Abstract
AIM: To determine the therapeutic efficacy of resveratrol on ulcerative colitis (UC) and its underlying mechanisms.
METHODS: The mouse UC model was developed using 5% dextran sulfate sodium. Mice were randomly divided into four groups: normal control, UC model group, resveratrol low-dose group (RLD; 50 mg/kg per day), and resveratrol high-dose group (RHD; 100 mg/kg per day).
RESULTS: The results showed that RLD regulates Treg/Th17 balance mainly through reducing the number of Th17 cells, whereas RHD regulates Treg/Th17 balance through both downregulating the number of Th17 cells and upregulating the number of Treg cells. Resveratrol can also regulate the level of plasma and intestinal mucosal cytokines including interleukin (IL)-10, transforming growth factor-β1, IL-6, and IL-17. The expressions of hypoxia inducible factor (HIF)-1α, mammalian target of rapamycin (mTOR), and signal transducer and activator of transcription 3 were significantly decreased in the intestinal tissues of mice treated with resveratrol.
CONCLUSION: The therapeutic efficacy of resveratrol in UC is dose dependent and closely associated with the regulation of Treg/Th17 balance and the HIF-1α/mTOR signaling pathway.
Keywords: Hypoxia inducible factor-α, Mammalian target of rapamycin, Resveratrol, Th17 cells, Treg cells, Ulcerative colitis
Core tip: Ulcerative colitis is an inflammatory bowel disease manifested by recurrent chronic diarrhea, bloody and mucous stools, and abdominal pain. The etiology and pathogenesis are not clear. Resveratrol is a natural and biologically active polyphenol with a variety of anti-inflammatory and antioxidant functions, which are beneficial to human health. This study aimed determine the therapeutic efficacy of resveratrol on ulcerative colitis and its underlying mechanisms. The results demonstrate that the therapeutic efficacy of resveratrol is dose dependent and closely associated with the regulation of Treg/Th17 balance and the hypoxia inducible factor-1α/mammalian target of rapamycin signaling pathway.
INTRODUCTION
Ulcerative colitis (UC) is a chronic nonspecific inflammatory disease of unknown cause involving the rectum and colon. It was hypothesized that UC is associated with genetic, infectious, immune, and environmental factors and intestinal dysbiosis[1]. In the early stage of UC, colonic mucosa lesions with visible diffuse inflammation, mucosal congestion, and edema can be observed. In severe cases, there are visible focal hemorrhages, and the tissues become brittle and bleed easily. In the acute phase of UC, there is an infiltration of lymphocytes, eosinophils, and neutrophils[2]. UC animal models can be developed by feeding the mice 5% DSS for a consecutive 7 d. After 4-7 d of DSS feeding, mice develop loose stools, bloody diarrhea, and weight loss with histologic changes similar to UC[3].
Previous studies have shown that abnormal intestinal mucosal immune response and inflammation disorders are present in UC and are closely associated with the imbalance of Treg and Th17 cells and disorders of cytokine levels[4]. Th17 cells affect the innate and acquired immune responses through the release of interleukin (IL)-17 and other inflammatory cytokines that are involved in the immune pathogenesis and prognosis of inflammatory bowel disease (IBD)[5]. Imbalances in Treg/Th17 and their secreted cytokines, e.g., IL-10, transforming growth factor (TGF)-β1, IL-17, and IL-6, play important roles in the development of IBD[4]. In the past 10-15 years, it was generally believed that Crohn’s disease is a Th1 cell-mediated intestinal inflammation, whereas UC is a Th2 cell-mediated inflammatory response[6]. Recently, Th17 cells were recognized as a new subset of helper T cells, and are closely associated with autoimmune diseases and IBD. Discovery of Th17 cells helps to explain some unusual phenomenon in Th1/Th2 responses in IBD[7]. Current evidence suggests that IBD is closely associated with Treg/Th17 imbalance[8,9]. Hypoxia-mammalian target of rapamycin (mTOR)-hypoxia inducible factor (HIF)-1α-Th17 and IL-6-signal transducer and activator of transcription (STAT)3-HIF-1α-Th17 pathways play important roles in Th17 development and the activation of IL-17 production. In addition, HIF-1α can bind Foxp3, leading to the accelerated degradation Foxp3, thus affecting Treg development and function[10]. Previous studies demonstrated that Treg/Th17 imbalance is present in many inflammatory and autoimmune diseases, e.g., rheumatoid arthritis and systemic lupus erythemoatosus[11-13].
