Abstract
AIM: To investigate alternative splicing in vascular endothelial growth factor A (VEGFA), amyloid beta precursor protein (APP), and Numb homolog (NUMB) in colorectal cancer (CRC).
METHODS: Real-time quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) and PCR-restriction fragment length polymorphism analyses were performed to detect the expression of VEGFA, APP, and NUMB mRNA in 20 CRC tissues and matched adjacent normal tissues, as well as their alternative splicing variants.
RESULTS: qRT-PCR analysis revealed that the expression of APP, NUMB, and VEGFA165b mRNA were significantly downregulated, while VEGFA mRNA was upregulated, in CRC tissues (all P < 0.05). PCR-restriction fragment length polymorphism analysis revealed that the expression of VEGFA165a/b in CRC tissues was significantly higher than in adjacent normal tissues (P < 0.05). Compared with adjacent normal tissues, the expression of NUMB-PRRS in CRC tissues was significantly decreased (P < 0.05), and the expression of NUMB-PRRL was increased (P < 0.05).
CONCLUSION: Alternative splicing of VEGFA, APP, and NUMB may regulate the development of CRC, and represent new targets for its diagnosis, prognosis, and treatment.
Keywords: Alternative splicing, Amyloid beta precursor protein, Colorectal cancer, Numb homolog, Vascular endothelial growth factor A
Core tip: This study was undertaken to investigate the effect of alternative splicing of vascular endothelial growth factor A (VEGFA), amyloid beta precursor protein (APP), and Numb homolog (NUMB) genes in colorectal cancer (CRC). We demonstrated that these genes and their alternative splice variants were different in CRC tissues and matched adjacent normal tissues using quantitative reverse transcriptase PCR and PCR-restriction fragment length polymorphism analyses. We conclude that alternative splicing of VEGFA, APP, and NUMB may be associated with the diagnosis, prognosis, and treatment of CRC. This study may help in understanding the relationship between alternative splicing and CRC.
INTRODUCTION
Alternative splicing (AS), through which different mRNA variants are produced from the splicing of a single gene[1], is a pivotal step in the generation of proteomic and functional diversity. At least 95% of human genes are predicted to generate alternatively spliced transcripts[2,3]. AS is performed by the spliceosome, which is composed of four small nuclear ribonucleoproteins (snRNPs; U1, U2, U4/U6, and U5) and a number of non-snRNP auxiliary proteins. There are several basic patterns of AS: exon skipping (cassette alternative exon), intron retention, mutually exclusive, alternative 5′SS, alternative 3′SS, alternative initiation sites, and alternative polyadenylation sites. AS is regulated by the interaction between cisregulatory sequences and transacting factors. Cisregulatory sequences include exonic and intronic splicing enhancers, as well as exonic and intronic splicing silencers. Trans-acting factors function by binding to splicing enhancers and silencers and include members of Ser/Argrich and heterogeneous nuclear (hn) RNP protein families. Ser/Argrich protein families are generally considered positive regulators of AS, but not all of them[4]. Similarly, not all hnRNP protein family members are negative regulators[5]. Previous research has demonstrated that AS and the RNA binding proteins and other factors which regulate this process are often disordered in human diseases, including cancers, spinal muscular atrophy, tauopathies, Hutchinson-Gilford progeria syndrome, hypercholesterolemia, familial dysautonomia, and frontotemporal lobar degeneration[6-14].
Due to its roles in promoting proliferation[15], increasing microvascular permeability[16], and inducing neovascularization, vascular endothelial growth factor A (VEGFA) is always associated with cancers, and is involved in tumor vascularity, metastasis development, and recurrence. Similarly, the expression of amyloid beta precursor protein (APP) is correlated with cell adhesion, motility, and proliferation. In addition, Numb (Drosophila melanogaster) homolog (NUMB) is a membrane-associated protein that plays critical roles in asymmetric cell division and is regarded as a determinant of cell fate. Previous studies have used AS microarray profiling and reverse transcriptase (RT)-PCR assays to detect enormously profiled alternative exons and demonstrated the relationship between the AS of VEGFA, APP, and NUMB genes and cancer, especially lung, breast, and colon tumors[10]. However, the definite mechanisms of AS underlying these genes have not been fully elucidated.
Colorectal cancer (CRC) is the third most common cancer and the third leading cause of cancer death in men and women[17]. Due to the improvement in living standards and changes in diet structure, CRC morbidity and mortality rates have increased rapidly year by year. Although the exact etiology and mechanism of CRC is unclear, high-risk factors, such as a higher intake of meat, fat, calcium, and vitamin D and lower intake of fiber, fruit, and vegetables, are associated with CRC[18]. Early stage CRC almost always has no symptoms and is difficult to diagnose accurately. When symptoms and metastasis appear, treatment becomes more difficult, and a complete cure may be impossible. Thus, improved efficiency in early diagnosis and treatment is crucial.
In this study, we performed quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) and PCR-restriction fragment length polymorphism (RFLP) analyses to determine AS changes in matched normal and tumor tissues from CRC patients. Our results reveal that AS of VEGFA, APP, and NUMB were closely related to CRC. These findings provide evidence that AS may contribute to the development of CRC and these markers may have potential clinical value.
MATERIALS AND METHODS
Patient samples
Matched pairs of primary tumor and adjacent normal tissue samples were obtained from 40 patients with CRC who underwent resection at the Department of Surgery, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital from 2012 through 2014. Six cases had synchronous hepatic metastases; the primary tumor and hepatic tumors were simultaneously resected during surgery. Postoperative pathology confirmed that all CRC and hepatic metastases samples were tubular adenocarcinoma. The pathologic stages, depth, histology, and lymphatic invasion of the primary tumors are shown in Table 1. None of the patients had received preoperative radiotherapy or chemotherapy. All tissue samples were flash frozen within 30 min of surgical removal and stored at -80 °C until further use.
Table 1.
