhibited the ability of induce decrease in leukocyte recruitment and protein extravazation. As expected, bocaiuva oil reduces both anti-inflammatory aspects, showing not only antiedematogenic effect but also leukocyte and protein extravazation.
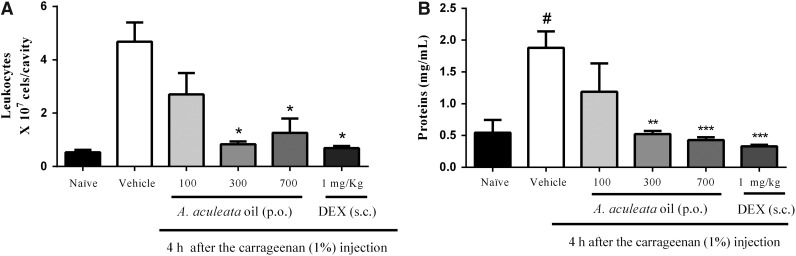
In Figure 2A, it can be verified that there was a significant increase of ∼8 times in the migration of leukocytes in the control group in relation to the naive group. In addition, results show that the oral administration of the bocaiuva oil at 300 or 700 mg/kg dose significantly inhibited the leukocyte migration induced by carrageenan to the pleural cavity in rats. The inhibitions equaled 91%±3% and 81%±16%, respectively. Administering the dexamethasone anti-inflammatory showed a 96%±2% inhibition (Fig. 2A).
FIG. 2.
Effect of the bocaiuva oil on the leukocyte migration (A) and plasmatic extravasation (B) induced by carrageenan (Cg) in the pleurisy model in rats. Rats were treated orally with bocaiuva oil, dexamethasone (s.c.), or vehicle. Data were expressed as mean±SEM. The asterisks denote the statistical significance levels between groups from control group while # indicates the differences of naive group from control group followed. One-way ANOVA followed by Newman-Keuls test: *P<.05, **P<.01, and ***P<.001.
In relation to the plasmatic extravasation, both doses, 300 and 700 mg/kg, were statistically significant to induce the reduction in the plasmatic extravasation in relation to the control group. The observed inhibitions were 98%±2% and 100%, respectively. As expected, in the group of animals treated with dexamethasone, it inhibited 100% of the pleural plasmatic extravasation induced by carrageenan (Fig. 2B).
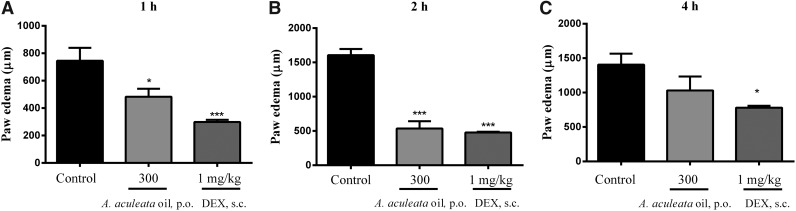
The treatment with the in natura bocaiuva oil inhibited the formation of the edema at 300 mg/kg dose, with inhibitions of 35%±8% after 1 h and 67%±7% after 2 h of the intraplantar injection of carrageenan, but after 4 h, there was no edema induction (Fig. 3).
FIG. 3.
Effect of the bocaiuva oil on the paw edema after 1 h (A), 2 h (B), and 4 h (C) of the intraplantar carrageenan injection. Animals received oil (300 mg/kg) by the oral route (p.o.) or control, or dexamethasone (DEX) 1 h before the intraplantar injection of Cg (300 μg/paw). Each column was expressed as mean±SEM. The asterisks denote the statistical significance levels between groups from control group followed. One-way ANOVA followed by Newman-Keuls test: *P<.05 and ***P<.001.
