Abstract
HIV may induce gastrointestinal (GI) mucosal immune dysregulation similar to inflammation observed in ulcerative colitis (UC). Colorectal biopsies from healthy controls (N=12) and from participants with HIV (N=20) or UC (N=9) were subjected to real time (RT)-PCR for selected cytokines, chemokines, antimicrobial peptides, Toll-like receptors, and inflammatory signaling and epithelial barrier proteins. HIV long terminal repeat relative copy number (RCN) in HIV participant biopsies was quantified by RT-PCR. Mean interleukin (IL)-6 mRNA levels did not differ significantly between HIV and UC participants (p=0.48) but were significantly higher relative to control mRNA levels only for HIV participants (p=0.03). Mean IL-8 and human defensin (HD) 5 mRNA levels were similar between HIV and UC participants (p=1.0 and p=0.35, respectively) and were significantly greater in both groups relative to controls (p<0.05 for all). Human beta-defensin (HBD)-2 mRNA levels were higher in UC relative to HIV and control participants (p<0.01 for both). Conversely, HBD-1 mRNA levels were downregulated in UC vs. HIV participants (p=0.01). Mediator gene expression did not differ significantly between HIV participants with detectable (N=10) or nondetectable (N=10) plasma viral loads. Tissue HIV relative copy number (RCN) correlated with plasma viral load (r=0.88, p<0.01) but not with mediator mRNA levels. The results of this study indicate that both chronic HIV infection and UC are associated with similar patterns of IL-6, IL- 8, and HD5 expression in colorectal biopsy tissue. These findings suggest overlapping mechanisms for GI mucosal inflammation in these two illnesses and merit further investigation in larger studies.
Introduction
HIV-induced inflammation and tissue injury in the gastrointestinal tract may impact viral pathogenesis by potentiating local HIV replication and recruitment of HIV target cells. Colorectal biopsy tissue from individuals with chronic HIV infection may not show evidence of significant cellular inflammation by qualitative histology, as concomitant CD4+ T-lymphocyte depletion and CD8+ T-lymphocyte influx may result in an overall normocellular appearance.1–3 However, prior studies have shown evidence of cellular inflammation by flow cytometry in intestinal biopsies from HIV-infected participants1 and have described upregulation of proinflammatory cytokines and chemokines in intestinal biopsies from HIV-infected individuals compared to uninfected individuals,1,4,5 with the highest levels of inflammatory cytokines observed in those with high levels of local HIV replication.5 The majority of participants in past studies lacked gastrointestinal symptoms. Use of antiretroviral therapy (ART) and average plasma viral loads varied among participants in these prior studies, thus it is difficult to evaluate the impact of these variables on mucosal inflammation. However, mucosal inflammation is abrogated by ART in some studies, suggesting a direct association between HIV replication and inflammation.5–7
Alterations of soluble immune mediators at the intestinal mucosa in the setting of HIV may overlap with those observed in patients with ulcerative colitis (UC) and Crohn's disease.1,8 One prior study identified upregulated expression of inflammatory cytokines and chemokines in intestinal biopsies from participants with HIV or inflammatory bowel disease (IBD).1 Although biopsies from HIV-infected participants in this study appeared similar to those from healthy controls by qualitative histology, gene expression for the HIV coreceptors CCR5 and CXCR4 and for the chemokines regulated on activation, normal T cell expressed and secreted (RANTES), macrophage inflammatory protein (MIP)-1β, and MIP-1α was upregulated relative to healthy controls and was similar between UC and HIV-infected participants.
Altered expression of genes integral to intestinal barrier integrity is well described in IBD9 and has also been described in studies of HIV-infected individuals,2,6,10,11 although prior studies have not directly compared intestinal barrier gene expression between these two groups. Loss of barrier integrity in the setting of IBD or HIV may promote microbial translocation and potentiate inflammation, thus exacerbating colitis and viral dissemination.12–16 Dysregulated intestinal mucosa expression of Toll-like receptors (TLR), inflammatory signaling proteins, and antimicrobial peptides such as defensins may also contribute to altered mucosal immunity in the setting of HIV or IBD.17–19 Altered gene expression of human beta-defensin (HBD)-2 and HBD-317 and multiple TLRs20 has been described in intestinal biopsies from IBD patients. Altered enteric defensin and TLR expression in the setting of HIV has been suggested based on the findings of animal studies21,22 but has not been studied in HIV-infected humans.
Few human studies have characterized alterations of soluble immune mediators at the intestinal mucosa that occur in the setting of HIV or have directly compared the changes that may occur in both HIV and IBD. Furthermore, the association between HIV replication and gastrointestinal antimicrobial peptides has not been well characterized in human models. A more thorough investigation of the similarities between IBD and HIV intestinal pathogenesis could expand our understanding of how mucosal inflammation in the setting of HIV contributes to systemic inflammation and disease progression and could inform the development of novel antiinflammatory therapies for both IBD and HIV.