Patient characteristics
| Characteristics | n (%) |
| Sex | |
| Male | 21 (52.5) |
| Female | 19 (47.5) |
| Primary tumor location | |
| Ascending colon | 5 (12.5) |
| Transverse colon | 7 (17.5) |
| Sigmoid colon | 15 (37.5) |
| Rectum | 13 (32.5) |
| TNM stage | |
| I | 7 (17.5) |
| II | 14 (35.0) |
| III | 13 (32.5) |
| IV | 6 (15.0) |
| T factor | |
| T1 | 3 (7.5) |
| T2 | 7 (17.5) |
| T3 | 11 (27.5) |
| T4 | 19 (47.5) |
| Lymphatic invasion | |
| N0 | 19 (47.5) |
| N1 | 9 (22.5) |
| N2 | 12 (30.0) |
| Distant metastasis | |
| M0 | 34 (85.0) |
| M1 | 6 (15.0) |
TNM: Tumor-node-metastasis.
RNA extraction and qRT-PCR
We performed qRT-PCR to estimate the expression of VEGFA, APP, NUMB, and VEGFA165b. The mucosal scrapes (30 mg) were homogenized for RNA extraction. RNA was extracted using a total RNA extraction kit (SLNCO, China), followed by RT using a qPCR-RT kit (Toyobo Co., Ltd., Osaka, Japan). cDNA was then evaluated by qRT-PCR using Real-Time PCR Master Mix (Toyobo Co., Ltd.) in a FTC-3000 PCR Cycler (Funglyn Biotech, Inc., Scarborough, ON, Canada) using the primers (Generay Biotech Co., Ltd., Shanghai, China) listed in Table 2. Denaturation, annealing, and extension temperatures were set at 94 °C, 61 °C, and 72 °C for 30 s each, respectively, for 40 cycles according to routine procedures.
Table 2.
Primers for quantitative reverse transcription-polymerase chain reaction
| Gene | Primers | Sequence (5'-3') | Products (bp) |
| GAPDH | Forward | TGAAGGTCGGAGTCAACGGA | 225 |
| Reverse | CCTGGAAGATGGTGATGGGAT | ||
| VEGFA | Forward | TTGCTGCTCTACCTCCACCAT | 270 |
| Reverse | GGTGATGTTGGACTCCTCAGTG | ||
| APP | Forward | GGCGGAGCAGACACAGACTA | 131 |
| Reverse | ACCTCATCACCATCCTCATCGT | ||
| NUMB | Forward | GCTGGATCTGTCACTGCTTCAT | 118 |
| Reverse | CCACATTCCTTCTCCCGCTTC | ||
| VEGFA165b | Forward | TCAGAGCGGAGAAAGCATTTGT | 130 |
| Reverse | TCCTGGTGAGAGATCTGCAAGT |
PCR-RFLP
PCR-RFLP analysis was performed to detect the expression of VEGFA, APP, and NUMB AS variants. RNA extracted from mucosal tissues served as a template for amplification of these genes using the total RNA extraction kit (SLNCO, China). The RT reaction using the ReverTra Ace qPCR RT kit (Toyobo Co., Ltd.) contained 8 μL H2O, 6 μL total RNA, 4 μL 5× RT buffer, 1 μL enzyme mix, and 1 μL RT primer, and was reacted at 42 °C for 18 min following inactivation of reverse transcriptase at 98 °C for 5 min. The PCR reaction included 7.2 μL RNase-free H2O, 10 μL 2× Taq Master Mix, 0.4 μL forward primer, 0.4 μL reverse primer, and 2 μL cDNA. The 5′ ends of the forward primers (Generay Biotech Co., Ltd.) were labeled with the fluorescent molecule, 6-carboxyfluorescein, listed in Table 3. The annealing temperatures of APP, NUMB, and VEGFA were 55 °C, 57 °C, and 58 °C, respectively. Denaturation and extension temperatures were set at 94 °C for 90 s and 72 °C for 60 s, respectively, for 38 cycles according to routine procedures. Amplified PCR products were verified by electrophoresis using a 1.5% agarose gel and detected by capillary electrophoresis (ABI3730XL sequencer, Applied Biosystems of Thermo Fisher Scientific, Waltham, MA, United States). The fragment peak size, area, and height were determined by GeneMapper (Applied Biosystems).
Table 3.
Primers for polymerase chain reaction-restriction fragment length polymorphism
| Gene | Primers | Sequence (5'-3') | Products (bp) |
| GAPDH | Forward | FAM-TGAAGGTCGGAGTCAACGGA | 225 |
| Reverse | CCTGGAAGATGGTGATGGGAT | ||
| VEGFA | Forward | FAM-TGAGCTTCCTACAGCACAAC | 206/338/410 |
| Reverse | TCGATGGTGATGGTGTGGTG | ||
| APP | Forward | FAM-CCTACGAAGAAGCCACAGAG | 162/330/387 |
| Reverse | GGGCATGTTCATTCTCATCC | ||
| NUMB | Forward | FAM-TGCTCCGATGACCAAACCAG | 157/301 |
| Reverse | CACCTCTTCTAACCATCGGTC |
Statistical analysis
Real-time qRT-PCR assays were performed in triplicate. Data are presented as the mean ± SE for three or more independent experiments. The differences in APP, NUMB, and VEGFA mRNA levels and their AS variants, between tumor and adjacent normal tissue were compared using a paired t test. Differences in mRNA expression levels in tissues inside and outside the serosal layer, tissues with and without lymph node metastasis, tissues in Tumor-node-metastasis (TNM) stages were compared by monofactor ANOVA analysis (GraphPad version 5.01, GraphPad Software, Inc., La Jolla, CA, United States). P < 0.05 was considered statistically significant. The statistical methods of this study were reviewed by Yi-Jun Zhao, Hua-Zhong Han, Chen-Zhang Shi, Qing-Chao Zhu, and Jun Yang from Department of Surgery, Sixth People’s Hospital affiliated to Shanghai Jiao Tong University.
