Abstract
Human nocardiosis, caused by Nocardia spp., an ubiquitous soil-borne bacteria, is a rare granulomatous disease close related to immune dysfunctions. Clinically can occur as an acute life-threatening disease, with lung, brain and skin being commonly affected. The infection was classically diagnosed in HIV infected persons, organ transplanted recipients and long term corticosteroid treated patients. Currently the widespread use of immunomodulators and immunossupressors in the treatment of inflammatory diseases changed this scenario. Our purpose is to review all published cases of nocardiosis in immunomodulated patients due to inflammatory diseases and describe clinical and laboratory findings. We reviewed the literature concerning human cases of nocardiosis published between 1980 and 2014 in peer reviewed journals. Eleven cases of nocardiosis associated with anti-tumor necrosis factor (TNF) prescription (9 related with infliximab and 2 with adalimumab) were identified; 7 patients had inflammatory bowel disease (IBD), 4 had rheumatological conditions; nocardia infection presented as cutaneous involvement in 3 patients, lung disease in 4 patients, hepatic in one and disseminated disease in 3 patients. From the 10 cases described in IBD patients 7 were associated with anti-TNF and 3 with steroids and azathioprine. In conclusion, nocardiosis requires high levels of clinical suspicion and experience of laboratory staff, in order to establish a timely diagnosis and by doing so avoid worst outcomes. Treatment for long periods tailored by the susceptibility of the isolated species whenever possible is essential. The safety of restarting immunomodulators or anti-TNF after the disease or the value of prophylaxis with cotrimoxazole is still debated.
Keywords: Nocardiosis, Immunomodulation, Nocardia spp., Inflammatory diseases
Core tip: Opportunistic infections in immunomodulated patients with inflammatory diseases has gained renewed interest because of the new biological therapies. Concerning inflammatory bowel disease, in particular anti-tumor necrosis factor drugs, turned granulomatous infection diseases a real risk. The awareness and knowledge about nocardiosis, a rare but severe granulomatous infection, is probably lacking for the majority of doctors treating these patients. Our aim is to increase the awareness about the infection and review the published cases in this particular group of patients. We would like that our reads increase knowledge about clinical manifestations and up-to-date treatment, be aware of the risk of the disease and when to suspect nocardiosis.
INTRODUCTION
Human nocardiosis is generally recognized as an opportunistic disease close related to immune dysfunctions, however any host may be affected. The infection can range from a sub-clinical infection to acute life-threatening disease[1].
Classically the infection was more common in patients living with human immunodeficiency virus (HIV) infection, organ transplant recipients and those on long-term corticosteroid therapy[2]. Concurrent use of immunosuppressants, preexisting pulmonary diseases and diabetes mellitus are also associated with increased risk of nocardiosis[3].
The incidence of Nocardia infection is low, nevertheless early diagnosis and treatment in immunosuppressed patients is essential, due to its high morbidity and mortality[4]. Nocardia infection causes granulomatous diseases and differential diagnosis should be made with more frequent granulomatous diseases, like tuberculosis[5] and Crohn’s disease. After the introduction of anti-tumor necrosis factor drugs (TNF-α) an increase in the incidence of granulomatous infections, including nocardiosis[5] was noticed.
Our purpose is to focus on the descriptions of nocardiosis in immunomodulated patients due to inflammatory diseases and to review published cases in this setting.
RESEARCH
We searched PubMed, B-On, OVID databases for articles till November 2014, using these key words alone or in combination: “Nocardia spp.”, “nocardiosis”, “immunosuppressed patients”, “nocardia diagnosis”, “nocardia treatment”, “nocardia sensibility”, “inflammatory bowel disease”, “Crohn Disease”, “ulcerative colitis”, “anti-TNF therapy”. We selected review articles of nocardiosis and 14 articles of case reports all in English language except one case report, all together 50 articles.
NOCARDIA SPP: THE BACTERIA AND PATHOGENIC MECHANISMS
Nocardia species are ubiquitous soil-borne aerobic microorganisms which belong to a large group of bacteria, aerobic actinomycetes, with more than 80 different species of Nocardia identified, of which at least 33 species are pathogenic[6]. The majority of Nocardia infections are caused by inhalation, but some may be acquired by percutaneous inoculation after direct contact with soil. Nocardia species can spread hematogenously from lung parenchyma, particularly within the upper lobes, or from cutaneous infection sites to the brain, kidneys, joints, bones, soft tissues and eyes causing disseminated nocardiosis[7]. Bacteria dissemination has been related to immunocompromising conditions as cell-mediated response and macrophages function[2]. Therefore, patients under corticosteroids, in which macrophage and T-cell function are decreased, and patients treated with infliximab, an inducer of apoptosis of macrophages and T cells, are at risk of developping nocardiosis[8]. The need for continuous immunosuppressive therapy, disseminated disease and central nervous system involvement[9] are factors associated with poor prognosis. In a review of 10 cases of nocardiosis occurring in rheumatic patients 6 out of 10 had disseminated disease when their pulmonary lesion was diagnosed[10].