To address these gaps, we used real time (RT)-PCR to quantify mRNA for a select panel of mucosal mediators in colorectal biopsies collected from participants with HIV infection, from participants with active UC disease (“positive inflammatory” control group) and from healthy participants (“negative inflammatory” control group). Mediators assessed by PCR were selected for their potential role in inhibiting or promoting local inflammation or for their relevance to intestinal barrier integrity. Biopsy samples from HIV-infected participants were also subjected to PCR for HIV long terminal repeat (LTR) gene expression to evaluate the relationship between local viral replication and inflammation. We hypothesized that chronic HIV infection is associated with intestinal mucosal inflammation similar to that described in UC and that we would observe similar levels of inflammatory gene expression when comparing biopsies from HIV-infected participants to biopsies from UC participants.
Materials and Methods
Participants
This study was approved by the UCLA Office of the Human Research Protection Program Institutional Review Board, and use of deidentified samples was approved by the Albert Einstein College of Medicine Institutional Review Board. Participants were recruited through the Mucosal Immunology Core Laboratory at UCLA, provided written informed consent, and underwent endoscopic evaluation. Colorectal biopsy tissue was obtained as previously described.1 To augment the study's sample size, colorectal biopsy samples from two healthy individuals and from five individuals with active UC were obtained from OriGene Technologies, Inc. (Rockville, MD).
Histological scoring
Histology scores for all samples (UCLA and OriGene) were assigned by a single blinded gastrointestinal pathologist according to a validated colonic inflammation scoring system that ranged from a score of 1 (normal histology) to 6 (severely abnormal histology with destruction of epithelium and minimal cellular elements) (Fig. 1).3,23 For UCLA-recruited participants, biopsies were mounted on mesh screens, fixed in 10% formalin, and embedded in paraffin. Sections (7 μm) were stained with hematoxylin and eosin and examined under light microscopy. Two slides with three sections per biopsy were evaluated at 4× and 10× power. This histology scoring system was also applied to photographed slides from the OriGene samples.
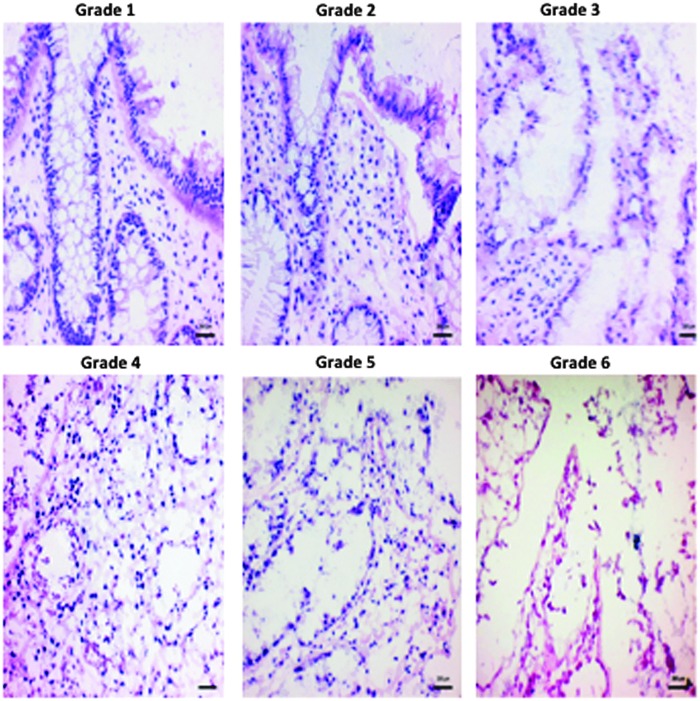
FIG. 1.
Histological grading scale (grades 1–6) illustrated using representative hematoxylin–eosin-stained sections of colonic mucosa. Grade 1: Intact epithelium and lamina propria. No structural abnormality. Grade 2: Detatched epithelium but intact lamina propria. Chronic inflammatory cell infiltrates. Grade 3: Detached epithelium and disaggregated lamina propria. Neutrophil infiltrates in lamina propria. Grade 4: Detached epithelium and disaggregated lamina propria with >30% karyorrhexis. Neutrophil infiltrates in epithelium. Grade 5: Detached epithelium and disaggregated lamina propria with >75% karyorrhexis and crypt destruction. Grade 6: Epithelium lost and minimal cellular remnants, with mostly structural elements remaining and erosions or ulcerations present. Biopsy samples scored as grade 1 were categorized as normal. Samples scored as grades 2–3 were categorized as having mild changes, grade 4 samples were categorized as moderate, and grades 5–6 were categorized as severe.