RESULTS
Different expression levels of VEGFA, APP, and NUMB mRNA between tumor and adjacent normal tissues
Compared with controls, the relative quantitative value of VEGFA mRNA was significantly higher in CRC tissues (P < 0.01), and the expression of APP and NUMB mRNAs were significantly lower (P < 0.01). The expression of VEGFA, APP, and NUMB mRNA was not correlated with the depth of tumor infiltration, the presence of lymph node metastasis, or the TNM stage (Figures 1, 2 and 3).
Figure 1.
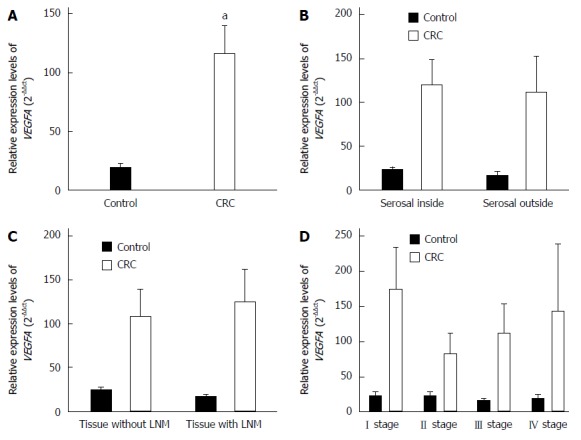
Expression levels of VEGFA mRNA assessed by quantitative reverse transcription-polymerase chain reaction. A: Colorectal cancer (CRC) tissues and normal intestinal mucosa tissues; B: Tissues inside and outside the serosal layer; C: Tissues with and without lymph node metastasis (LNM); D: Tissues of tumor-node-metastasis (TNM) stages. aP < 0.05 vs control.
Figure 2.
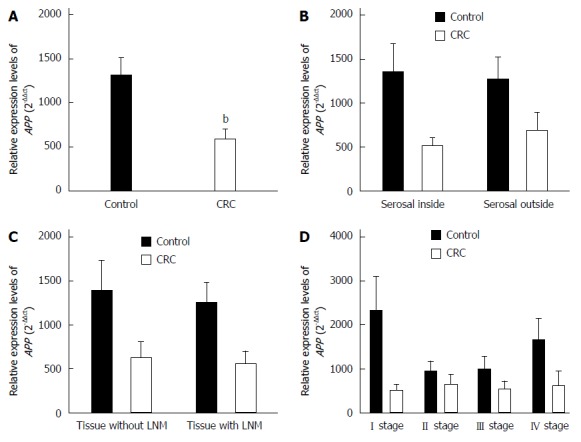
Expression levels of APP mRNA assessed by quantitative reverse transcription-polymerase chain reaction. A: Colorectal cancer (CRC) tissues and normal intestinal mucosa tissues; B: Tissues inside and outside the serosal layer; C: Tissues with and without lymph node metastasis (LNM); D: Tissues of tumor-node-metastasis (TNM) stages. bP < 0.01 vs control.
Figure 3.

Expression levels of NUMB mRNA assessed by quantitative reverse transcription-polymerase chain reaction. A: Colorectal cancer (CRC) tissues and normal intestinal mucosa tissues; B: Tissues inside and outside the serosal layer; C: Tissues with and without lymph node metastasis (LNM); D: Tissues of tumor-node-metastasis (TNM) stages. bP < 0.01 vs control.
Expression of VEGFA AS variants
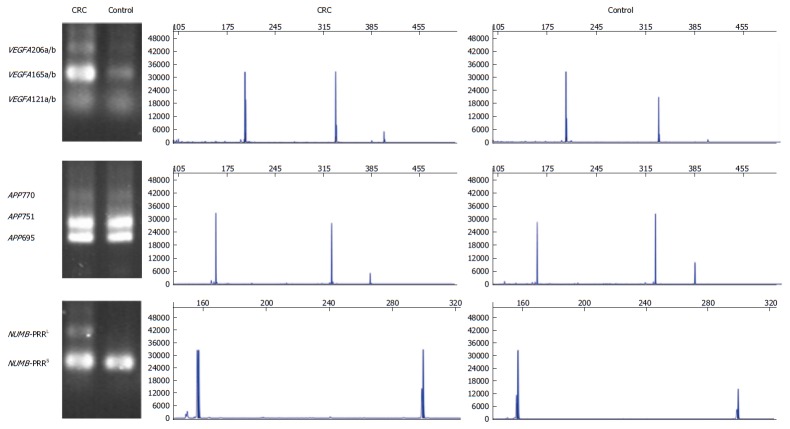
VEGFA amplified gene fragments of 206 bp, 338 bp, and 410 bp represent VEGFA121a/b, VEGFA165a/b, and VEGFA206a/b, respectively. PCR-RFLP analysis revealed that the expression of VEGFA AS variant VEGFA165a/b in CRC tissues was significantly higher than that in adjacent normal tissues (P < 0.05), the expression of VEGFA AS variant VEGFA121a/b was not significantly different between CRC tissues and adjacent normal tissues, and the expression of VEGFA AS variant VEGFA206a/b was only found in a few samples (Figures 4 and 5).
Figure 4.
Electrophoregram of alternative splicing variants in VEGFA, APP and NUMB. CRC: Colorectal cancer.
Figure 5.
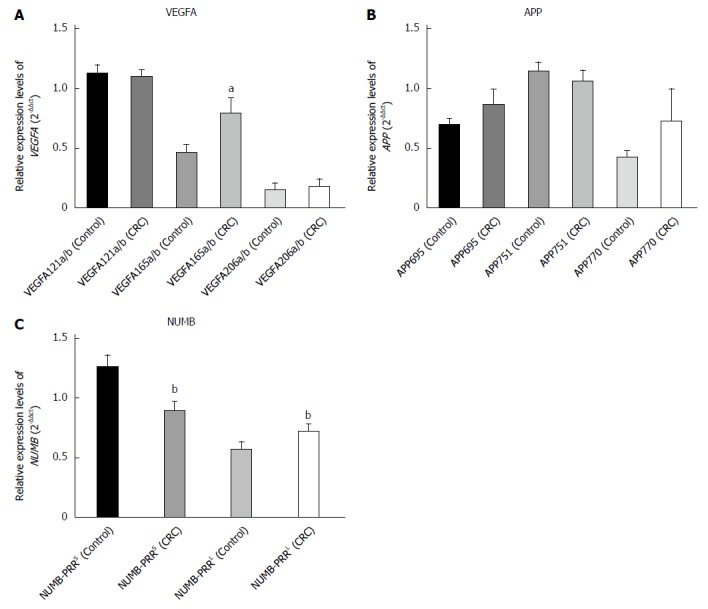
Expression levels of alternative splice variants using polymerase chain reaction-restriction fragment length polymorphism analysis. Expression levels of A: VEGFA; B: APP; and C: NUMB alternative splice variants in colorectal cancer (CRC) tissues and normal intestinal mucosa tissues. aP < 0.05 and bP < 0.01 vs the control group.