CLINICAL ASPECTS
Nocardiosis may have several clinical presentations[7]. (1) pulmonary Nocardiosis: in more than two-thirds of cases the lungs are the primary site of nocardial infection; the onset of the disease may be subacute or chronic and it is not distinguished by any specific signs or symptoms. Fever, weight loss, anorexia, dyspnea, cough, and haemoptysis[2] may be present. Radiographic findings of lung involvement may include single or multiple nodules, lung masses (with or without cavitation), reticulonodular infiltrates, interstitial infiltrates, lobar consolidation[7] (Figure 1). Brain imaging should be performed in all patients with pulmonary nocardiosis as cerebral dissemination is frequent and the bacteria seems to have a special tropism for neural tissue[9]; (2) cerebral Nocardiosis: CNS is involved in approximately 20 percent of nocardiosis and in 44 percent of disseminated cases[9]. Most commonly it results from dissemination of infection from a pulmonary or cutaneous site. Cerebral lesions are parenchymal abscess that can occur in any region of the brain[9] (Figure 2). Signs and symptoms of nocardial brain abscess are diverse and nonspecific: fever, headache, meningismus, seizures, and/or focal neurologic deficits. Nocardial meningitis is rare and can occur with or without an associated brain abscess[11]. The clinical presentation is a subacute or chronic meningitis and the cerebrospinal fluid is similar to other bacterial meningitis[11]; and (3) skin and cutaneous Nocardiosis: cutaneous disease most commonly results from direct inoculation of organisms into the skin after trauma in immunocompromised individuals. Primary infections usually present as superficial painless cellulitis or abscess with localized lymphadenopathy, and progress slowly[7].
Figure 1.
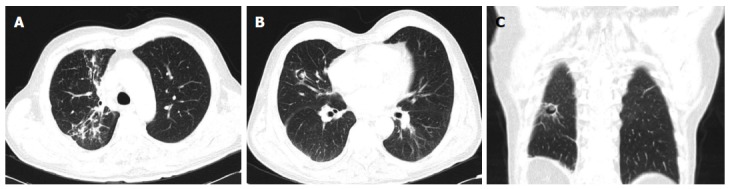
Torax computed tomography scan 73-year-old man with disseminated nocardiosis (cutaneous, pulmonary and cerebral involvements). A: Consolidations in upper right pulmonary lobe; B: Cavitation with heterogenous filling in right upper pulmonary lobe; C: Coronal image showing a cavitation in right lower pulmonary lobe.
Figure 2.
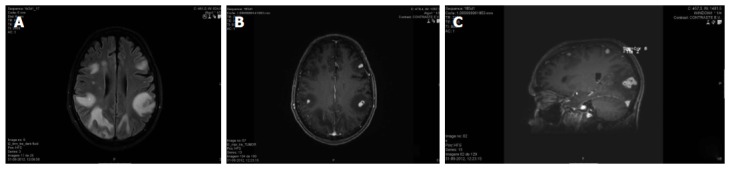
Cerebral magnetic resonance of 73-year-old man with disseminated nocardiosis (cutaneous, pulmonary and cerebral involvements). A: Axial T2 FLAIR (Fluid attenuated inversion recovery). Multiple focal lesions in bilateral fronto-parietal subcortical white matter, with proeminent edema and mass effect resulting in sulcal effacement; B: Post-gadolinium T1 3D-MPRAGE, axial reformat. Ring enhancing lesions were depicted in post-contrast images; C: Post gadolinium T1 3D-MPRAGE, sagittal reformat. Ring enhancing lesions were depicted in post-contrast images, forming clusters in right occipital lobe.
Disseminated nocardiosis is defined as two or more noncontiguous sites of involvement that may or may not include a pulmonary focus. There are no pathognomonic signs or symptoms of nocardiosis. The infection should be suspected in any patient who has brain, soft tissue, or cutaneous lesions, and a concurrent or recent pulmonary lesion. Pulmonary nocardiosis may mimic an exacerbation of an underlying lung disease, like chronic obstructive pulmonary disease[12] and pulmonary sarcoidosis[13]. Nocardiosis may be misdiagnosed as tuberculosis (since upper lobe involvement is common and Nocardia spp. are weakly acid fast), invasive fungal disease and malignancy[2].