Total RNA extraction and RT-PCR amplification to quantify mRNA
Total RNA was extracted from deidentified and homogenized UCLA biopsy tissue using an Absolutely RNA RT-PCR Miniprep Kit (Strategene, La Jolla, CA). RNA (200 ng) was then reverse transcribed with a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). RT-PCR amplification was performed in duplicate using an ABI PRISM 7000 detection system and analyzed using sequence detector software. PCR cycling conditions were as follows: 1 cycle, 50°C for 2 min; 1 cycle, 95°C for 15 min; 45 cycles, 95°C for 10 s; and 1 cycle, 60°C for 1 min. Gene expression levels were calculated as 2ddCt (where Ct indicates cycle threshold) using large ribosomal protein (RPLPO) as the endogenous control. Gene expression levels for UC and HIV-infected participants were compared to healthy control participants. Specimens with undetectable RNA levels were attributed a Ct value of 45.
The following commercially available probes (Applied Biosystems, Foster City, CA) were used for all specimens: human SLPI (Hs00268204_m1), human beta-defensin 1 (HBD-1) (Hs00174765_m1), human beta-defensin 2 (HBD-2) (Hs00175474_m1), human beta-defensin 3 (HBD-3) (Hs00218678_mL), human defensin 5 (HD5) (Hs00360716_mL), human neutrophil peptides 1–3 (HNP1-3) (Hs00234383_mL), (Hs00175474_m1), interleukin 6 (IL-6) (Hs00174131_m1), interleukin 8 (IL-8) (Hs00174103_m1), interleukin 17 (IL-17) (Hs00174383), interleukin 1β (IL-1β) (Hs00174097_mL), tumor necrosis factor-α (TNF-α) (Hs00174128_m1), toll-like receptor 2 (TLR2) (Hs00152932_m1), toll-like receptor 4 (TLR4) (Hs00152939_mL), toll-like receptor 5 (TLR5) (Hs00152825_mL), toll-like receptor 9 (TLR9) (Hs00152973_mL), myeloid differentiation primary response gene (88) (MyD88) (Hs00182082_m1), tight junction protein 1 (TJP1) (Hs01551876_mL), CHUK (IKK alpha) (Hs00175141_m1), and ribosomal large protein subunit (RPLPO) (4333761F).
HIV RNA quantitation in biopsy tissue collected from HIV-infected participants was interpolated using a calibration curve generated from serial dilutions of cell lysate prepared from U1 cells, a monocytic cell line infected with HIV.24 HIV relative copy number (RCN) was calculated as the ratio between HIV LTR and RPLPO copy numbers as previously described.25
Statistical analysis
Differences in participant demographics between groups were compared using analysis of variance (ANOVA) or t-tests for continuous variables and chi-square or Fisher's exact tests for categorical variables. Gene expression levels of mediators were log10 transformed to reduce skewness. Differences in mediators between HIV, UC, and control groups were compared using one-way ANOVA, followed by Bonferroni correction for post hoc pairwise comparisons. Spearman correlation coefficients were used to assess correlations between HIV plasma viral load, HIV RCN in biopsy tissue, and mRNA of immune mediators. All analyses were performed using GraphPad Prism version 6 (San Diego, CA.). All tests for significance were two-sided, and a value of p<0.05 was considered significant.
Results
Participants
Forty-one study participants provided a total of 41 colorectal biopsy samples and included 20 individuals with HIV infection, nine individuals with active UC, and 12 healthy control participants (Table 1). Ten HIV-infected participants had nondetectable (<50 copies/ml) plasma viral load (PVL) (HIVNDVL). Ten HIV-infected participants had detectable PVL (HIVDVL), with a mean±standard deviation (SD) viral load of 4.38±0.53 log10 copies/ml (Table 1). Nine of 10 HIVNDVL participants and six of 10 HIVDVL participants received ART at the time of biopsy (p=0.30). HIVNDVL participants had a significantly higher mean peripheral CD4+ T lymphocyte count compared to HIVDVL participants (692.4 cells/μl±351.7 vs. 301.6±232.3, p<0.001). Participant medications, including ART regimens, are detailed in Table 2.
Table 1.
Participant Demographics
| UC a N=9 | All HIV N=20 | Controls N=12 | p value | |
|---|---|---|---|---|
| Sex | ||||
| Male, number (%) | 7 (77.8) | 19 (95) | 10 (83.3) | 0.36 |
| Female, number | 2 (22.2) | 1 (5) | 2 (16.7) | |
| Age, years | ||||
| Mean±SDb | 39.3±14 | 45.4±10.3 | 42.5±12.1 | 0.29 |
| Race | ||||
| White, number (%) | 9 (100) | 15 (75) | 6 (50) | 0.15 |
| Nonwhite, number | 0 (0) | 5 (25) | 6 (50) | |
| Histology scorec | ||||
| Number with normal to mild (%) | 0 (0) | 19 (95) | 12 (100) | <0.001 (UC vs. HIV) |
| Number with moderate to severe (%) | 9 (100) | 1 (5) | 0 (0) | <0.001 (UC vs. controls) |
| 1.0 (HIV vs. controls) | ||||
| HIV-infected participantsd | |||
|---|---|---|---|
| Characteristic | HIVDVL N=10 | HIVNDVL N=10 | p value |
| Plasma viral load | |||
| Mean log10 copies±SD | 4.4±0.5 | n/a | n/a |
| CD4+ T-lymphocyte count | |||
| Mean cells/μl±SD | 301.6±232.3 | 692.4±351.7 | <0.001 |
| ARTe at the time of biopsy | |||
| Yes, number (%) | 6 (60) | 9 (90) | 0.30 |
| No | 4 (40) | 1 (10) | |
| Number of years from HIV diagnosisf | n=6 | n=7 | |
| Mean±SD | 17.5±5.2 | 10.1±3.3 | 0.01 |
| Histology score | |||
| Normal, number (%) | 5 (50) | 10 (100) | 0.03 |
| Mild | 4 (40) | 0 (0) | |
| Moderate | 0 (0) | 0 (0) | |
| Severe | 1 (10) | 0 (0) | |
Ulcerative colitis.