Expression of APP AS variants
APP generated amplicons of 162 bp, 330 bp, and 387 bp represent APP770, APP751, and APP695, respectively. We detected three APP AS variants named APP695, APP751 and APP770, which were not significantly different between CRC tissues and adjacent normal tissues (Figures 4 and 5).
Expression of NUMB AS variants
The products of 157 bp and 301 bp represent NUMB-PRRS and NUMB-PRRL. The expression of NUMB AS variant NUMB-PRRS in CRC tissues was significantly lower than that in normal intestinal mucosa tissues (P < 0.05), and the expression of NUMB AS variant NUMB-PRRL in CRC tissues was significantly higher than that in normal intestinal mucosa tissues (P < 0.05) (Figures 4 and 5).
Decreased VEGFA165b expression in CRC tissues
qRT-PCR analysis revealed that the expression of VEGFAl65b in CRC tissues was significantly lower than that in normal intestinal mucosa tissues (P < 0.05). The expression of VEGFA165b was not correlated with depth of tumor infiltration, the presence of lymph node metastasis, or TNM stage (Figure 6).
Figure 6.
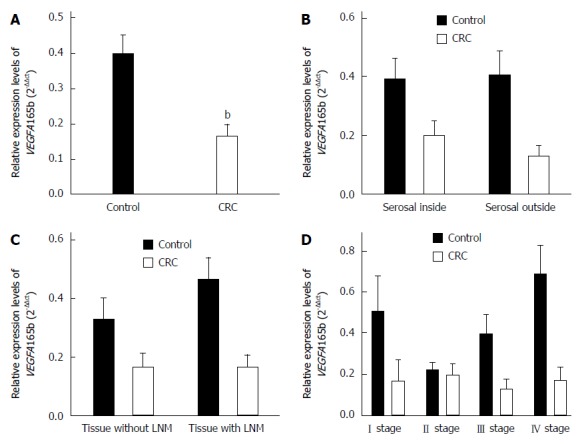
Expression levels of VEGFA165b assessed by quantitative reverse transcription-polymerase chain reaction. Expression levels of VEGFA165b in A: Colorectal cancer (CRC) tissues and normal intestinal mucosa tissues; B: Tissues inside and outside the serosal layer; C: Tissues with and without lymph node metastasis (LNM); and D: Tissues of tumor-node-metastasis (TNM) stages. bP < 0.01 vs control.
DISCUSSION
Previous studies have confirmed that the expression of VEGFA correlates with vascularity, metastasis, and proliferation of CRC[19]. The VEGFA gene consists of eight exons separated by seven introns, including three alternative exons (6, 7 and 8), and thus is composed of multiple isoforms. Due to alternative selection of the 3′ splice site in exon 8 resulting in a six amino acid substitution (Cys-Asp-Lys-Pro-Arg-Arg to Ser-Leu-Thr-Arg-Lys-Asp), VEGFA isoforms are classified into VEGFAxxx (pro-angiogenic) and VEGFAxxxb (anti-angiogenic), where xxx denotes the amino acid number[20]. AS of VEGFA exons 6 and 7 generates isoforms including VEGFA121a/b, VEGFA165a/b, and VEGFA206a/b. Cheung et al[21] found that VEGFA121a/b and VEGFA165a/b represented a proportion of the total VEGFA in both lung and colon samples including normal and tumor tissues, however, VEGFA206a/b was not detected in any samples. The protein product of VEGFA121a/b is unable to bind to heparin, whereas VEGFA165a/b and VEGFA206a/b are heparin-binding isoforms[22,23]. As previous studies demonstrated that VEGFA121a/b, VEGFA165a/b, and VEGFA206a/b are closely associated with melanoma[24,25], lung cancer[26,27], colon cancer[21], breast cancer[28], ovarian cancer[29], prostate cancer[30], and bladder cancer[31], our study mainly focused on the expression of these isoforms in CRC. Based on the results of qRT-PCR and PCR-RFLP analyses, we found significant upregulation of VEGFA in CRC tissues, with VEGFA121a/b and VEGFA165a/b being the predominant isoforms, and VEGFA206a/b only detected in a few samples. The upregulation of VEGFA and VEGFA165a/b in CRC tissues is suggestive of their role in promoting the development of CRC. Furthermore, we determined the expression of AS isoform VEGFA165b and found that it was downregulated in CRC tissues using qRT-PCR. However, the expressions of VEGFA and VEGFA165b were not significantly associated with depth of infiltration, lymphatic invasion, or TNM stage, which was likely due to an insufficient number of samples. VEGFA165b functions as a tumor suppressor of CRC. Due to AS of exon 8, the residual two arginines of VEGFA165b are substituted by lysine and aspartic acid, resulting in loss of one disulfide bond and the inability of VEGFA165b to bind the vascular endothelial growth factor receptor 2 and neuropilin 1. The properties of VEGFA165b generate inefficient autophosphorylation of the receptor, and account for a series of changes in the downstream pathway. With regard to the major anti-angiogenic isoform, VEGFA165b was identified in diverse normal and abnormal human tissues including various tumors[32-35]. It was previously reported that VEGFA165b is downregulated in several human cancers, and its overexpression delayed the growth of these cancers[32,35-37]. Recombinant human VEGFA165b (rhVEGF165b) treatment in vivo has an inhibitory effect on growth; consequently, VEGFA165b is deemed a potential anti-cancer target. In addition, the VEGFAxxx/VEGFAxxxb ratio affects the sensitivity of tumors to bevacizumab, and VEGFAxxxb can inhibit the effect of bevacizumab by competitive binding[35]. Hence, bevacizumab may be a feasible treatment by increasing the amount of VEGFAxxxb or decreasing the amount of VEGFAxxx. In brief, the differential expression of VEGFA isoforms, including VEGF165a and VEGF165b in CRC, appears to be a possible prognostic factor and potential target in the treatment of CRC.