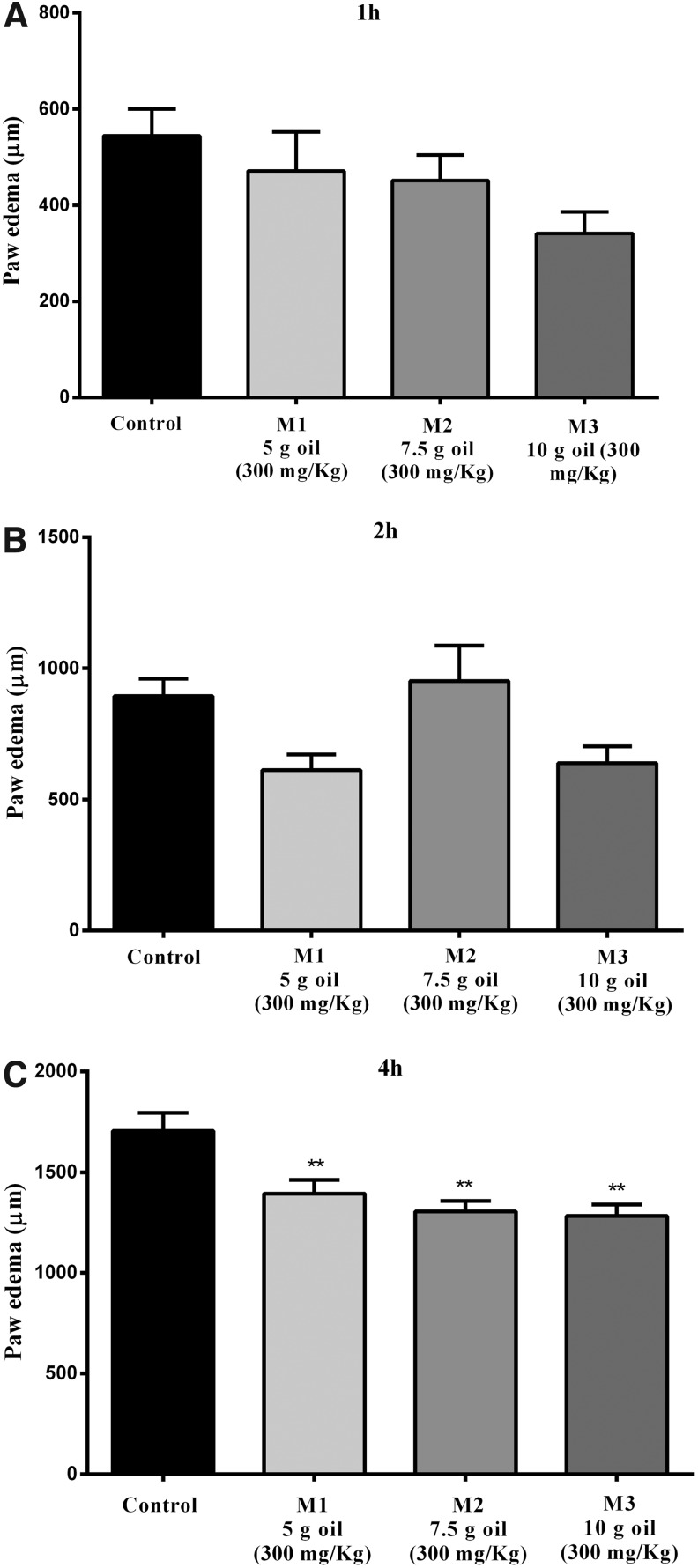
On the other hand, the treatment with microcapsules M1, M2, and M3 of the bocaiuva oil significantly inhibited the edema formation at a dose of 300 mg/kg, with inhibitions of 18%±4% (M1), 23%±3% (M2), and 25%±3% (M3), after 4 h of the intraplantar carrageenan injection, but not in other measuring points of the edema (Fig. 4).
FIG. 4.
Effect of microcapsules of the bocaiuva oil on the paw edema after 1 h (A), 2 h (B), and 4 h (C) of the intraplantar injection of carrageenan. The animals were treated with the microencapsulated oil at a 300 mg/kg oral route (p.o.) or vehicle 1 h before the intraplantar injection of carrageenan (300 μg/paw). Each column was expressed as mean±SEM. The asterisks denote the statistical significance levels between groups from control group followed. One-way ANOVA followed by Newman-Keuls test: *P<.05 and **P<.01.
This shows that the formulation of the microcapsule kept the stability of the active ingredient and showed the antiedematogenic action. The active ingredient was probably released more slowly, because only after 4 h of the carrageenan injection the activity could be verified.
Dexamethasone, a reference drug used as positive control, inhibited the formation of the edema in 62%±1% after 1 h and 70%±1% after 2 h of the intraplantar injection of carrageenan (Fig. 4).
Summary
The anti-inflammatory and diuretic actions of the bocaiuva oil were verified on paw and pleural edema induced by carrageenan, probably due to the presence of bioactive compounds present in oil as fatty acids that interfere with inflammatory parameters. The microencapsulated oil maintained the stability of the active ingredient and showed antiedematogenic action, as well as diuretic activity. In this context, this study provides information about A. aculeata as a source of bioactive compounds in preventing diseases from inflammatory processes and strengthens the development of new products based on natural products. Further investigations are needed to identify the active components that possess such biological activities.
Acknowledgments
The authors thank the Brazilian Federal Agency for Support and Evaluation of Graduate Education CAPES for the financial help. The authors also thank Espaço da Escrita–Coordenadoria Geral da Universidade–UNICAMP for the language services provided.
Author Disclosure Statement
The authors declare that there is no conflict of interest.