Standard deviation.
Normal histology score=grade 1, mild histology score=grade 2–3, moderate histology score=grade 4, severe histology score=grade 5–6.
HIV-infected subjects were dichotomized according to detectable plasma viral load (HIVDVL) or nondetectable (<50 copies/ml) (HIVNDVL) plasma viral load.
Antiretroviral therapy.
Time from diagnosis was not available for all participants.
Table 2.
Participant Medications
| UC participant number, medications | HIVDVL participant number, medications | HIVNDVL participant number, medications | |||
|---|---|---|---|---|---|
| 1 | Dicyclomine, metronidazole, prednisone | 1 | Lamivudine, nevirapine, tenofovir DF | 1 | Lamivudine/zidovudine, saquinavir, ritonavir, nevirapine |
| 2 | 5-ASA, prednisone, AZA, fludrocortisone | 2 | 5-ASA, lamivudine/zidovudine, tenofovir DF, efavirenz | 2 | Lamivudine, lopinavir/ritonavir, tenofovir DF |
| 3 | Infliximab | 3 | 5-ASA, lamivudine/zidovudine, tenofovir DF, efavirenz | 3 | Abacavir/lamivudine/zidovudine, tenofovir DF |
| 4 | Infliximab, prednisone | 4 | No medications | 4 | Lamivudine/zidovudine, efavirenz, acyclovir |
| 5 | Unknown | 5 | No medications | 5 | No medications |
| 6 | 5-ASA | 6 | Emtricitabine/tenofovir DF/efavirenz | 6 | Raltegravir, lopinavir/ritonavir, etravirine |
| 7 | 5-ASA | 7 | No medications | 7 | Lamivudine, didanosine, nevirapine, stavudine |
| 8 | Fish oil | 8 | No medications | 8 | Lamivudine/zidovudine, saquinavir, nelfinavir |
| 9 | 5-ASA, prednisone, 6-MP | 9 | Lopinavir/ritonavir, tenofovir DF, abacavir | 9 | Lamivudine, stavudine, tenofovir DF |
| 10 | Lopinavir/ritonavir, lamivudine | 10 | Lamivudine, stavudrine, efavirenz | ||
HIV-infected participants were stratified according to detectable plasma viral load (HIVDVL) vs. nondetectable plasma viral load (HIVNDVL).
5-ASA, 5-aminosalicylic acid; AZA, azathioprine; 6-MP, 6-mercaptopurine; DF, disoproxil fumarate.
Histological scoring
All healthy control participants had normal (grade 1) histological scores on biopsy (Table 1). All UC participants had moderate (grade 4) (N=6) or severe (grade 5–6) (N=3) histological scores (Table 1). All HIVNDVL participants had normal scores. Nine of the 10 HIVDVL participants had normal or mildly abnormal (grade 2–3) scores. UC participants were significantly more likely to have a moderate or severe histological score when compared to all HIV participants or to control participants (p<0.001 for both). The proportions of subjects with normal vs. abnormal histological scores did not differ significantly between all HIV participants and control participants (p=1.0). Neutrophil counts were obtained from histology sections from seven UC, 11 HIV, and five control participants; samples from UC participants were more likely to have neutrophil infiltrates compared to samples from HIV or control participants (p=0.03). Five of the seven samples from UC participants had neutrophil infiltrates [mean 41.08 neutrophils per high power field (HPF)±30.92], while only two samples from HIV participants had identifiable neutrophils (mean 0.7 neutrophils per HPF±0.71), and no neutrophils were identified in samples from control participants.