APP is a Type I integral and widely expressed membrane protein that normally functions in neuroprotection and neurite outgrowth[38]. As a result, APP is considered a crucial step in the molecular cascade of events resulting in the pathogenesis of Alzheimer’s disease[39]. Previous research has demonstrated that the expression of APP is associated with cell adhesion, motility, and proliferation. Increased expression in prostate, pancreatic, thyroid, and oral squamous cell cancers showed that APP promotes the growth of these cancers[40-43]. In our research, the expression of APP decreased in CRC tissues, which indicates that APP may be involved in the regulation of proliferation and invasion. The reduced expression of APP leads to increased activity of tyrosine kinases, a change in p53-associated cell apoptosis signaling pathways, reduced cell adhesion, and increased liquidity, which are linked to cell proliferation and tumorigenesis[44,45]. The differential expression of APP was the result of the properties of the cancers compared to CRC. However, the expression of APP was not significantly associated with depth of infiltration, lymphatic invasion, or TNM stage, likely due to an insufficient number of samples. APP contains 19 exons separated by 18 introns and encodes different isoforms according to differential splicing of alternative exons 7, 8, and 15[46]. Due to the AS of exons 7 and 8, APP can be spliced to produce isoforms such as APP695 (skipped exons 7 and 8), APP751 (skipped exon 8), and APP770 (full-length). The 751 and 770 isoforms contain a 56-amino acid Kunitz-type protease inhibitor domain, whereas APP695 excludes this region. Ko et al[41] demonstrated that APP770 and APP751, but not APP695, were upregulated in oral keratinocytes and oral squamous cell carcinoma. Our results showed that APP695, APP751, and APP770 were not significantly different between CRC tissues and adjacent normal tissues, likely due to an insufficient number of samples. Consequently, the relationship between the isoforms of APP and CRC still need to be determined in the future.
NUMB contains an amino-terminal phosphotyrosine-binding (PTB) domain and C-terminal proline-rich (PRR) and Eps15 homology regions. Related research has shown that NUMB was involved in inhibiting the development of cancer through the suppression of epithelial-mesenchymal transition, and Notch and Hedgehog pathways, as well as activation of the p53 gene[47-50]. In our research, the expression of NUMB in CRC tissues was significantly lower than that in adjacent normal intestinal mucosa tissues, which suggests that NUMB may have a role in inhibiting the development of CRC. However, the expression of NUMB was not significantly associated with depth of infiltration, lymphatic invasion, or TNM stage, due to an insufficient number of samples. Due to the AS of exons 3 and 9, generating two splicing variant sites in the PTB and PRR domains, NUMB gives rise to four alternatively spliced isoforms, PTBLPRRL (p72), PTBSPRRL (p71), PTBLPRRS (p66), and PTBSPRRS (p65). Previous studies have provided evidence that exon 3 is not significantly different between patient tumor and normal samples in non-small cell lung cancer[10]. Thus, our research focused mainly on the AS of exon 9, which is the distinction between NUMB-PRRL (PTBLPRRL and PTBSPRRL, exon 9-included) and NUMB-PRRS (PTBLPRRS and PTBSPRRS, exon 9-skipped). Our results show that NUMB-PRRL splice variants are specifically expressed at elevated levels in CRC tissues. In contrast, the expression of the NUMB-PRRS splice variant was reduced in CRC tissues. Combined with the increase in NUMB expression in CRC tissues, we suggest that NUMB-PRRS may have a role in inhibiting the development of CRC, while NUMB-PRRL may have a role in promoting the development of CRC. NUMB-PRRS appears to be more functional than NUMB-PRRL, and consequently, NUMB mRNA plays a role in inhibiting the development of CRC. Therefore, the PRR region encoded by exon 9 is closely correlated with the development of CRC. The increased level of NUMB-PRRL results in reduced levels of overall NUMB with subsequent Notch activation. It is also possible that NUMB-PRRL plays a dominant role in suppressing the Notch inhibitory activity of NUMB-PRRS[10]. It has been reported that increased levels of NUMB-PRRL may promote cell proliferation, whereas NUMB-PRRS mainly has an important role in promoting cell differentiation[51,52]. A study has provided evidence that the splicing regulators, Nova and Fox2, act in a dominant manner in the regulation of NUMB exon 9 AS[13,53]. However, the mechanism is unclear, and further studies are essential.
In summary, AS in VEGFA, APP, and NUMB is closely associated with CRC, however, most cancer-associated AS events have not been functionally characterized on any level, and many others remain to be detected. APP functions as a tumor suppressor at the integral level, whereas VEGFA and NUMB play crucial roles in the development of cancer at the integral and individual level. The AS in VEGFA, APP, and NUMB probably provides a possible prognostic factor and potential therapeutic target for CRC. Investigations of these genes at the protein level and their mechanism of action in CRC are urgently needed.
COMMENTS
Background
Alternative splicing (AS) is a pivotal step in the generation of proteomic and functional diversity. Previous research has provided evidence that AS and the RNA binding proteins and other factors, which regulate this process, are often disordered in cancers and other human diseases. Although the link between AS and human diseases has been established, there is little understanding of the effects of AS in the development of colorectal cancer (CRC). Therefore, this study was undertaken to explore the effect of AS of vascular endothelial growth factor A (VEGFA), amyloid beta (A4) precursor protein (APP) and the Numb (Drosophila melanogaster) homolog (NUMB) genes in CRC.