NOCARDIA LABORATORIAL DIAGNOSIS
Nocardia spp. appear as delicate, filamentous, branching gram-positive rods in clinical specimens[6].The bacteria, like Mycobacterium, Corynebacterium, Rhodococcus, Gordona and Tsukamurella , members of the Nocardiform actinomycetes subgroup, are all variably acid-fast on appropriate staining[7]. However acid-fast staining property of Nocardia is often lost in older cultures[1]. Growing of Nocardia species in culture is slow and incubation should be carried out for at least two weeks, and ideally cultures should be maintained for 4-6 wk before they are red as negative. Therefore, when Nocardia infection is suspected, the laboratory should be notified for specific culture media and staining procedures. For instance some sputum decontamination solutions are toxic to Nocardia spp., particularly sodium hydroxide, N-acetylcysteine and benzalkonium chloride[2]. Curiously Nocardia spp. only rarely can be recovered in blood cultures despite frequent hematogenous dissemination[14]. Most cases of bacteremia are associated with central venous catheters or other endovascular devices[14]. Nocardia species identification is essential as not all species are pathogenic and different Nocardia species and strains often have markedly different susceptibility patterns[15]. Identification of the species is difficult when using routine phenotypic tests but identification based on conventional phenotypic and enzymatic tests enables for the rapid identification of the most common[16]. Alternatively, polymerase chain reaction (PCR) for identification of Nocardia spp. permits faster results than the conventional methods[17]. Susceptibility patterns of Nocardia varies among different species; the most common patterns of sensibility and resistance are detailed on Table 1. Results of laboratorial antimicrobial susceptibility testing of Nocardia should be interpreted with caution because few studies correlated in-vitro data with clinical outcome. Nevertheless, it should be pointed that susceptibility of all Nocardia spp. to trimethoprim plus sulphamethoxazole, amikacin and linezolid has been confirmed by several studies[6,15,18-20], whereas susceptibilities to beta-lactams, other aminoglycosides, ciprofloxacin and clarithromycin varied markedly[15]. From 93 Nocardia isolates in clinical specimens, belonging to 15 strains of Nocardia spp., activity of beta-lactams was variable, with 89% of isolates being susceptible to imipenem, 84% to amoxicillin + clavulanate, 55% to ceftriaxone, 50% to amoxicillin and 9% to piperacillin + tazobactam[15]. High-level of resistance to beta-lactams, including ceftriaxone and imipenem, was found in reference strains of N. brasiliensis, N. otitidiscaviarum and N. niigatensis[15]. Also N. farcinica characteristically demonstrates resistance to third-generation cephalosporins and is often resistant to imipenem[15,21,22]. Antimicrobial susceptibility testing is recommended as a guide to therapy for severe disease, refractory cases and for patients who are intolerant to treatment with trimetroprim-sulphamethoxazole[15]. Susceptibility testing is particularly important in patients infected with Nocardia species that have high frequencies of antimicrobial resistance, such as N. farcinica. Drugs to be tested by microdilution are: amikacin, amoxicillin-clavulanate, ceftriaxone, ciprofloxacin, clarithromycin, imipenem, linezolid, minocycline (which predicts doxycycline susceptibility), sulfamethoxazole or trimethoprim-sulfamethoxazole, and tobramycin[23].
Table 1.
Usual susceptibility patterns of common Nocardia species in human diseases
| Nocardia species | Susceptibility | Resistance | Ref. |
| N. asteroids sensu stricto | TMP-SMX, TGC, Amikacin | TGC | Lerner et al[2] |
| Imipenem (64%-98%) | Sorrel et al[43] | ||
| N. farcinica | Amikacin | Tobramycin | Wallace et al[21] |
| TMP-SMX, Minocycline | TMP-SMX (80%), TGC | Lerner et al[2] | |
| Uhde et al[44] | |||
| N. nova | TMP-SMX, TGC, Imipenem, Amikacin | TMP-SMX, TGC (53%) | Uhde et al[44] |
| Clarithromycin (96%) | Wallace et al[21] | ||
| Larruskain et al[46] | |||
| N. brasiliensis | TMP-SMX, Amikacin | Ceftriaxone (81%) | Uhde et al[44] |
| TGC (88%-100%), Imipenem (20%-30%) | Sorrel et al[43] | ||
| N. transvalensis | TMP-SMX (88%), Imipenem (90%), TGC (50%) | Amikacin | Sorrel et al[43] |
| McNeil et al[47] | |||
| N. otitidiscaviarum | Amikacin, Minocycline | TMP-SMX | Lerner et al[2] |
TMP-SMX: Trimethropim-sulfamethoxazol; TGC: Third generation cephalosporins.
THERAPY
Trimethoprim-sulphamethoxazole (TMP-SMX) is the first line option and can therefore be used as an initial empirical treatment in patients with extensive disease, including brain abscess. It has been reported that this drug has excellent penetration into most tissue compartments, including the central nervous system, and high serum concentrations even after oral administration (recommended oral dose: 2.5-5 mg/kg of the trimethoprim component orally twice daily). Concerning endovenous TMP-SMX the doses should be similar to the treatment of pneumocystosis (15 mg/kg per day of the trimethoprim component in two to four divided doses ). There have been few reports of patients failing to respond to TMP-SMX[24]. Although, in patients with life-threatening disease and in those failing treatment, sulfonamide levels should be monitored. A sulfonamide level measured two hours after a dose should have a serum concentration between 100 and 150 mcg/mL[2]. If the patient is allergic to sulfonamides, desensitization should be performed. For patients infected with sulfonamide-resistant Nocardia spp. or those who are allergic to sulfonamides, imipenem (500 mg IV every six hours) plus amikacin (7.5 mg/kg iv every 12 h) is an option.