References
- 1.Hiane PA, Baldasso PA, Marangoni S, Macedo MLR: Chemical and nutritional evaluation of kernels of bocaiuva, Acrocomia aculeata (Jacq.) Lodd. Ciênc Tecnol Aliment 2006;26:683–689 [Google Scholar]
- 2.Chuba CAM, Sanjinez-Argandoña EJ: Caracterização biométrica, física e química de frutos da palmeira bocaiuva Acrocomia aculeata (Jacq) Lodd. Rev Bras Frutic 2011;33:1023–1028 [Google Scholar]
- 3.Dessimoni-Pinto NAV, Silva VM, Batista AG, Vieira G, Souza CR, Dumont PV, Santos GKM: Características físico-químicas da amêndoa de macaúba e seu aproveitamento na elaboração de barras de cereais. Alim Nutr 2010;21:77–84 [Google Scholar]
- 4.Bora PS, Rocha RVM: Macaiba palm: fatty and amino acids composition of fruits. Ciênc Tecnol Aliment 2004;4:158–162 [Google Scholar]
- 5.Ramos MIL, Ramos-Filho MM, Hiane PA, Braga-Neto JA, Siqueira EMA: Qualidade nutricional da polpa da bocaiuva Acrocomia aculeata (Jacq.) Lodd. Ciênc Tecnol Aliment 2008;28:90–94 [Google Scholar]
- 6.Sosnowska J, Balslev H: American palms used for medicine, in the ethnobotanical and pharmacological publications. Rev Peru Biol 2008;15:143–146 [Google Scholar]
- 7.Agra MDF, Freitas PFD, Barbosa-Filho JM: Synopsis of the plants known as medicinal and poisonous in Northeast of Brazil. Rev Bras Farmacogn 2007;17:114–140 [Google Scholar]
- 8.Estevan AO, Silva MA, Arena AC, Argandoña EJS, Breda CA, Kassuya CAL: Estudo do potencial antiinflamatório dos extratos de Acrocomia aculeata no processo inflamatório agudo e crônico em modelos experimentais. 10 Simpósio Brasil Japão-Campo Grande, 2010:1–6 [Google Scholar]
- 9.Hartman L, Lago RCA: Rapid preparation of fatty acids methyl esters. Lab Pract 1973;22:475–476 [PubMed] [Google Scholar]
- 10.NIH: Guide for the Care and Use of Laboratory Animals. Public Health Service, National Institutes of Health, NIH Publ. No. 86–23, Revised 1985. Bethesda, MD [Google Scholar]
- 11.Alvim ID, Grosso CRF: Microparticles obtained by complex coacervation: Influence of the type of reticulation and the drying process on the release of the core material. Ciênc Tecnol Aliment 2005;30:1069–1076 [Google Scholar]
- 12.Kau ST, Keddie JR, Andrews D: A method for screening diuretic agents in the rat. J Pharmacol Methods 1984;11:67–75 [DOI] [PubMed] [Google Scholar]
- 13.Benjumea D, Abdala S, Hernandez-Luis F, Pérez-Paz P, Martin-Herrera D: Diuretic activity of Artemisia thuscula, an endemic Canary species. J Ethnopharmacol 2005;100:205–209 [DOI] [PubMed] [Google Scholar]
- 14.Kassuya CA, Cremoneze A, Barros LF, et al. : Antipyretic and anti-inflammatory properties of the ethanolic extract, dichloromethane fraction and costunolide from Magnolia ovata (Magnoliaceae). J Ethnopharmacol 2009;124:369–376 [DOI] [PubMed] [Google Scholar]
- 15.Bradford MM: Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–254 [DOI] [PubMed] [Google Scholar]
- 16.Menendéz R, Carvajal D, Mas R, et al. : Efectos del D-004, extracto lipídico de los frutos de la palma Real (Roystonea regia), sobre El granuloma inducido por algodón en ratas y sobre La lipoxigenasa presente en leucocitos polimorfonucleares (PMNs). Acta Farm Bonaer 2006;25:213–218 [Google Scholar]
- 17.Weatherill AR, Lee JY, Zhao L, Lemay DG, Youn HS, Hwang DH: Saturated and polyunsaturated fatty acids reciprocally modulate dendritic cell functions mediated through TLR4. J Immunol 2005;174:5390–5397 [DOI] [PubMed] [Google Scholar]
- 18.Debmandal M, Mandal S: Coconut (Cocos nucifera L.: Arecaceae): In health promotion and disease prevention. Asian Pac J Trop Med 2011;4:241–247 [DOI] [PubMed] [Google Scholar]
- 19.Hiane PA, Ramos MMF, Ramos MIL, Macedo MLR: Bocaiúva, Acrocomia Aculeata (Jacq.) Lodd., pulp and kernel oils: characterization and fatty acid composition. Braz J Food Technol 2005;3:56–59 [Google Scholar]
