Highlights
-
•
Lynch syndrome (LS) is an uncommon, genetic disorder which predisposes affected individuals to colorectal, endometrial and ovarian malignancies.
-
•
We report a case of cervical gastric-type adenocarcinoma in a patient with LS.
-
•
Immunohistochemistry for mismatch repair proteins is a useful screening tool in tumours suspected to be associated with LS.
Keywords: Lynch syndrome, Cervical gastric-type adenocarcinoma, MSH6
Introduction
Lynch syndrome (LS), also known as hereditary non-polyposis colorectal cancer (HNPCC), is an uncommon, but not rare, autosomal dominant familial cancer syndrome (Barrow et al., 2013). This condition predisposes individuals to colorectal carcinoma (CRC) as well as various extra-colonic tumours including cancer of the endometrium, ovaries, small bowel, stomach and urothelium (Barrow et al., 2013).
Tumours arise as a result of inactivating mutations in genes encoding essential proteins in the DNA mismatch repair (MMR) pathway (Barrow et al., 2013, Masuda et al., 2011). This pathway is principally involved in the repair of replication errors, such as base mismatches and small insertions or deletions, in highly repetitive base sequences known as microsatellites (Masuda et al., 2011). Accumulation of these mutations, in genes involved in carcinogenesis, predisposes cellular malignant transformation; hence individuals with LS have an increased incidence of specific tumours (Barrow et al., 2013, Masuda et al., 2011). Four human MMR genes have been implicated in this condition; MLH1, MSH2, MSH6 and PSM2 (Masuda et al., 2011). Mutations icbun MLH1 and MSH2 account for approximately 90% of all LS cases (Barrow et al., 2013).
In women with LS there is an increased risk of developing both endometrial carcinoma and ovarian carcinoma; indeed, endometrial carcinoma is seen as commonly as CRC in these individuals and is often the sentinel malignancy. In contrast, predisposition to cervical cancer is not a feature of LS (Barrow et al., 2013). There have been only occasional cases of cervical cancer reported in the literature in patients with LS; one patient with a germline-mutation in MSH2, in which the tumour was immunohistochemically deficient for MSH2 and MSH6 with high levels of MSI (Mongiat-Artus et al., 2006) and the other with the Muir–Torre variant of LS, where individuals have defects in either MSH2 or MLH1 (Mongiat-Artus et al., 2006, Nair et al., 2012). In the first case, there was no mention of the tumour morphology (Mongiat-Artus et al., 2006) while in the second the neoplasm was described as an adenocarcinoma with focal serous papillary features (Nair et al., 2012). In the second case, there was no mention of testing the tumour for MSI or undertaking immunohistochemistry for mismatch repair proteins.
In this case report, we describe a case of gastric-type cervical adenocarcinoma in a patient with LS secondary to an MSH6 mutation. We discuss the possibility that LS may be associated with rare non-HPV related cervical adenocarcinomas.
Case report
A 50 year-old female underwent a supracervical abdominal hysterectomy and bilateral salpingo-oophorectomy as a prophylactic procedure for previously diagnosed LS. The patient was asymptomatic at the time of surgery.
Eight years previously, a family history of early onset colonic cancer led to genetic testing, which subsequently revealed a germline mutation in the MSH6 gene on chromosome 2, consistent with the diagnosis of LS. The patient had no history of malignancy, with the last colonoscopy for colonic cancer surveillance undertaken 2 years ago and reported as normal. Cervical smears were up-to-date and normal with no evidence of a squamous or glandular abnormality.
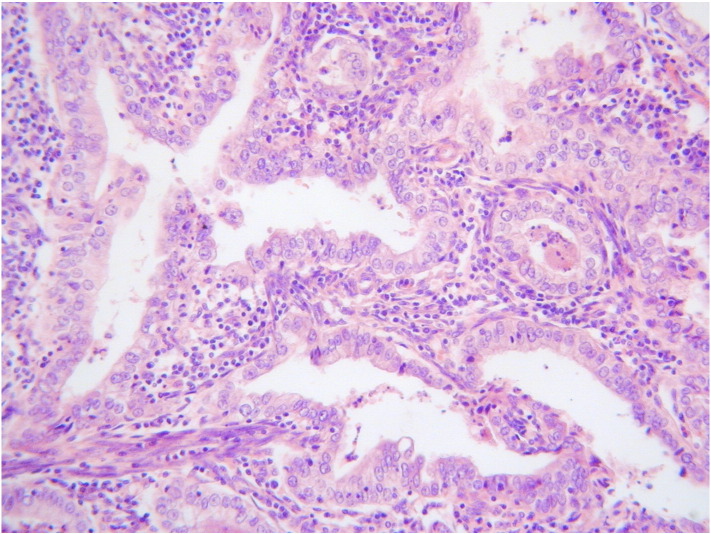
Gross pathological examination revealed no abnormality in the uterus, cervix, ovaries or fallopian tubes. The entire endometrium and cervix were examined histologically. Focal atypical endometrial hyperplasia was identified with no evidence of endometrial adenocarcinoma. The cervix was incompletely excised without representation of the ectocervix or the transformation zone. Within the endocervix, two separate foci of abnormal glandular proliferation were present. These measured 8 mm and 3 mm in their maximum horizontal dimension and both had a depth of invasion of 1.5 mm. Both areas comprised moderately differentiated adenocarcinomas composed of tumour cells with atypical nuclei, clear cytoplasm and prominent cell membranes. There was an associated brisk inflammatory infiltrate, mainly consisting of lymphocytes (Fig. 1). Immunohistochemically, both foci were largely negative for p16 and were negative for oestrogen receptor (ER), progesterone receptor (PR) and carcinoembryonic antigen (CEA). Both foci were clear of the margins and there was no lymphovascular invasion.