Resveratrol is a naturally occurring active ingredient that is present in grapes, peanuts, and other plants. Resveratrol contains a variety of biologic activities, including immune regulation, anti-inflammation, antioxidation, antiangiogenesis, and reduction of tissue damage[14,15]. Our previous studies demonstrated that resveratrol exhibits anti-inflammatory effects on colitis in mice via antioxidant activities[14]. Recently, it has been shown that resveratrol has excellent therapeutic efficacy on UC by reducing neutrophilic exudate, inhibiting adhesion molecules, and regulating cytokine levels[16,17]. Clinical studies have shown that anti-inflammatory treatment has a very good therapeutic effect on UC patients. Salazosulfapyridine and 5-aminosalicyclic acid have recently been used in the treatment of colitis[18,19]. These drugs can effectively relieve intestinal inflammation, but have some adverse effects. Recent studies suggest that UC patients can benefit from anti-inflammatory treatments, e.g., the use of infliximab to inhibit tumor necrosis factor-α or anti-IL-6-based therapy[20-22]. Adverse effects of biologic agents include increased antibody reaction, increased risk of infection and hypersensitivity, and an unknown risk of mutagenesis[23,24]. Numerous studies reported that resveratrol had favorable biologic activities that can be used for the treatment of rheumatoid arthritis and pancreatitis[25,26]. Resveratrol is a natural biologic extracts with few toxic side effects and its multiple therapeutic effects have been demonstrated. In this study, we demonstrate that colitis mice have severe Treg/Th17 imbalance, decrease of anti-inflammatory factors (e.g., TGF-β1, and IL-10), increased proinflammatory cytokines (e.g., IL-6, and IL-17), and elevation of nuclear HIF-1α and cytoplasmic mTOR and STAT3. Our results also show that resveratrol can regulate the rebalancing of Treg/Th17, increase TGF-β1 and IL-10 levels, decrease IL-6 and IL-17 levels, and inhibit hypoxia-mTOR-HIF-1α-Th17 and IL-6-STAT3-HIF-1α-Th17 pathways. Regulation of immune disorder in mice with colitis further demonstrates that resveratrol has an excellent therapeutic effect on UC, which provides a potential new treatment for UC.
MATERIALS AND METHODS
Animals
Specific pathogen-free BALB/c mice (males, 6-7-wk-old, weight: 22-26 g, n = 40) (Experimental Animal Center of Southern Medical University, Certificate number: SCXK Guangdong 2011-0015) were maintained in a clean animal room with a temperature of 22 °C-25 °C and relative humidity of approximately 55% under a 12 h light/dark cycle. Mice were provided controlled access to water and free access to food.
Reagents
Dextran sulfate sodium (DSS; MW: 5000) was purchased from Woka Company (Japan), and dissolved in sterile distilled water to make a 5% solution. Flow cytometry reagents for analysis of Th17/Treg were purchased from BD Biosciences (Becton, Dickenson, and Company, Franklin Lakes, NY, United States). ELISA detection kits for IL-17, IL-10, IL-6, and TGF-β1 were purchased from Beijing Dakota Biotechnology Co., Ltd. (Beijing, China). Anti-mouse antibodies against STAT3 and HIF-1α were purchased from Cell Signaling Technology, Inc. (Danvers, MA, United States), and anti-mouse antibody against mTOR was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, United States). Resveratrol (purity ≥ 99%) was purchased from Guangzhou Qiyunsheng Biotechnology Co., Ltd. (China) and was dissolved in ethanol to make a 0.5% solution as required.
Experimental design
After adaptive feeding for one week, 40 mice were randomly divided into four groups (n = 10/group): normal controls (NC), model (MD) group, resveratrol 50 mg/kg per day treatment group (RLD), and resveratrol 100 mg/kg per day treatment group (RHD). The NC group was given free access to sterile distilled water for 14 d; MD group and the treatment groups were given 5% DSS for the first 7 d and distilled water for a subsequent 7 d. Starting from the 7th day, mice in the NC and MD groups were given 0.5% ethanol (0.2 mL) daily via gavage for 7 d, while mice in the RLD and RHD groups were given the same amount of ethanol containing 50 mg/kg per day and 100 mg/kg per day resveratrol, respectively. After 14 d, blood samples were collected from the eye and mice were subsequently sacrificed by cervical dislocation. The plasma, spleen, and colon tissue samples were collected from the sacrificed mice.