- 20.Coimbra MC, Jorge N: Characterization of the pulp and kernel oils from Syagrus oleracea, Syagrus romanzoffiana, and Acrocomia aculeata. J Food Sci 2011;76:1156–1161 [DOI] [PubMed] [Google Scholar]
- 21.Teres S, Barceló-Coblijn G, Benet M, Álvarez R, Bressani R, Halver JE, Escribá PV: Oleic acid content is responsible for the reduction in blood pressure induced by olive oil. PNAS 2008;105:13811–13816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huertas EL: Health effects of oleic acid and long chain omega-3 fatty acids (EPA and DHA) enriched milks. A review of intervention studies. Pharmacol Res 2010;61:200–207 [DOI] [PubMed] [Google Scholar]
- 23.Kapoor R, Huang YS: Gamma linolenic acid: an antiinflammatory omega-6 fatty acid. Curr Pharm Biotechnol 2006;7:531–534 [DOI] [PubMed] [Google Scholar]
- 24.Grimm H, Mayer K, Mayser P, Eigenbrodt E: Regulatory potential of n-3 fatty acids in immunological and inflammatory processes. Br J Nutr 2002;87:59–67 [DOI] [PubMed] [Google Scholar]
- 25.Calder PC, Yaqoob P, Thies F, Wallace FA, Miles EA: Fatty acids and lymphocyte functions. Br J Nutr 2002;87:31–48 [DOI] [PubMed] [Google Scholar]
- 26.Bouanani S, Henchiri C, Migianu-Griffoni E, Aouf N, Lecouvey M: Pharmacological and toxicological effects of Paronychia argentea in experimental calcium oxalate nephrolithiasis in rats. J Ethnopharmacol 2010;129:38–45 [DOI] [PubMed] [Google Scholar]
- 27.Rose BD: Diuretics. Kidney Int 1991;39:336–352 [DOI] [PubMed] [Google Scholar]
- 28.Carter BL: Guidelines for use of diuretics: a view from a member of JNC 7. J Clin Hypertens 2012;14:273–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frazier HS, Yager H: The Clinical use of diuretics. New Engl J Med 1973;288:455–457 [DOI] [PubMed] [Google Scholar]
- 30.Perez S, Perez RM, Perez C, Zavala MA, Vargas R: Coyolosa, a new hypoglycemic from Acrocomia mexicana. Pharm Acta Helv 1997;72:105–111 [DOI] [PubMed] [Google Scholar]
- 31.Leuschner J: Anti-inflammatory, spasmolytic and diuretic effects of a commercially available Solidago gigantea Herb. Extract. Arzneimittelforschung Drug Res 1995;45:165–168 [PubMed] [Google Scholar]
- 32.Coimbra MC, Jorge N: Proximate composition of guariroba (Syagrus oleracea), jerivá (Syagrus romanzoffiana) and macaúba (Acrocomia aculeata) palm fruits. Food Res In 2011;44:2139–2142 [Google Scholar]
- 33.Jackson EK: Diuréticos. In: As Bases Farmacológicas das Terapêuticas, (Hardman JG, Molinoff PB, Goodman GA, eds.). McGraw-Hill, New York, 1996, pp. 502–522 [Google Scholar]
- 34.Wright SJ, Stoner KE, Beckman N, et al. : The plight of large animals in tropical forests and the consequences for plant regeneration. Biotrop 2007;39:289–291 [Google Scholar]
- 35.Sousa LP, Alessandri AL, Pinho V, Teixeira MM: Pharmacological strategies to resolve acute inflammation. Curr Opin Pharmacol 2013;13:625–631 [DOI] [PubMed] [Google Scholar]
- 36.Springer TA: Traffic signals for lymphocyte recirculation and leukocyte emigration: the multi step paradigm. Cell 1994;76:301–314 [DOI] [PubMed] [Google Scholar]
- 37.Brown EJ: Adhesive interactions in the immune system. Trends Cell Biol 1997;7:289–295 [DOI] [PubMed] [Google Scholar]
- 38.Worthylake RA, Burridge K: Leukocyte transendothelial migration: orchestrating the underlying molecular machinery. Curr Opin Cell Biol 2001;13:569–577 [DOI] [PubMed] [Google Scholar]
- 39.Johnson-Léger C, Aurrand-Lions M, Imhof BA: The parting of the endothelium: miracle, or simply a junctional affair? J Cell Sci 2000;113:921–933 [DOI] [PubMed] [Google Scholar]
- 40.Pinheiro RM, Calixto JB: Effect of the selective COX-2 inhibitors, celecoxib and rofecoxib in rat acute models of inflammation. Inflamm Res 2002;51:603–610 [DOI] [PubMed] [Google Scholar]