Research frontiers
AS is an important post-transcriptional regulatory mechanism and it has been reported to be associated with human disease. The current research hotspots are the exact biologic function, precise regulation and mechanism of AS in human disease, as well as the effects of signal pathways, and the factors in human disease and gene therapies that are based on AS.
Innovations and breakthroughs
Previous research has provided evidence that AS is associated with human diseases, however, there is still little understanding concerning the effects of AS in the development of CRC. In this study, molecular biologic technology was used to detect the expression of VEGFA, APP, and NUMB in CRC, as well as their AS variants. The authors demonstrated the important role of AS in regulating the expression of VEGFA, APP, and NUMB. These findings are crucial in providing gene therapy strategies for CRC.
Applications
This study demonstrates that AS of VEGFA, APP, and NUMB is closely associated with CRC and could be used to provide possible prognostic factors and potential therapeutic targets for CRC.
Terminology
AS, through which diverse mRNA variants are produced from the splicing of a single gene, is a pivotal step in the generation of proteomic and functional diversity. Regarded as an important regulatory mechanism between transcription and translation, AS affects nearly 95% of mammalian genes and multiple regulatory processes. The process of AS is performed by the spliceosome and regulated by the interaction between cisregulatory sequences and transacting factors. Aberrant regulation of AS results in multiple human diseases, including various cancers.
Peer-review
This is a good study in which authors investigated the relationship between alternative splicing of VEGFA, APP, and NUMB and CRC. The results are reliable and suggest that there is a significant role of alternative splicing of VEGFA, APP, and NUMB in the development of CRC. This study represents a further step in understanding the mechanisms involved in this relationship.
Footnotes
Ethics approval: The study was reviewed and approved by the Institutional Review Board of the Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University.
Conflict-of-interest: The authors declare that they have no conflicts of interest related to this work.
Data sharing: Technical appendix, statistical code, and dataset available from the corresponding author at yangjun_sh@163.com. Participants gave informed consent for data sharing.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: November 24, 2014
First decision: January 8, 2015
Article in press: March 12, 2015
P- Reviewer: Huang ZH, Ramia J, Seicean A, Tomizawa M, Yu Z S- Editor: Qi Y L- Editor: AmEditor E- Editor: Ma S
References
- 1.Kornblihtt AR, Schor IE, Alló M, Dujardin G, Petrillo E, Muñoz MJ. Alternative splicing: a pivotal step between eukaryotic transcription and translation. Nat Rev Mol Cell Biol. 2013;14:153–165. doi: 10.1038/nrm3525. [DOI] [PubMed] [Google Scholar]
- 2.Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat Genet. 2008;40:1413–1415. doi: 10.1038/ng.259. [DOI] [PubMed] [Google Scholar]
- 3.Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, Kingsmore SF, Schroth GP, Burge CB. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008;456:470–476. doi: 10.1038/nature07509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cowper AE, Cáceres JF, Mayeda A, Screaton GR. Serine-arginine (SR) protein-like factors that antagonize authentic SR proteins and regulate alternative splicing. J Biol Chem. 2001;276:48908–48914. doi: 10.1074/jbc.M103967200. [DOI] [PubMed] [Google Scholar]
- 5.Martinez-Contreras R, Fisette JF, Nasim FU, Madden R, Cordeau M, Chabot B. Intronic binding sites for hnRNP A/B and hnRNP F/H proteins stimulate pre-mRNA splicing. PLoS Biol. 2006;4:e21. doi: 10.1371/journal.pbio.0040021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tazi J, Bakkour N, Stamm S. Alternative splicing and disease. Biochim Biophys Acta. 2009;1792:14–26. doi: 10.1016/j.bbadis.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klinck R, Bramard A, Inkel L, Dufresne-Martin G, Gervais-Bird J, Madden R, Paquet ER, Koh C, Venables JP, Prinos P, et al. Multiple alternative splicing markers for ovarian cancer. Cancer Res. 2008;68:657–663. doi: 10.1158/0008-5472.CAN-07-2580. [DOI] [PubMed] [Google Scholar]
- 8.Kim E, Goren A, Ast G. Alternative splicing and disease. RNA Biol. 2008;5:17–19. doi: 10.4161/rna.5.1.5944. [DOI] [PubMed] [Google Scholar]
- 9.Thorsen K, Sørensen KD, Brems-Eskildsen AS, Modin C, Gaustadnes M, Hein AM, Kruhøffer M, Laurberg S, Borre M, Wang K, et al. Alternative splicing in colon, bladder, and prostate cancer identified by exon array analysis. Mol Cell Proteomics. 2008;7:1214–1224. doi: 10.1074/mcp.M700590-MCP200. [DOI] [PubMed] [Google Scholar]
- 10.Misquitta-Ali CM, Cheng E, O’Hanlon D, Liu N, McGlade CJ, Tsao MS, Blencowe BJ. Global profiling and molecular characterization of alternative splicing events misregulated in lung cancer. Mol Cell Biol. 2011;31:138–150. doi: 10.1128/MCB.00709-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper TA, Wan L, Dreyfuss G. RNA and disease. Cell. 2009;136:777–793. doi: 10.1016/j.cell.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dutertre M, Vagner S, Auboeuf D. Alternative splicing and breast cancer. RNA Biol. 2010;7:403–411. doi: 10.4161/rna.7.4.12152. [DOI] [PubMed] [Google Scholar]