Fig. 1.
Adenocarcinoma composed of cells with abundant clear or eosinophilic cytoplasm and with an associated pronounced inflammatory infiltrate.
Linear array HPV genotyping (Roche Molecular Diagnostics, Pleasanton, California, USA) was performed on paraffin blocks of the areas containing the abnormal cervical glandular proliferation. Linear array HPV genotyping involves PCR amplification of target DNA followed by hybridization for the detection of 37 HPV types; 18 high risk types (16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 82) and 19 low risk types (6, 11, 40, 42, 54,55, 61, 62, 64, 67, 69, 70, 71, 72, 81, 83, 84, IS39, CP108). HPV testing was negative.
Given the morphology, immunohistochemistry and the absence of HPV, a diagnosis of primary cervical gastric-type adenocarcinoma was made. Immunohistochemistry for mismatch repair proteins was performed and this demonstrated normal nuclear staining with MLH1, PMS2 and MSH2. MSH6 was difficult to interpret due to cytoplasmic immunoreactivity but there was loss of nuclear staining with a positive internal control in the form of nuclear staining of some lymphoid and stromal cells.
The ovaries and fallopian tubes showed no histological abnormality.
Following the histological results, the residual cervix was assessed by examination under anaesthesia (EUA), cystoscopy and pelvic MRI scan. No cervical abnormality was detected on MRI and the 3 cm cervical stump was mobile on EUA, with no evidence of parametrial, bladder or rectal involvement. The tumour was provisionally staged as FIGO 1B1 (based on the histological measurements). Both surgery, in the form of a radical hysterectomy with lymphadenectomy, and chemoradiation were considered as equally effective treatment options (Landoni et al., 1997). After discussion with the patient, laparotomy with excision of the cervical stump and vaginal cuff with bilateral pelvic node dissection was undertaken along with omentectomy, given the high rate of intraabdominal dissemination described with cervical gastric-type adenocarcinoma (to be discussed later) (Kojima et al., 2007). There was no evidence of macroscopic disease at laparotomy and histological assessment of all resected specimens, including the cervical stump, parametria, vaginal cuff, omentum, infundibular pelvic ligaments, pelvic nodes and common iliac nodes, was unremarkable with no evidence of residual or metastatic adenocarcinoma.
No adjuvant therapy was recommended and routine follow was arranged with the surgical gynaecological team. She has now been in follow up for 18 months with no evidence of disease.
Discussion
We report a rare case of gastric-type adenocarcinoma of the cervix in a patient with LS secondary to a germline mutation in the MSH6 MMR gene. As this patient had coexisting endometrial atypical hyperplasia, initially it was considered whether the cervical glandular proliferation could have represented spread from the endometrium. However, a thorough histological examination of the entire endometrium was performed and no evidence of invasive endometrial carcinoma was identified. Moreover, the morphology and immunophenotype (hormone receptor negative) were not that of an endometrioid adenocarcinoma of the endometrium.
The morphological features were in keeping with a cervical gastric-type adenocarcinoma. This subtype is an uncommon variant of primary cervical adenocarcinoma which exhibits gastric differentiation (on the basis of mucin histochemistry and immunohistochemistry) and, in contrast to the majority of cervical cancers, is not associated with HPV infection. Histologically, gastric type adenocarcinoma is characterised by tumour cells with atypical nuclei and abundant clear or eosinophilic cytoplasm, typically with distinct cell borders. There is sometimes a marked associated inflammatory infiltrate, including lymphocytes, neutrophils and eosinophils. p16 is usually negative or only focally positive, in contrast to HPV associated adenocarcinomas where diffuse positive staining is seen. Although the clinical features have not been extensively studied, it is thought that cervical gastric type adenocarcinoma is associated with aggressive behaviour and a poor prognosis, including a possible propensity for peritoneal, omental and adnexal dissemination (Kojima et al., 2007). Kojima et al. found that gastric type adenocarcinomas had a 5 year disease free survival of 30% compared to 74% for usual endocervical-type adenocarcinomas (Kojima et al., 2007).