Cutaneous infections
Oral TMP-SMX or amoxicillin-clavulanic acid are the drugs of choice for 1 to 3 mo in the case of mild cutaneous disease. Immunocompromised patients must be treated for a minimum of six months to one year.
Severe nocardia infections
Severe nocardiosis refers to some cases of pulmonary disease, all cases of disseminated disease or central nervous system disease, and infections involving more than one site in immunocompromised patients. In severely ill patients combined therapy with endovenous drugs with activity against Nocardia, like amikacin, imipenem, meropenem, ceftriaxone or cefotaxime is advisable[7]. Initially treatment with two intravenous drugs is recommended by some authors[2,6]. For more severe infection, even three intravenous drugs should be used[6]. When the severe infection does not involve CNS initial treatment may consist of TMP-SMX (15 mg/kg iv of the trimethropim per day in two to four divided doses) plus amikacin (7.5 mg/kg iv every 12 h). An alternative would be imipenem (500 mg IV every 6 h) plus amikacin[2]. When there is CNS disease TMP-SMX plus imipenem is an option. Amikacin may be associated in case of multiorgan involvement.
SURGERY
In several settings surgical intervention may be needed: (1) cerebral or some large soft tissue abscesses that do not respond to antibiotic therapy[25]; (2) empyemas and mediastinal infection with fluids; and (3) pulmonary nocardiosis complicated by pericarditis, which is almost always fatal if pericardial drainage is not performed[26].
TREATMENT DURATION
The optimal duration of antimicrobial treatment for severe disease has not been clearly settled. Drugs should be switched to oral medication 3 to 6 wk after initial endovenous therapy and maintained for at least 6 to 12 mo in the case of cerebral or extensive disease. Immunocompromised patients may require longer courses of initial IV therapy (3 to 6 mo, depending on the extent of the disease, and clinical response) and the addition of amikacin, or a carbapenem or ceftriaxone may be advisable. All immunocompromised patients (except those with isolated cutaneous infection) and all patients with CNS involvement should be treated for at least one year[2].
RISK OF NOCARDIA RELAPSES
Because of the relapsing nature of Nocardia infection, long duration antimicrobial treatment is recommend. The need for continuation therapy in those who need re-introduction of immunosupressors is not well settled. Some authors recommend prolonged oral maintenance therapy to prevent relapse of nocardiosis in patients who continue to be immunosuppressed as a result of their disease or treatment[27]. TMP-SMX is the drug of choice but protection is not complete and it is not known the most appropriate regimen[27]. Thus, in patients whose immunosuppression cannot be reversed, a maintenance regimen of TMP-SMX one single strength tablet daily if the Nocardia isolate is susceptible to TMP-SMX is the choice[28]. Alternative maintenance regimens have not been systematically evaluated, although doxycycline 100 mg daily is a possible alternative.
NOCARDIOSIS IN PATIENTS UNDER ANTI-TNF-α
Nocardiosis is an infrequent complication in patients with chronic inflammatory diseases under anti- TNF-α agents. A total of eight cases of nocardiosis were identified among approximately 300000 patients treated with anti-TNF agents with a rate of 3.55 and 0.88 per 100000 treated patients with infliximab or etanercept, respectively[5,29]. To our knowledge 10 cases were published (Table 2). Following infliximab therapy three cutaneous cases of nocardiosis were published: two in IBD patients[30,31]. and the other in a rheumatic patient[32]. Three pulmonary nocardiosis cases[33-35] and one hepatic nocardiosis[36] have been reported in IBD patients under infliximab therapy. Disseminated nocardiosis was described following infliximab treatment in one case of psoriasis[37] and in another of rheumatoid arthritis[38]. One patient with disseminated nocardiosis did not survived: he was a man, 66-year-old, on infliximab due to psoriasis[37]. Outside immunologic diseases it was reported a disseminated nocardiosis in a patient with alcoholic hepatitis treated with etanercept[39].
Table 2.
Clinical forms of Nocardiosis related to anti-TNF therapy in inflammatory bowel disease, rheumatic and psoriatic patients
| Clinical form |
Anti-TNF |
|||||
| IFX or ADA duration of therapy | Age | Associated treatment | Nocardia isolation | Outcome | Ref. | |
| Cutaneous | ||||||
| IBD-P | IFX -3 infusions | 45 | No | Nocardia spp. | Favourable | Singh et al[30] |
| IFX- 1,5 yr | 61 | No | Nocardia spp. | Favourable | Ali et al[31] | |
| R-P | IFX -3 yr | 70 | Metothrexate + steroids | N. otitidiscaviarum | Favourable | Fabre et al[32] |
| Pulmonary | ||||||
| IBD-P | IFX - 8 mo -6 infusions | 77 | Steroids | N. asteroids | Favourable | Stratakos et al[33] |
| IFX - 3 infusions | 53 | Azathioprine + steroids | N.cyriacigeorgica | Favourable | Parra et al[34] | |
| IFX - 6 mo | 81 | 6-mercapto-purine | Nocardia spp | Favourable | Saleemuddin et al[35] | |
| R-P | ADA - 4 mo | 63 | Steroids (DPOC) | N. asteroids | Favourable | Doraiswamy et al[48] |
| Disseminated | ||||||
| R-P | ADA1- 4 mo | 63 | Metotrexate | N. farcinica | Favourable | Wendling et al[38] |
| P-P | IFX2- 2 mo | 66 | Alefacet 6 mo before | N. farcinica | death | Al-Tawfiq et al[37] |
| IBD-P | IFX - 5 infusions | 73 | Prednisolone methrotexate | N. asteroids | Favourable with sequelae | Sidney et al[49] |
| Hepatic | ||||||
| IBD-P | IFX ≤ 1 mo | 23 | Steroids | N. farcinica | Favourable | Nakahara et al[36] |
Previously was treated with 3 mo of etanercept;
Diabetic patient. IBD-P: IBD-patients; R-P: Rheumatologic patients; P-P: Psoriatic patients.