- 13.Venables JP, Klinck R, Koh C, Gervais-Bird J, Bramard A, Inkel L, Durand M, Couture S, Froehlich U, Lapointe E, et al. Cancer-associated regulation of alternative splicing. Nat Struct Mol Biol. 2009;16:670–676. doi: 10.1038/nsmb.1608. [DOI] [PubMed] [Google Scholar]
- 14.Kelemen O, Convertini P, Zhang Z, Wen Y, Shen M, Falaleeva M, Stamm S. Function of alternative splicing. Gene. 2013;514:1–30. doi: 10.1016/j.gene.2012.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 16.Karathanasis E, Chan L, Karumbaiah L, McNeeley K, D’Orsi CJ, Annapragada AV, Sechopoulos I, Bellamkonda RV. Tumor vascular permeability to a nanoprobe correlates to tumor-specific expression levels of angiogenic markers. PLoS One. 2009;4:e5843. doi: 10.1371/journal.pone.0005843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 18.Key TJ, Allen NE, Spencer EA, Travis RC. The effect of diet on risk of cancer. Lancet. 2002;360:861–868. doi: 10.1016/S0140-6736(02)09958-0. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi Y, Kitadai Y, Bucana CD, Cleary KR, Ellis LM. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res. 1995;55:3964–3968. [PubMed] [Google Scholar]
- 20.Harper SJ, Bates DO. VEGF-A splicing: the key to anti-angiogenic therapeutics? Nat Rev Cancer. 2008;8:880–887. doi: 10.1038/nrc2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheung N, Wong MP, Yuen ST, Leung SY, Chung LP. Tissue-specific expression pattern of vascular endothelial growth factor isoforms in the malignant transformation of lung and colon. Hum Pathol. 1998;29:910–914. doi: 10.1016/s0046-8177(98)90195-2. [DOI] [PubMed] [Google Scholar]
- 22.Ferrara N. The role of vascular endothelial growth factor in pathological angiogenesis. Breast Cancer Res Treat. 1995;36:127–137. doi: 10.1007/BF00666035. [DOI] [PubMed] [Google Scholar]
- 23.Zhang HT, Scott PA, Morbidelli L, Peak S, Moore J, Turley H, Harris AL, Ziche M, Bicknell R. The 121 amino acid isoform of vascular endothelial growth factor is more strongly tumorigenic than other splice variants in vivo. Br J Cancer. 2000;83:63–68. doi: 10.1054/bjoc.2000.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu JL, Rak JW, Klement G, Kerbel RS. Vascular endothelial growth factor isoform expression as a determinant of blood vessel patterning in human melanoma xenografts. Cancer Res. 2002;62:1838–1846. [PubMed] [Google Scholar]
- 25.Pritchard-Jones RO, Dunn DB, Qiu Y, Varey AH, Orlando A, Rigby H, Harper SJ, Bates DO. Expression of VEGF(xxx)b, the inhibitory isoforms of VEGF, in malignant melanoma. Br J Cancer. 2007;97:223–230. doi: 10.1038/sj.bjc.6603839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zygalaki E, Tsaroucha EG, Kaklamanis L, Lianidou ES. Quantitative real-time reverse transcription PCR study of the expression of vascular endothelial growth factor (VEGF) splice variants and VEGF receptors (VEGFR-1 and VEGFR-2) in non small cell lung cancer. Clin Chem. 2007;53:1433–1439. doi: 10.1373/clinchem.2007.086819. [DOI] [PubMed] [Google Scholar]
- 27.Matsuyama M, Chijiwa T, Inoue Y, Abe Y, Nishi M, Miyazaki N, Furukawa D, Mukai M, Suemizu H, Sekido Y, et al. Alternative splicing variant of vascular endothelial growth factor-A is a critical prognostic factor in non-small cell lung cancer. Oncol Rep. 2009;22:1407–1413. doi: 10.3892/or_00000582. [DOI] [PubMed] [Google Scholar]
- 28.Hyder SM, Chiappetta C, Stancel GM. Pharmacological and endogenous progestins induce vascular endothelial growth factor expression in human breast cancer cells. Int J Cancer. 2001;92:469–473. doi: 10.1002/ijc.1236. [DOI] [PubMed] [Google Scholar]
- 29.Stimpfl M, Tong D, Fasching B, Schuster E, Obermair A, Leodolter S, Zeillinger R. Vascular endothelial growth factor splice variants and their prognostic value in breast and ovarian cancer. Clin Cancer Res. 2002;8:2253–2259. [PubMed] [Google Scholar]
- 30.Catena R, Muniz-Medina V, Moralejo B, Javierre B, Best CJ, Emmert-Buck MR, Green JE, Baker CC, Calvo A. Increased expression of VEGF121/VEGF165-189 ratio results in a significant enhancement of human prostate tumor angiogenesis. Int J Cancer. 2007;120:2096–2109. doi: 10.1002/ijc.22461. [DOI] [PubMed] [Google Scholar]
- 31.Fauconnet S, Bernardini S, Lascombe I, Boiteux G, Clairotte A, Monnien F, Chabannes E, Bittard H. Expression analysis of VEGF-A and VEGF-B: relationship with clinicopathological parameters in bladder cancer. Oncol Rep. 2009;21:1495–1504. doi: 10.3892/or_00000380. [DOI] [PubMed] [Google Scholar]
- 32.Woolard J, Wang WY, Bevan HS, Qiu Y, Morbidelli L, Pritchard-Jones RO, Cui TG, Sugiono M, Waine E, Perrin R, et al. VEGF165b, an inhibitory vascular endothelial growth factor splice variant: mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res. 2004;64:7822–7835. doi: 10.1158/0008-5472.CAN-04-0934. [DOI] [PubMed] [Google Scholar]
- 33.Perrin RM, Konopatskaya O, Qiu Y, Harper S, Bates DO, Churchill AJ. Diabetic retinopathy is associated with a switch in splicing from anti- to pro-angiogenic isoforms of vascular endothelial growth factor. Diabetologia. 2005;48:2422–2427. doi: 10.1007/s00125-005-1951-8. [DOI] [PubMed] [Google Scholar]
- 34.Díaz R, Peña C, Silva J, Lorenzo Y, García V, García JM, Sánchez A, Espinosa P, Yuste R, Bonilla F, et al. p73 Isoforms affect VEGF, VEGF165b and PEDF expression in human colorectal tumors: VEGF165b downregulation as a marker of poor prognosis. Int J Cancer. 2008;123:1060–1067. doi: 10.1002/ijc.23619. [DOI] [PubMed] [Google Scholar]