IL-6 and IL-8 expression in HIV-infected participants did not differ significantly from UC participants and was significantly upregulated relative to controls
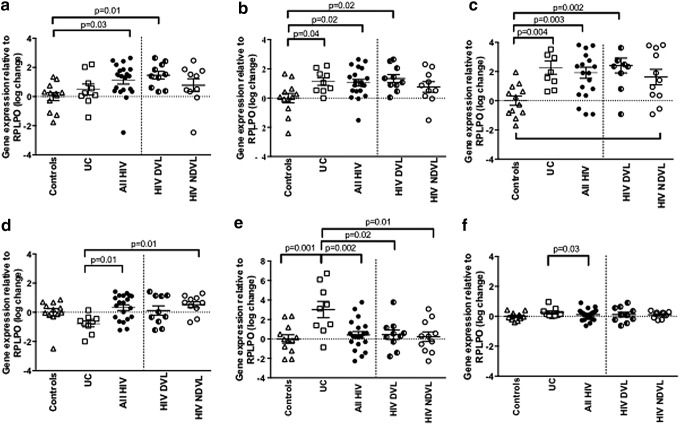
Mean IL-6 mRNA levels were modestly but not significantly higher in HIV-infected compared to UC participants (1.12±1.17 vs. 0.49±1.13, respectively; p=0.48) and were significantly upregulated relative to controls only for HIV-infected participants (p=0.03) (Fig. 2a). Mean IL-8 mRNA levels were similar between all HIV-infected and UC participants (1.14±0.77 vs. 1.05±1.02, respectively; p=1.0) and were significantly higher relative to controls for both HIV-infected (p=0.02) and UC participants (p=0.04) (Fig. 2b). When HIV-infected patients were then stratified by PVL, mean IL-8 and IL-6 mRNA levels were significantly higher only in the HIVDVL group relative to controls (p=0.01 for IL-6, p=0.02 for IL-8). Expression of IL-1β, IL-17, and TNF did not differ significantly between participants with HIV, UC, or controls (p>0.05 for all, data not shown).
FIG. 2.
mRNA for interleukin (IL)-6, IL-8, human defensin (HD)5, human β defensin (HBD)-1, HBD-2, and MyD88 was quantified from biopsy tissue by real time (RT)-PCR. mRNA levels were log-transformed, expressed relative to large ribosomal protein (RPLPO), and compared across groups by one-way analysis of variance (ANOVA) followed by Bonferroni correction for post hoc pairwise comparisons. As a secondary analysis, HIV-infected participants (all HIV) were stratified by detectable (HIVDVL) vs. nondetectable (HIVNDVL) plasma viral load; mRNA levels did not differ significantly for any mediator between the HIVDVL and HIVNDVL groups (p>0.05 for all). (a) Mean IL-6 mRNA levels did not differ significantly between ulcerative colitis (UC) and all HIV participants (p=0.48) but were significantly higher in all HIV participants vs. controls. (b) IL-8 mRNA levels were similar between UC and all HIV participants (p=1.0) and differed significantly in both groups from those observed in controls. (c) Mean HD5 mRNA levels were similar between UC and all HIV participants (p=1.0) but were significantly higher in both groups relative to controls. (d) Mean HBD-1 mRNA levels were significantly lower in UC compared to all HIV participants. (e) Mean HBD-2 levels were significantly higher in UC compared to all HIV and control participants. (f) Mean MyD88 mRNA levels were significantly higher in UC compared to all HIV participants.
Expression of human defensin 5, but not of human beta-defensins-1 or -2, was similar between HIV-infected and UC participants
Mean HD5 mRNA levels were similar between all HIV and UC participants (1.92±1.69 vs. 2.27±1.37, respectively; p=0.35) and were significantly upregulated in both groups relative to controls (p=0.004 for UC, p=0.003 for all HIV) (Fig. 2c). Again, when HIV-infected participants were stratified by PVL, only the HIVDVL group had significantly higher mean levels of HD5 mRNA relative to controls (p=0.002). HBD-1 expression was significantly downregulated in UC relative to all HIV-infected participants (−0.79±0.64 vs. 0.32±0.85, respectively; p=0.01), and HBD-1 mRNA levels were significantly higher in the HIVNDVL group relative to participants with UC (Fig. 2d). In contrast, mean HBD-2 mRNA levels were significantly higher in UC participants (3.02±2.54) relative to all HIV-infected (0.41±1.54, p=0.002) and control participants (p=0.001) (Fig. 2e). A trend was noted toward higher mean HNP 1–3 mRNA levels in UC compared to HIV-infected participants (1.35±1.03 vs. 0.23±1.42, respectively), but no significant difference was identified between UC, HIV, and control participants (p=0.05) (data not shown). No significant differences were observed between groups for HBD-3 mRNA (p>0.05 for all, data not shown).
Mean MyD88 expression was significantly higher in UC compared to all HIV-infected participants (0.25±0.31 vs. 0.11±0.38, p=0.03), although expression for both groups was similar to that observed in healthy control participants (p>0.05 for both) (Fig. 2f). No statistically significant differences between groups were observed for expression of CHUK, TJP-1, or SLPI (p>0.05 for all, data not shown) or for TLR2, TLR4, TLR5, and TLR9 (p>0.05 for all, data not shown).