NOCARDIOSIS IN PATIENTS WITH IBD
In patients with inflammatory bowel diseases to the best of our knowledge nine cases had been reported. Three cases were reported in patients under immunomodulation (steroids in two associated with 6-mercaptopurine/azathioprine and in one with cyclosporine[40-42]) and two of them were disseminated. Six other cases were associated with anti-TNF: two cutaneous forms were under anti-TNF alone[30,31] and four with anti-TNF combined with immunomodulators (steroids, thiopurines), with pulmonary disease in three cases and hepatic disease in one[33-36] (Table 3).
Table 3.
Clinical cases of nocardia disease in inflammatory bowel disease patients: Literature review
| Ref. | IBD | Age (yr) | Sex | Medication | N. species | Clinical form | Treatment (duration) | Evolution |
| Vohra et al[40] | CD | 16 | F | 6-Mercaptopurine 6 wk steroids | N. asteroids | Brain abcess; calf abscess | TMP-SMX + ceftriaxon: not established | Favourable |
| Stack et al[41] | UC | 68 | M | Cyclosporine, steroids | N. asteroids | Pulmonary (abcess) | Amikacin + cefotaxime-3 wk followed by cefuroxime 3 mo | Favourable |
| Singh et al[30] | CD | 45 | M | Infliximab 6 wk | N. spp. (polymerase chain reaction) | Cutaneous | TMP-SMX, 3 yr | Favourable |
| Stratakos et al[33] | CD (DM) | 77 | F | Infliximab 8 mo, steroids | N asteroids (+Pneumocystis jiroveci) | Pulmonary | TMP-SMX, 6 mo | Favourable |
| Parra et al[34] | CD | 53 | F | Infliximab, azathioprine, steroids | N. cyriacigeorgica | Pulmonary | TMP-SMX + amikacin + imipnem - 6 wk followed by TMP-SMX, 7.5 mo | Favourable |
| Arora et al[42] | UC | 61 | F | Azathioprine, steroids | N. nova | Cutaneous, abscess: brain lung, renal, pancreatic | TMP-SMX, 1 yr | Favourable, remission 2 yr after treatment |
| Nakahara et al[36] | CD | 23 | M | Infliximab, < 3 wk, steroids | N. farcinia | Liver nocardiosis | TMP-SMX for? not known | Favourable |
| Ali et al[31] | CD | 61 | M | Infliximab > 1 yr | N. spp. | Cutaneous | TMP-SMX for 6 mo | Favourable, restarted anti-TNF after therapy |
| Saleemuddin et al[35] | CD | 81 | M | Infliximab (3 mo) 6-mercapto-purine | Nocardia spp. | Pulmonary | TMP-SMX for? not known | Favourable; 5 mo after restarted anti-TNF under TMP-SMX; ok 1 yr after diagnosis |
| Sidney et al[49] | CD | 73 | F | Infliximab (5 infusions) Prednisolone methotrexate | Nocardia asteroids | Disseminated: Pulmonary cerebral | TMP-SMX for? not known | Favourable with sequelae |
TMP-SMX: Trimethoprim- sulfamethoxazole; DM: Diabetes mellitus; CD: Crohn's disease; UC: Ulcerative colitis.
Seven out of nine patients had a diagnosis of Crohn colitis with median age of 49 (61 if not considering the teenager) years-old and 5 were male. All but two patients, with cutaneous forms, were under two or more immunomodulatory drugs. Six patients were under steroids and six under anti-TNF. Pulmonary nocardiosis was the most common clinical form of Nocardiosis, described in 44% of patients. N asteroids was isolated in 3 patients, N. farcinica, N. cyriacigeorgica and N. nova in one case each. Two patients restarted anti-TNF therapy: one under TMP-SMX, 5 mo after starting Nocardia therapy and the other after 6 mo of therapy with TMP-SMX.