- 35.Varey AH, Rennel ES, Qiu Y, Bevan HS, Perrin RM, Raffy S, Dixon AR, Paraskeva C, Zaccheo O, Hassan AB, et al. VEGF 165 b, an antiangiogenic VEGF-A isoform, binds and inhibits bevacizumab treatment in experimental colorectal carcinoma: balance of pro- and antiangiogenic VEGF-A isoforms has implications for therapy. Br J Cancer. 2008;98:1366–1379. doi: 10.1038/sj.bjc.6604308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rennel E, Waine E, Guan H, Schüler Y, Leenders W, Woolard J, Sugiono M, Gillatt D, Kleinerman E, Bates D, et al. The endogenous anti-angiogenic VEGF isoform, VEGF165b inhibits human tumour growth in mice. Br J Cancer. 2008;98:1250–1257. doi: 10.1038/sj.bjc.6604309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rennel ES, Hamdollah-Zadeh MA, Wheatley ER, Magnussen A, Schüler Y, Kelly SP, Finucane C, Ellison D, Cebe-Suarez S, Ballmer-Hofer K, et al. Recombinant human VEGF165b protein is an effective anti-cancer agent in mice. Eur J Cancer. 2008;44:1883–1894. doi: 10.1016/j.ejca.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haass C, Schlossmacher MG, Hung AY, Vigo-Pelfrey C, Mellon A, Ostaszewski BL, Lieberburg I, Koo EH, Schenk D, Teplow DB. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992;359:322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- 39.Yankner BA. Mechanisms of neuronal degeneration in Alzheimer’s disease. Neuron. 1996;16:921–932. doi: 10.1016/s0896-6273(00)80115-4. [DOI] [PubMed] [Google Scholar]
- 40.Hansel DE, Rahman A, Wehner S, Herzog V, Yeo CJ, Maitra A. Increased expression and processing of the Alzheimer amyloid precursor protein in pancreatic cancer may influence cellular proliferation. Cancer Res. 2003;63:7032–7037. [PubMed] [Google Scholar]
- 41.Ko SY, Lin SC, Chang KW, Wong YK, Liu CJ, Chi CW, Liu TY. Increased expression of amyloid precursor protein in oral squamous cell carcinoma. Int J Cancer. 2004;111:727–732. doi: 10.1002/ijc.20328. [DOI] [PubMed] [Google Scholar]
- 42.Krause K, Karger S, Sheu SY, Aigner T, Kursawe R, Gimm O, Schmid KW, Dralle H, Fuhrer D. Evidence for a role of the amyloid precursor protein in thyroid carcinogenesis. J Endocrinol. 2008;198:291–299. doi: 10.1677/JOE-08-0005. [DOI] [PubMed] [Google Scholar]
- 43.Takayama K, Tsutsumi S, Suzuki T, Horie-Inoue K, Ikeda K, Kaneshiro K, Fujimura T, Kumagai J, Urano T, Sakaki Y, et al. Amyloid precursor protein is a primary androgen target gene that promotes prostate cancer growth. Cancer Res. 2009;69:137–142. doi: 10.1158/0008-5472.CAN-08-3633. [DOI] [PubMed] [Google Scholar]
- 44.Cáceres J, Brandan E. Interaction between Alzheimer’s disease beta A4 precursor protein (APP) and the extracellular matrix: evidence for the participation of heparan sulfate proteoglycans. J Cell Biochem. 1997;65:145–158. doi: 10.1002/(sici)1097-4644(199705)65:2<145::aid-jcb2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 45.Sandhu FA, Kim Y, Lapan KA, Salim M, Aliuddin V, Zain SB. Expression of the C terminus of the amyloid precursor protein alters growth factor responsiveness in stably transfected PC12 cells. Proc Natl Acad Sci USA. 1996;93:2180–2185. doi: 10.1073/pnas.93.5.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ponte P, Gonzalez-DeWhitt P, Schilling J, Miller J, Hsu D, Greenberg B, Davis K, Wallace W, Lieberburg I, Fuller F. A new A4 amyloid mRNA contains a domain homologous to serine proteinase inhibitors. Nature. 1988;331:525–527. doi: 10.1038/331525a0. [DOI] [PubMed] [Google Scholar]
- 47.Wang Z, Sandiford S, Wu C, Li SS. Numb regulates cell-cell adhesion and polarity in response to tyrosine kinase signalling. EMBO J. 2009;28:2360–2373. doi: 10.1038/emboj.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng X, Huber TL, Chen VC, Gadue P, Keller GM. Numb mediates the interaction between Wnt and Notch to modulate primitive erythropoietic specification from the hemangioblast. Development. 2008;135:3447–3458. doi: 10.1242/dev.025916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ho L, Alman B. Protecting the hedgerow: p53 and hedgehog pathway interactions. Cell Cycle. 2010;9:506–511. doi: 10.4161/cc.9.3.10552. [DOI] [PubMed] [Google Scholar]
- 50.Colaluca IN, Tosoni D, Nuciforo P, Senic-Matuglia F, Galimberti V, Viale G, Pece S, Di Fiore PP. NUMB controls p53 tumour suppressor activity. Nature. 2008;451:76–80. doi: 10.1038/nature06412. [DOI] [PubMed] [Google Scholar]
- 51.Bani-Yaghoub M, Kubu CJ, Cowling R, Rochira J, Nikopoulos GN, Bellum S, Verdi JM. A switch in numb isoforms is a critical step in cortical development. Dev Dyn. 2007;236:696–705. doi: 10.1002/dvdy.21072. [DOI] [PubMed] [Google Scholar]
- 52.Verdi JM, Bashirullah A, Goldhawk DE, Kubu CJ, Jamali M, Meakin SO, Lipshitz HD. Distinct human NUMB isoforms regulate differentiation vs. proliferation in the neuronal lineage. Proc Natl Acad Sci USA. 1999;96:10472–10476. doi: 10.1073/pnas.96.18.10472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang C, Frias MA, Mele A, Ruggiu M, Eom T, Marney CB, Wang H, Licatalosi DD, Fak JJ, Darnell RB. Integrative modeling defines the Nova splicing-regulatory network and its combinatorial controls. Science. 2010;329:439–443. doi: 10.1126/science.1191150. [DOI] [PMC free article] [PubMed] [Google Scholar]