HIV relative copy number is highest in biopsies from participants with detectable plasma viral loads
Mean HIV RCN from biopsy tissue was significantly higher in HIVDVL compared to HIVNDVL participants (0.04±0.01 vs. 4.7e-005±2.67e-005, p=0.01) and correlated positively with plasma viral load (r=0.88, p<0.01). We identified no significant correlations between plasma viral loads and mediator gene expression in biopsy tissue or between biopsy tissue HIV RCN and mediator gene expression (data not shown). No significant differences in mediator gene expression were observed when participants were stratified by HIVDVL vs. HIVNDVL or by ART vs. no ART use (data not shown).
Discussion
In this cohort of chronically infected HIV participants, we observed patterns of gastrointestinal mucosal mediator expression that were similar to those observed in participants with active UC, although expression of beta-defensins differed between the two groups. While the majority of HIV-infected participants received ART and had normal to mildly abnormal histology scores, soluble mediator expression in HIV-infected participants differed significantly from control participants. Together, these findings suggest evidence of GI mucosal inflammation in the setting of HIV and underscore the findings of prior studies in which biopsies from HIV-infected participants were found to have increased expression of inflammatory chemokines and evidence of cellular inflammation by flow cytometry and immunohistochemistry, despite normal qualitative histology scores.1,3
Gene expression of proinflammatory IL-6 and IL-8 did not differ significantly between HIV-infected and UC participants, and both IL-6 and IL-8 were significantly upregulated in HIV-infected participants relative to controls. IL-6 upregulation in the GI mucosa of HIV-infected individuals is consistent with prior studies, which found that IL-6 upregulation persisted after initiation of ART26 and in the setting of undetectable mucosal viral load.5 We did not observe a significant difference in levels of IL-6 or IL-8 mRNA between HIVDVL and HIVNDVL participants or between participants who received or did not receive ART. Thus, ART and peripheral viral suppression may not fully suppress IL-6 and IL-8 gene expression, and chronic upregulation may lead to ongoing mucosal inflammation, target cell recruitment, direct stimulation of HIV replication, and lymphocyte depletion.27–29
To our knowledge, our study is the first to examine defensin and TLR expression in the gastrointestinal mucosa of HIV-infected individuals. Although HD5 expression was similar between HIV-infected and UC participants, HBD-1 and -2 expression differed between these two groups. Interestingly, HBD-2 mRNA levels in HIV-infected participants were significantly lower than those observed in UC participants. HBD-2 exhibits activity against HIV-1 in a human oral epithelial cell culture model.30 HIV infection is thought to induce expression of HBD-2 by epithelial cells, and significant upregulation of HBD-2 gene expression was observed in the oral epithelial mucosa (but not the genital tract mucosa) of HIV-exposed, seronegative individuals.31 Our small sample size may have limited our ability to detect significant upregulation of HBD-2 gene expression in HIV-infected participants, especially because only 10 participants had detectable plasma viral loads and evidence of local HIV replication by HIV RCN. As the majority of our HIV-infected participants received ART, an alternative theory is that use of ART abrogates upregulation of HBD-2 in the setting of HIV.
In contrast, the alpha-defensin HD5 is thought to be constitutively expressed by intestinal Paneth cells and by genitourinary tract epithelial cells.32,33 Thus, upregulation of HD5 in both HIV-infected and UC participants relative to controls is intriguing. One prior study identified higher concentrations of HD5 by immunoblot from urethral lavage from individuals with gonococcal or chlamydial urethritis, suggesting that mucosal inflammation may induce HD5 secretion.34 HD5 may exert anti-HIV activity in the serum-free environment of the intestinal lumen35 but the in vivo roles of both alpha- and beta-defensins against viral infection and replication remain unclear, and elevated levels could also promote inflammation. The role of alpha- and beta-defensins as protective antimicrobial peptides vs. promoters of mucosal inflammation requires investigation in larger cohorts of individuals with HIV or UC and may provide insight into the pathogenesis of these two disease states.
Along with crypt architecture destruction and plasmacellular infiltrates, neutrophil infiltrates are included in the histological criteria for diagnosis of inflammatory bowel disease.36–38 Neutrophil infiltrates were identified in histological sections from UC participants; only scant neutrophils were identified in available histological sections from HIV participants and were not identified in sections from control participants. Neutrophil degranulation in UC biopsy samples may provide a rationale for the observed trend of increased HNP 1–3 gene expression in UC participants. MyD88-dependent signaling may induce neutrophil recruitment in the setting of acute intestinal infection;39 however, MyD88 gene expression was relatively low across all three groups and was similar between UC and control participants.
Although we found that HIV RCN in biopsy tissue correlated positively and significantly with plasma viral load, we did not identify significant correlations between HIV RCN and mediator gene expression. The majority of HIV-infected participants received ART, which limited our ability to assess how mediator expression may vary between those who do or do not receive ART. ART may impact the soluble mucosal immune environment by reducing gastrointestinal antigen challenge and infection and reducing local viral replication, thus permitting T-lymphocytes to reestablish mucosal homeostasis. One prior study described a reduction in gastrointestinal mucosal cytokine expression after initiation of ART and suggested that those with nondetectable mucosal viral loads on ART had downregulation of mucosal cytokine expression relative to healthy controls.5 Additional studies are needed to address the impact of different antiretroviral therapy regimens on mucosal inflammation. A more comprehensive understanding of the relationship between systemic and mucosal inflammation and replication could impact the development of therapies to eradicate HIV reservoirs. Furthermore, identification of common pathways of mucosal inflammation among HIV-infected and IBD patients could lead to novel antiinflammatory therapies that offer therapeutic benefit for both diseases.