Disease activity index and spleen index
Daily disease activity index (DAI) was recorded using the Murthy scoring system[27] (Table 1). The DAI was calculated as the total scores of weight loss rate, feces viscosity, and occult/visible bloody stools divided by 3. After the mice were sacrificed, spleens were dissected, and the blood on the surface of spleen was washed with physiologic saline. The spleen was dried and weighed. The spleen index (SI) was calculated as spleen weight (mg) divided by the body weight (g)[28].
Table 1.
Murthy scoring system
| Scores | Rate of body weight loss (%) | Viscosity of the stools | Occult and visible bloody stools |
| 0 | (-) | Normal (particles) | Normal |
| 1 | 1-5 | ||
| 2 | 6-10 | Soft (paste, do not adhere to the anus) | Occult blood (+) |
| 3 | 11-15 | Diarrhea (Water, adhere to the anus) | |
| 4 | > 15 | Bloody stools |
Pathologic assessment of colonic tissues in mice
Fresh colon tissues obtained from each experimental group were immediately placed in a 10% formalin solution at room temperature and fixed for 48 h in a dark environment. Tissue dehydration and embedding were performed, and tissue sections were stained with hematoxylin-eosin. Colonic tissues were assessed according to the histologic scoring criteria[29] (Table 2).
Table 2.
Histologic scoring system
| Scores | Histologic features |
| 0 | Normal intestinal mucosa |
| 1 | 1/3 crypts missing |
| 2 | 2/3 crypts missing |
| 3 | Lamina propria contains infiltration of mild inflammatory cells |
| 4 | Significant inflammatory cell infiltration |
Flow cytometry analysis of mouse spleen Treg/Th17 cells
Appropriate amounts of spleen tissues were placed in 5 mL lymphocyte separation solution and ground for 2 min on 200 mesh nylon using a 5 mL syringe piston. Spleen tissue homogenate was centrifuged at 800 × g for 30 min, and the lymphocyte layer was aspirated. After washing with 10 mL RPMI-1640 solution, a single lymphocyte cell suspension was obtained. The single cell suspension was resuspended in RPMI1640 medium containing 10% serum, 50 ng/mL of phorbol myristate acetate and 1 μg/mL of ionomycin (Sigma-Aldrich, St. Louis, MO, United States), and 2 μg/mL of monensin (BD Biosciences), and incubated for 5 h at 37 °C. The cell number was adjusted to 2 × 106/mL, and centrifuged at 300 g for 5 min. The collected cells were treated with 20 μL pre-chilled 1 × BD Mouse Foxp3 Fixation Buffer (BD Biosciences), and fixed at 4 °C in a dark room for 30 min. The fixative was then washed and the cells were collected. Subsequently, 200 μL preheated 1 × BD Mouse Foxp3 Permeabilization Buffer (BD Biosciences) was added, and the cells were incubated at 37 °C in a dark room for 30 min. The permeabilization Buffer was washed away and cells were incubated with 20 μL of mouse Treg/Th17 phenotype antibody reagents (BD Biosciences) or isotype control antibody at room temperature for 30 min. The antibody was then washed away and cells were resuspended in 200 μL FBS for flow cytometry analysis.
Cytokine analysis by ELISA
Heparin was added to blood samples for anticoagulation. The plasma samples were obtained by centrifugation at 3000 rpm for 20 min at 4 °C, and stored at -80 °C for subsequent analysis. Colon tissue (50 mg) was added to 2 mL cold saline and homogenized using a glass homogenizer. The homogenates were centrifuged at low temperature for 20 min at 3000 rpm. The protein concentration in the supernatant was quantified on a Nanodrop 2000 (Thermo Fisher Scientific, Waltham, MA, United States). The concentration of IL-6, IL-10, IL-17, and TGF-β1 was determined according to the manufacturer’s instruction.