CONCLUSION
Nocardiosis is an uncommon disease caused by a Gram positive bacteria with acid-fast staining proprieties and diagnosis requires high levels of suspicion and experience of laboratory staff. The clinical impact of the disease is partly unknown, suggesting an underestimating of the real role of nocardiosis in human diseases. Persons under immunomodulation or immunossupressive therapy require, as those with HIV, organ transplant recipients, patients with pulmonary diseases and diabetes mellitus, special attention concerning the risks of nocardiosis. Remarkably double or triple immunosuppression seems to represent a higher risk for the disease. When concerning patients treated with anti TNF alone, just two cases of cutaneous forms were described. The most common clinical presentations are pulmonary and cutaneous, but the bacteria has the ability to disseminate and affect any organ, in particular the central nervous system. Laboratorial diagnosis is based on the identification of the bacteria (that growths slowly) on biological products, rarely on blood, and in alternative by PCR. The optimal duration of antimicrobial treatment for severe disease is not established but a prolonged course (one year) is advisable, because of the relapsing nature. There are several unanswered questions in nocardiosis infection as the safety of restarting immunomodulators or anti-TNF or the value of prophylaxis with TMP-SMX claiming urgent attention from physicians and investigators devoted to infection and immunological diseases.
Footnotes
Conflict-of-interest: No potential conflicts of interest relevant to this article were reported.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 8, 2015
First decision: January 22, 2015
Article in press: March 31, 2015
P- Reviewer: Naftali T S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
References
- 1.McNeil MM, Brown JM. The medically important aerobic actinomycetes: epidemiology and microbiology. Clin Microbiol Rev. 1994;7:357–417. doi: 10.1128/cmr.7.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lerner PI. Nocardiosis. Clin Infect Dis. 1996;22:891–903; quiz 904-905. doi: 10.1093/clinids/22.6.891. [DOI] [PubMed] [Google Scholar]
- 3.Peleg AY, Husain S, Qureshi ZA, Silveira FP, Sarumi M, Shutt KA, Kwak EJ, Paterson DL. Risk factors, clinical characteristics, and outcome of Nocardia infection in organ transplant recipients: a matched case-control study. Clin Infect Dis. 2007;44:1307–1314. doi: 10.1086/514340. [DOI] [PubMed] [Google Scholar]
- 4.Hardak E, Yigla M, Berger G, Sprecher H, Oren I. Clinical spectrum and outcome of Nocardia infection: experience of 15-year period from a single tertiary medical center. Am J Med Sci. 2012;343:286–290. doi: 10.1097/MAJ.0b013e31822cb5dc. [DOI] [PubMed] [Google Scholar]
- 5.Wallis RS, Broder MS, Wong JY, Hanson ME, Beenhouwer DO. Granulomatous infectious diseases associated with tumor necrosis factor antagonists. Clin Infect Dis. 2004;38:1261–1265. doi: 10.1086/383317. [DOI] [PubMed] [Google Scholar]
- 6.Brown-Elliott BA, Brown JM, Conville PS, Wallace RJ. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev. 2006;19:259–282. doi: 10.1128/CMR.19.2.259-282.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simpson RS, Read RC. Nocardiosis and actinomycosis. Medicine. 2014;42:23–25. [Google Scholar]
- 8.Morelli J, Wilson FA. Does administration of infliximab increase susceptibility to listeriosis? Am J Gastroenterol. 2000;95:841–842. doi: 10.1111/j.1572-0241.2000.01872.x. [DOI] [PubMed] [Google Scholar]
- 9.Beaman BL, Beaman L. Nocardia species: host-parasite relationships. Clin Microbiol Rev. 1994;7:213–264. doi: 10.1128/cmr.7.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamagata M, Hirose K, Ikeda K, Nakajima H. Clinical characteristics of Nocardia infection in patients with rheumatic diseases. Clin Dev Immunol. 2013;2013:818654. doi: 10.1155/2013/818654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bross JE, Gordon G. Nocardial meningitis: case reports and review. Rev Infect Dis. 1991;13:160–165. doi: 10.1093/clinids/12.5.160. [DOI] [PubMed] [Google Scholar]
- 12.Khare V, Gupta P, Himanshu D, Kumar D. Emergence of co-trimoxazole resistant Nocardia brasiliensis causing fatal pneumonia. BMJ Case Rep. 2013;2013:bcr2013009069. doi: 10.1136/bcr-2013-009069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zuk J, Bazan-Socha S, Zarychta J, Leclercq A, Lecuit M, Le Flèche-Matéos A, Orłowska-Heitzman J, Musiał J. Disseminated nocardiosis mimicking exacerbation of pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2013;30:65–69. [PubMed] [Google Scholar]