Acknowledgments
The authors wish to thank Dr. Peter Anton and Dr. Betsy C. Herold for their editorial assistance, Dr. Yanhua Wang for her histopathology assistance, and Dr. Gloria C. Preza for her assistance in figure preparation. The authors also wish to thank the Mucosal Immunology Core of the UCLA Center for AIDS Research (CFAR) (NIH/NIAID AI-028697) for providing the biopsy samples and demographic and histology data utilized in this study. This work was supported by NIH/NIAID K23AI-089271 (to R.P.M.), by NIH/NIAID T32 AI070117 (awarded to the Albert Einstein College of Medicine), and by the Albert Einstein College of Medicine/Montefiore Medical Center CFAR (NIH/NIAID AI-051519). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Olsson J, Poles M, Spetz AL, et al. : Human immunodeficiency virus type 1 infection is associated with significant mucosal inflammation characterized by increased expression of CCR5, CXCR4, and beta-chemokines. J Infect Dis 2000;182(6):1625–1635 [DOI] [PubMed] [Google Scholar]
- 2.Sankaran S, George MD, Reay E, et al. : Rapid onset of intestinal epithelial barrier dysfunction in primary human immunodeficiency virus infection is driven by an imbalance between immune response and mucosal repair and regeneration. J Virol 2008;82(1):538–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGowan I, Elliott J, Cortina G, et al. : Characterization of baseline intestinal mucosal indices of injury and inflammation in men for use in rectal microbicide trials (HIV Prevention Trials Network-056). J Acquir Immune Defic Syndr 2007;46(4):417–425 [DOI] [PubMed] [Google Scholar]
- 4.McGowan I, Radford-Smith G, and Jewell DP: Cytokine gene expression in HIV-infected intestinal mucosa. AIDS 1994;8(11):1569–1575 [DOI] [PubMed] [Google Scholar]
- 5.McGowan I, Elliott J, Fuerst M, et al. : Increased HIV-1 mucosal replication is associated with generalized mucosal cytokine activation. J Acquir Immune Defic Syndr 2004;37(2):1228–1236 [DOI] [PubMed] [Google Scholar]
- 6.Epple HJ, Schneider T, Troeger H, et al. : Impairment of the intestinal barrier is evident in untreated but absent in suppressively treated HIV-infected patients. Gut 2009;58(2):220–227 [DOI] [PubMed] [Google Scholar]
- 7.Verhoeven D, Sankaran S, Silvey M, and Dandekar S: Antiviral therapy during primary simian immunodeficiency virus infection fails to prevent acute loss of CD4+ T cells in gut mucosa but enhances their rapid restoration through central memory T cells. J Virol 2008;82(8):4016–4027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brenchley JM. and Douek DC: HIV infection and the gastrointestinal immune system. Mucosal Immunol 2008;1(1):23–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinugasa T, Akagi Y, Yoshida T, et al. : Increased claudin-1 protein expression contributes to tumorigenesis in ulcerative colitis-associated colorectal cancer. Anticancer Res 2010;30(8):3181–3186 [PubMed] [Google Scholar]
- 10.Estes JD, Harris LD, Klatt NR, et al. : Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS Pathog 2010;6(8):e1001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epple HJ, Allers K, Troger H, et al. : Acute HIV infection induces mucosal infiltration with CD4+ and CD8+ T cells, epithelial apoptosis, and a mucosal barrier defect. Gastroenterology 2010;139(4):1289–1300 [DOI] [PubMed] [Google Scholar]
- 12.Sandler NG. and Douek DC: Microbial translocation in HIV infection: Causes, consequences and treatment opportunities. Nat Rev Microbiol 2012;10(9):655–666 [DOI] [PubMed] [Google Scholar]
- 13.Brenchley JM. and Douek DC: The mucosal barrier and immune activation in HIV pathogenesis. Curr Opin HIV AIDS 2008;3(3):356–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki T, Yoshinaga N, and Tanabe S: Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J Biol Chem 2011;286(36):31263–31271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swidsinski A, Ladhoff A, Pernthaler A, et al. : Mucosal flora in inflammatory bowel disease. Gastroenterology 2002;122(1):44–54 [DOI] [PubMed] [Google Scholar]
- 16.d'Ettorre G, Paiardini M, Zaffiri L, et al. : HIV persistence in the gut mucosa of HIV-infected subjects undergoing antiretroviral therapy correlates with immune activation and increased levels of LPS. Curr HIV Res 2011;9(3):148–153 [DOI] [PubMed] [Google Scholar]
- 17.Wehkamp J, Harder J, Weichenthal M, et al. : Inducible and constitutive beta-defensins are differentially expressed in Crohn's disease and ulcerative colitis. Inflamm Bowel Dis 2003;9(4):215–223 [DOI] [PubMed] [Google Scholar]