Western blot analysis
Nuclear and cytoplasmic proteins were obtained from 100 mg of colon tissue, and quantified on a Nanodrop 2000, and the appropriate amount of protein 6 × loading buffer and phosphatase and protease inhibitors were added. Protein samples were separated by 10% SDS-PAGE and transferred to a membrane. Following blocking, primary antibodies including STAT3 (1:500), HIF-1α (1:200), mTOR (1:200), lamin, α-tubulin, and β-actin were added. After extensive washing, membranes were incubated with appropriate secondary antibodies and developed with enhanced chemiluminescence reagents.
Statistical analysis
The experimental data are expressed as mean ± SD. Statistical analysis was performed using SPSS 13.0 (SPSS Inc., Chicago, IL, United States) statistical software. Differences between groups were analyzed using single-factor analysis of variance and P < 0.05 was considered statistically significance. The statistical methods of this study were reviewed by Wei-Seng Zeng from Southern Medical University in China.
RESULTS
DAI and SI assessments of mice
DAI of mice in the NC group was 0. In the first 7 d, mice in the MD, RLD, and RHD groups had bloody stool, weight loss, and decreased activities. Starting from the 10th day, visible bloody stool was not observed in the RLD and RHD groups. Furthermore, the RLD and RHD groups had reduced body weight loss. From day 10 to day 14, the DAI score in RHD group was significantly lower than that in the MD group and RLD group (P < 0.05) (Figure 1A). The mice in the MD group had markedly enlarged spleens (Figure 1B). The SI in the RLD and RHD groups was lower than that in the MD group (Figure 1C). Compared to the RLD group, mice in the RHD group had a significant reduction of SI (P < 0.05).
Figure 1.
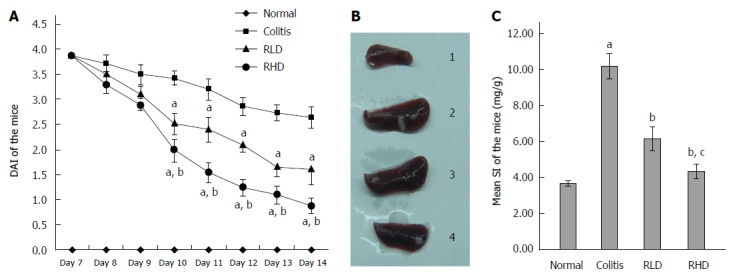
Effect of resveratrol on ulcerative colitis in mice. A: Disease activity index (DAI); B: Spleens from normal mice (1), mice with colitis (2), mice with colitis treated with low-dose resveratrol (RLD; 3), and mice with colitis treated with high-dose resveratrol (RHD; 4); and C: Spleen index (SI); aP < 0.05 vs normal; bP < 0.05 vs colitis; cP < 0.05 vs RLD.
Pathologic assessment of colon tissues
Compared to the NC group (Figure 2A), mice in the MD group (Figure 2B) exhibited acute inflammation, accompanied by mucosal erosions, edema, reduction of crypts, and presence of neutrophils and other inflammatory cells in the mucosa, submucosa, and lamina propria. Compared with the MD group, mice in the RLD (Figure 2C) and RHD (Figure 2D) groups exhibited relieved colonic inflammatory cell infiltration, erosion, and edema.
Figure 2.
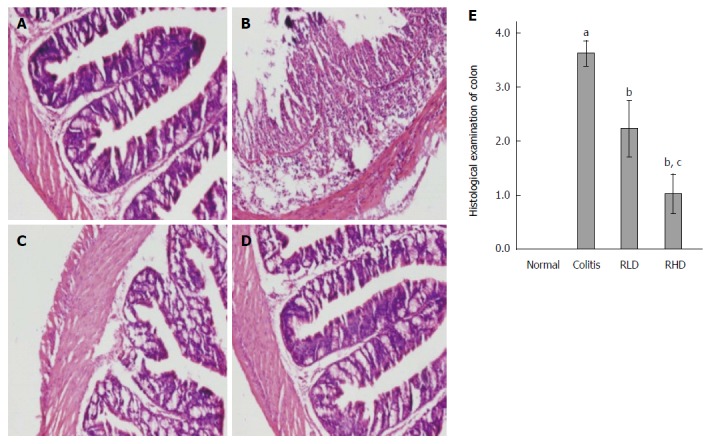
Histologic assessment of resveratrol effects. Hematoxylin and eosin staining in normal mice (A), mice with colitis (B), mice with colitis treated with low-dose resveratrol (RLD; C), and mice with colitis treated with high-dose resveratrol (RHD; D); E: Histologic scores; aP < 0.05 vs normal; bP < 0.05 vs colitis; cP < 0.05 vs RLD.