In this case, the tumour exhibited loss of nuclear expression of MSH6 thus supporting the association of this adenocarcinoma to the patient's known MSH6 germline mutation, rather than representing a sporadic tumour. This is somewhat unusual as cervical adenocarcinoma is not one of the tumours typically considered to be associated with LS. Indeed, a recent study undertaken by Mills et al. demonstrated intact MMR protein in 60 cervical adenocarcinomas (Mills et al., 2012).
The mutations in the MMR genes vary between LS patients and are often family-specific. It is plausible that certain mutations confer a susceptibility to the development of unusual tumours. Indeed, this theory has been suggested in a separate case report describing an unusual spectrum of tumours in a family of patients with LS (Yu et al., 2009). Another recent study described the occurrence of four unusual tumours in patients with LS, suggesting that the spectrum of tumours associated with this syndrome may be wider than is currently appreciated (Karamurzin et al., 2012). Germane to the current case, it is interesting that LS associated endometrial carcinomas have been reported to more frequently having a lower uterine segment (LUS) location (Westin, 2008). Given that non-HPV related cervical adenocarcinomas, including gastric-type, may arise proximal to the transformation zone and extend to involve the LUS, it is possible that some of the LUS carcinomas reported in patients with LS represent non-HPV related cervical adenocarcinomas.
Our usual practice would be to always carry out a total hysterectomy for patients with LS but this patient was initially treated at a referring hospital by a non gynaecological oncology surgeon and thus underwent a supracervical hysterectomy.
This case highlights the fact that unusual tumour types may occur in patients with LS. MMR immunohistochemistry is a useful screening tool in unusual tumours when LS is suspected and loss of immunoreactivity with 1 or more of the MMR proteins may direct further investigations to confirm or exclude LS. MMR immunohistochemistry also has the advantage of suggesting which MMR gene is likely to be mutated and thus focusing further molecular tests.
Conclusion
We describe an unusual case of a LS associated cervical gastric-type adenocarcinoma. Further study of unusual non-HPV related variants of cervical adenocarcinoma are warranted to ascertain if there is an association with LS.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- Barrow E., Hill J., Evans D.G. Cancer risk in Lynch syndrome. Familial Cancer. 2013;12(2):229–240. doi: 10.1007/s10689-013-9615-1. [DOI] [PubMed] [Google Scholar]
- Karamurzin Y. Unusual DNA mismatch repair-deficient tumors in Lynch syndrome: a report of new cases and review of the literature. Hum. Pathol. 2012;43(10):1677–1687. doi: 10.1016/j.humpath.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Kojima A. Gastric morphology and immunophenotype predict poor outcome in mucinous adenocarcinoma of the uterine cervix. Am. J. Surg. Pathol. 2007;31(5):664–672. doi: 10.1097/01.pas.0000213434.91868.b0. [DOI] [PubMed] [Google Scholar]
- Landoni F. Randomised study of radical surgery versus radiotherapy for stage Ib–IIa cervical cancer. Lancet. 1997;350(9077):535–540. doi: 10.1016/S0140-6736(97)02250-2. [DOI] [PubMed] [Google Scholar]
- Masuda K. Relationship between DNA mismatch repair deficiency and endometrial cancer. Mol. Biol. Int. 2011;2011:256063. doi: 10.4061/2011/256063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills A.M. Are women with endocervical adenocarcinoma at risk for lynch syndrome? Evaluation of 101 cases including unusual subtypes and lower uterine segment tumors. Int. J. Gynecol. Pathol. 2012;31(5):463–469. doi: 10.1097/PGP.0b013e31824a1dad. [DOI] [PubMed] [Google Scholar]
- Mongiat-Artus P. Spectrum of molecular alterations in colorectal, upper urinary tract, endocervical, and renal carcinomas arising in a patient with hereditary non-polyposis colorectal cancer. Virchows Arch. 2006;449(2):238–243. doi: 10.1007/s00428-006-0182-9. [DOI] [PubMed] [Google Scholar]
- Nair N. Cervical adenocarcinoma in a patient with Lynch syndrome, Muir–Torre variant. J. Clin. Oncol. 2012;30(2):E5–E6. doi: 10.1200/JCO.2011.36.3325. [DOI] [PubMed] [Google Scholar]
- Westin S.N.e.a. Carcinoma of the lower uterine segment: a newly described association with Lynch syndrome. J. Clin. Oncol. 2008;26(36):5965–5971. doi: 10.1200/JCO.2008.18.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu V.P. Unusual presentation of Lynch syndrome. Hered. Cancer Clin. Pract. 2009;7(1):12. doi: 10.1186/1897-4287-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]