- 18.Wehkamp J, Harder J, Weichenthal M, et al. : NOD2 (CARD15) mutations in Crohn's disease are associated with diminished mucosal alpha-defensin expression. Gut 2004;53(11):1658–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brenchley JM, Price DA, Schacker TW, et al. : Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006;12(12):1365–1371 [DOI] [PubMed] [Google Scholar]
- 20.Stanislawowski M, Wierzbicki PM, Golab A, et al. : Decreased Toll-like receptor-5 (TLR-5) expression in the mucosa of ulcerative colitis patients. J Physiol Pharmacol 2009;60(Suppl 4):71–75 [PubMed] [Google Scholar]
- 21.Zaragoza MM, Sankaran-Walters S, Canfield DR, et al. : Persistence of gut mucosal innate immune defenses by enteric alpha-defensin expression in the simian immunodeficiency virus model of AIDS. J Immunol 2011;186(3):1589–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klatt NR, Funderburg NT, and Brenchley JM: Microbial translocation, immune activation, and HIV disease. Trends Microbiol 2013;21(1):6–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geboes K, Riddell R, Ost A, et al. : A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut 2000;47(3):404–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Folks TM, Justement J, Kinter A, et al. : Cytokine-induced expression of HIV-1 in a chronically infected promonocyte cell line. Science 1987;238(4828):800–802 [DOI] [PubMed] [Google Scholar]
- 25.Mesquita PM, Rastogi R, Segarra TJ, et al. : Intravaginal ring delivery of tenofovir disoproxil fumarate for prevention of HIV and herpes simplex virus infection. J Antimicrob Chemother 2012;67(7):1730–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schulbin H, Bode H, Stocker H, et al. : Cytokine expression in the colonic mucosa of human immunodeficiency virus-infected individuals before and during 9 months of antiretroviral therapy. Antimicrob Agents Chemother 2008;52(9):3377–3384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poli G, Bressler P, Kinter A, et al. : Interleukin 6 induces human immunodeficiency virus expression in infected monocytic cells alone and in synergy with tumor necrosis factor alpha by transcriptional and post-transcriptional mechanisms. J Exp Med 1990;172(1):151–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lane BR, Lore K, Bock PJ, et al. : Interleukin-8 stimulates human immunodeficiency virus type 1 replication and is a potential new target for antiretroviral therapy. J Virol 2001;75(17):8195–8202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haase AT: Targeting early infection to prevent HIV-1 mucosal transmission. Nature 2010;464(7286):217–223 [DOI] [PubMed] [Google Scholar]
- 30.Quinones-Mateu ME, Lederman MM, Feng Z, et al. : Human epithelial beta-defensins 2 and 3 inhibit HIV-1 replication. AIDS 2003;17(16):F39–48 [DOI] [PubMed] [Google Scholar]
- 31.Zapata W, Rodriguez B, Weber J, et al. : Increased levels of human beta-defensins mRNA in sexually HIV-1 exposed but uninfected individuals. Curr HIV Res 2008;6(6):531–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bevins CL. and Salzman NH: Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol 2011;9(5):356–368 [DOI] [PubMed] [Google Scholar]
- 33.Selsted ME. and Ouellette AJ: Mammalian defensins in the antimicrobial immune response. Nat Immunol 2005;6(6):551–557 [DOI] [PubMed] [Google Scholar]
- 34.Porter E, Yang H, Yavagal S, et al. : Distinct defensin profiles in Neisseria gonorrhoeae and Chlamydia trachomatis urethritis reveal novel epithelial cell-neutrophil interactions. Infect Immun 2005;73(8):4823–4833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson SS, Wiens ME, and Smith JG: Antiviral mechanisms of human defensins. J Mol Biol 2013;425(24):4965–4980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morson B: Rectal and colonic biopsy in inflammatory bowel disease. Am J Gastroenterol 1977;67(5):417–426 [PubMed] [Google Scholar]
- 37.Surawicz CM. and Belic L: Rectal biopsy helps to distinguish acute self-limited colitis from idiopathic inflammatory bowel disease. Gastroenterology 1984;86(1):104–113 [PubMed] [Google Scholar]
- 38.Jenkins D, Balsitis M, Gallivan S, et al. : Guidelines for the initial biopsy diagnosis of suspected chronic idiopathic inflammatory bowel disease. The British Society of Gastroenterology Initiative. J Clin Pathol 1997;50(2):93–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jarchum I, Liu M, Shi C, et al. : Critical role for MyD88-mediated neutrophil recruitment during Clostridium difficile colitis. Infect Immun 2012;80(9):2989–2996 [DOI] [PMC free article] [PubMed] [Google Scholar]