Effect of resveratrol on Treg/Th17
Compared with the NC group, the MD group showed significantly increased ratio of CD4+IL-17+(Th17)/CD4+ lymphocytes and decreased CD4+Foxp3 + (Treg)/CD4+ lymphocytes (both P < 0.05) (Figure 3). Compared with the MD group, the RHD group had significantly reduced Th17 lymphocytes and increased Treg lymphocytes (both P < 0.05). Th17 cells were significantly decreased (P < 0.05), but Treg was not changed in the RLD group compared with the MD group.
Figure 3.
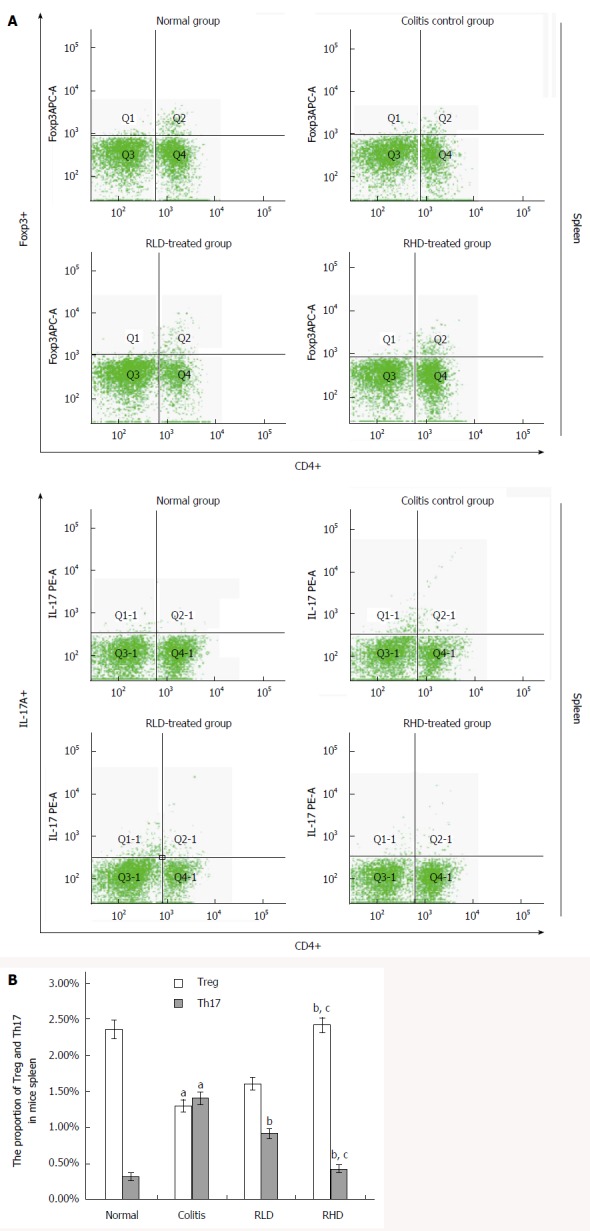
Effect of resveratrol on spleen Treg and Th17 cells. A: Flow cytometric sorting of Treg and Th17 cells; B: Quantification of flow cytometry. aP < 0.05 vs normal; bP < 0.05 vs colitis; cP < 0.05 vs RLD. RHD: Resveratrol high-dose group; RLD: Resveratrol low-dose group.
Effect of resveratrol on cytokines in the plasma and colon tissue
Compared with the NC group, the MD group showed significantly increased levels of proinflammatory cytokines (IL-6, IL-17) and decreased levels of anti-inflammatory cytokines (IL-10, TGF-β1) (both P < 0.05) (Figure 4). IL-6 and IL-17 in RLD and RHD groups were significantly lower than in the MD group (both P < 0.05), while IL-10 and TGF-β1 in the RLD and RHD groups were significantly lower than those in the MD group (both P < 0.05). Furthermore, more dramatic decreases of IL-6 and IL-17 and increases of IL-10 and TGF-β1 were observed in RHD group compared to those in the RLD group.
Figure 4.