- 14.Kontoyiannis DP, Ruoff K, Hooper DC. Nocardia bacteremia. Report of 4 cases and review of the literature. Medicine (Baltimore) 1998;77:255–267. doi: 10.1097/00005792-199807000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Glupczynski Y, Berhin C, Janssens M, Wauters G. Determination of antimicrobial susceptibility patterns of Nocardia spp. from clinical specimens by Etest. Clin Microbiol Infect. 2006;12:905–912. doi: 10.1111/j.1469-0691.2006.01460.x. [DOI] [PubMed] [Google Scholar]
- 16.Wauters G, Avesani V, Charlier J, Janssens M, Vaneechoutte M, Delmée M. Distribution of nocardia species in clinical samples and their routine rapid identification in the laboratory. J Clin Microbiol. 2005;43:2624–2628. doi: 10.1128/JCM.43.6.2624-2628.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Couble A, Rodríguez-Nava V, de Montclos MP, Boiron P, Laurent F. Direct detection of Nocardia spp. in clinical samples by a rapid molecular method. J Clin Microbiol. 2005;43:1921–1924. doi: 10.1128/JCM.43.4.1921-1924.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biehle JR, Cavalieri SJ, Saubolle MA, Getsinger LJ. Comparative evaluation of the E test for susceptibility testing of Nocardia species. Diagn Microbiol Infect Dis. 1994;19:101–110. doi: 10.1016/0732-8893(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 19.Ambaye A, Kohner PC, Wollan PC, Roberts KL, Roberts GD, Cockerill FR. Comparison of agar dilution, broth microdilution, disk diffusion, E-test, and BACTEC radiometric methods for antimicrobial susceptibility testing of clinical isolates of the Nocardia asteroides complex. J Clin Microbiol. 1997;35:847–852. doi: 10.1128/jcm.35.4.847-852.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown-Elliott BA, Ward SC, Crist CJ, Mann LB, Wilson RW, Wallace RJ. In vitro activities of linezolid against multiple Nocardia species. Antimicrob Agents Chemother. 2001;45:1295–1297. doi: 10.1128/AAC.45.4.1295-1297.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wallace RJ, Tsukamura M, Brown BA, Brown J, Steingrube VA, Zhang YS, Nash DR. Cefotaxime-resistant Nocardia asteroides strains are isolates of the controversial species Nocardia farcinica. J Clin Microbiol. 1990;28:2726–2732. doi: 10.1128/jcm.28.12.2726-2732.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torres OH, Domingo P, Pericas R, Boiron P, Montiel JA, Vázquez G. Infection caused by Nocardia farcinica: case report and review. Eur J Clin Microbiol Infect Dis. 2000;19:205–212. doi: 10.1007/s100960050460. [DOI] [PubMed] [Google Scholar]
- 23.National Committee for Clinical Laboratory Standards. Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes. Approved standard. Wayne: NCCLS document; 2003. pp. M24–A. [PubMed] [Google Scholar]
- 24.Brown-Elliott BA, Biehle J, Conville PS, Cohen S, Saubolle M, Sussland D, Wengenack N, Kriel K, Bridge L, McNulty S, et al. Sulfonamide resistance in isolates of Nocardia spp. from a US multicenter survey. J Clin Microbiol. 2012;50:670–672. doi: 10.1128/JCM.06243-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee GY, Daniel RT, Brophy BP, Reilly PL. Surgical treatment of nocardial brain abscesses. Neurosurgery. 2002;51:668–671; discussion 671-672. [PubMed] [Google Scholar]
- 26.Poland GA, Jorgensen CR, Sarosi GA. Nocardia asteroides pericarditis: report of a case and review of the literature. Mayo Clin Proc. 1990;65:819–824. doi: 10.1016/s0025-6196(12)62573-7. [DOI] [PubMed] [Google Scholar]
- 27.Wilson JP, Turner HR, Kirchner KA, Chapman SW. Nocardial infections in renal transplant recipients. Medicine (Baltimore) 1989;68:38–57. doi: 10.1097/00005792-198901000-00003. [DOI] [PubMed] [Google Scholar]
- 28.King CT, Chapman SW, Butkus DE. Recurrent nocardiosis in a renal transplant recipient. South Med J. 1993;86:225–228. doi: 10.1097/00007611-199302000-00018. [DOI] [PubMed] [Google Scholar]
- 29.Wallis RS, Broder M, Wong J, Beenhouwer D. Granulomatous infections due to tumor necrosis factor blockade: correction. Clin Infect Dis. 2004;39:1254–1255. doi: 10.1086/424455. [DOI] [PubMed] [Google Scholar]
- 30.Singh SM, Rau NV, Cohen LB, Harris H. Cutaneous nocardiosis complicating management of Crohn’s disease with infliximab and prednisone. CMAJ. 2004;171:1063–1064. doi: 10.1503/cmaj.1040563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ali T, Chakraburtty A, Mahmood S, Bronze MS. Risk of nocardial infections with anti-tumor necrosis factor therapy. Am J Med Sci. 2013;346:166–168. doi: 10.1097/MAJ.0b013e3182883708. [DOI] [PubMed] [Google Scholar]
- 32.Fabre S, Gibert C, Lechiche C, Jorgensen C, Sany J. Primary cutaneous Nocardia otitidiscaviarum infection in a patient with rheumatoid arthritis treated with infliximab. J Rheumatol. 2005;32:2432–2433. [PubMed] [Google Scholar]
- 33.Stratakos G, Kalomenidis I, Papas V, Malagari K, Kollintza A, Roussos C, Anagnostopoulou M, Paniara O, Zakynthinos S, Papiris SA. Cough and fever in a female with Crohn’s disease receiving infliximab. Eur Respir J. 2005;26:354–357. doi: 10.1183/09031936.05.00005205. [DOI] [PubMed] [Google Scholar]
- 34.Parra MI, Martinez MC, Remacha MA, Saéz-Nieto JA, Garcia E, Yagüe G, Guardiola J. Pneumonia due to Nocardia cyriacigeorgica in a patient with Crohn’s disease treated with infliximab. J Crohns Colitis. 2008;2:331–332. doi: 10.1016/j.crohns.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Saleemuddin A, Govender P, Farraye FA. Nocardia pneumonia in a patient with Crohn’s disease receiving 6-mercaptopurine and infliximab. J Crohns Colitis. 2014;8:708–709. doi: 10.1016/j.crohns.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 36.Nakahara T, Kan H, Nakahara H, Asamoto Y, Komatsu H, Tokumo H, Ishida K. [A case of liver nocardiosis associated with Crohn’s disease while treating infliximab] Nihon Shokakibyo Gakkai Zasshi. 2011;108:619–626. [PubMed] [Google Scholar]
- 37.Al-Tawfiq JA, Al-Khatti AA. Disseminated systemic Nocardia farcinica infection complicating alefacept and infliximab therapy in a patient with severe psoriasis. Int J Infect Dis. 2010;14:e153–e157. doi: 10.1016/j.ijid.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 38.Wendling D, Murad M, Mathieu S, Berger E, Rumbach L. Systemic nocardiosis in a case of rheumatoid arthritis treated with tumor necrosis factor blockers. J Rheumatol. 2008;35:539–542. [PubMed] [Google Scholar]
- 39.Menon KV, Stadheim L, Kamath PS, Wiesner RH, Gores GJ, Peine CJ, Shah V. A pilot study of the safety and tolerability of etanercept in patients with alcoholic hepatitis. Am J Gastroenterol. 2004;99:255–260. doi: 10.1111/j.1572-0241.2004.04034.x. [DOI] [PubMed] [Google Scholar]
- 40.Vohra P, Burroughs MH, Hodes DS, Norton KI, Kaufman DM, LeLeiko NS, Benkov KJ. Disseminated nocardiosis complicating medical therapy in Crohn’s disease. J Pediatr Gastroenterol Nutr. 1997;25:233–235. doi: 10.1097/00005176-199708000-00021. [DOI] [PubMed] [Google Scholar]
- 41.Stack WA, Richardson PD, Logan RP, Mahida YR, Hawkey CJ. Nocardia asteroides lung abscess in acute ulcerative colitis treated with cyclosporine. Am J Gastroenterol. 2001;96:2255–2256. doi: 10.1111/j.1572-0241.2001.03971.x. [DOI] [PubMed] [Google Scholar]
- 42.Arora G, Friedman M, Macdermott RP. Disseminated Nocardia nova infection. South Med J. 2010;103:1269–1271. doi: 10.1097/SMJ.0b013e3181faec65. [DOI] [PubMed] [Google Scholar]
- 43.Sorrel TC, Iredell JR, Chen SCA. Nocardia Species. 7th ed. Bennett JE, Dolin R, editors. Philadelphia: Churchill Livingstone Elsevier; 2010. p. 3199. [Google Scholar]
- 44.Uhde KB, Pathak S, McCullum I, Jannat-Khah DP, Shadomy SV, Dykewicz CA, Clark TA, Smith TL, Brown JM. Antimicrobial-resistant nocardia isolates, United States, 1995-2004. Clin Infect Dis. 2010;51:1445–1448. doi: 10.1086/657399. [DOI] [PubMed] [Google Scholar]
- 45.Wallace RJ, Brown BA, Tsukamura M, Brown JM, Onyi GO. Clinical and laboratory features of Nocardia nova. J Clin Microbiol. 1991;29:2407–2411. doi: 10.1128/jcm.29.11.2407-2411.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larruskain J, Idigoras P, Marimón JM, Pérez-Trallero E. Susceptibility of 186 Nocardia sp. isolates to 20 antimicrobial agents. Antimicrob Agents Chemother. 2011;55:2995–2998. doi: 10.1128/AAC.01279-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McNeil MM, Brown JM, Georghiou PR, Allworth AM, Blacklock ZM. Infections due to Nocardia transvalensis: clinical spectrum and antimicrobial therapy. Clin Infect Dis. 1992;15:453–463. doi: 10.1093/clind/15.3.453. [DOI] [PubMed] [Google Scholar]
- 48.Doraiswamy VA. Nocardia infection with adalimumab in rheumatoid arthritis. J Rheumatol. 2008;35:542–543. [PubMed] [Google Scholar]
- 49.Sidney G, Smith RSB. Disseminated Nocardia infection associated with infliximab (Abstract) USA: American College of Gastroenterology 69th Annual Scientific Meeting Orlando; 2004. [Google Scholar]