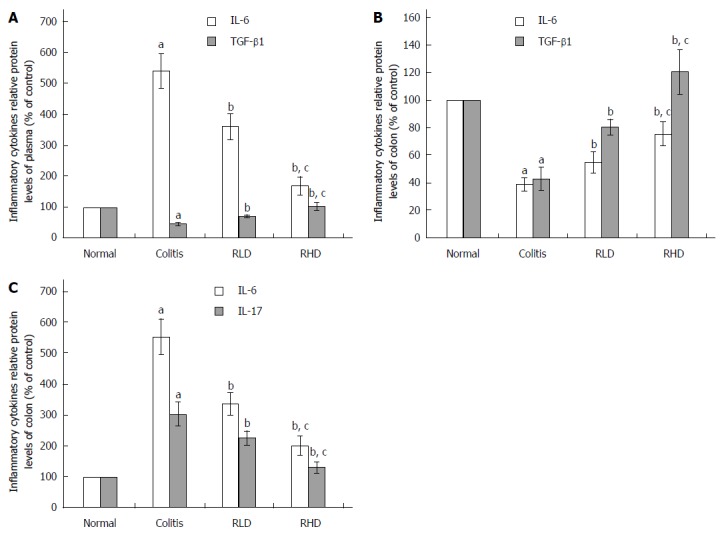
Effect of resveratrol on the cytokines in the plasma and colonic tissues. The effect of resveratrol on the level of A: Transforming growth factor (TGF)-β1 and interleukin (IL)-6 in plasma; B: TGF-β1 and interleukin (IL)-6 in colon tissues; and C: IL-6 and IL-17 in colon tissues. aP < 0.05 vs normal; bP < 0.05 vs colitis; cP < 0.05 vs RLD. RHD: Resveratrol high-dose group; RLD: Resveratrol low-dose group.
Effect of resveratrol on the expression of HIF-1α, mTOR, and STAT3
Nuclear HIF-1α and cytoplasmic mTOR and STAT3 in the MD group were significantly increased in comparison with those in the NC group (both P < 0.05) (Figure 5). Levels of HIF-1α, mTOR, and STAT3 in the RLD and RHD groups were lower than those in the MD group. Furthermore, the reduction of HIF-1α, mTOR, and STAT3 in RHD groups was greater than that in the RLD group, suggesting that resveratrol downregulates their expression in colitis mice in a dose-dependent manner.
Figure 5.
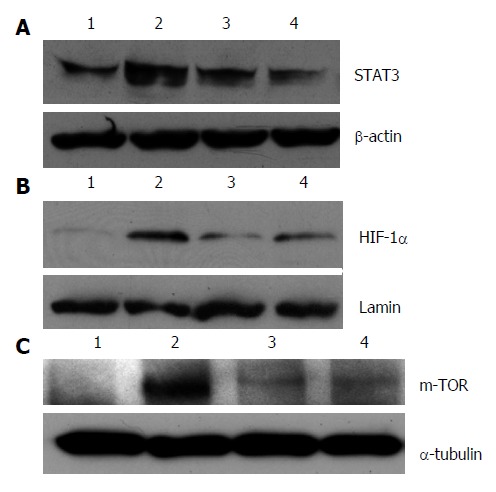
Effect of resveratrol on protein expression of HIF-1α, mTOR, STAT3. A: Signal transducer and activator of transcription 3 (STAT3); B: Hypoxia inducible factor (HIF)-1α; and C: Mammalian target of rapamycin (mTOR). Lane 1: Normal mice; Lane 2: Mice with colitis; Lane 3: Mice with colitis treated with low-dose resveratrol; Lane 4: Mice with colitis treated with high-dose resveratrol.
DISCUSSION
The molecular mechanisms of IBD pathogenesis are not fully understood. Studies have suggested that the environmental factors act on genetically susceptible populations, resulting in excessive immune response disorders, intestinal inflammation, and ultimately the intestinal injury[28,30]. It was previously believed that Th1/Th2 imbalance plays a dominant role in the pathogenesis of UC. The discovery of Th17 cells explained the unusual phenomenon of Th1/Th2 imbalance in UC[6,31]. In this study, we successfully developed a UC mouse model using 5% DSS. The colitis mice experienced bloody stools, loss of body weight, and decreased activities. DAI and SI of the colitis mice were significantly increased and the histologic alterations were similar to those observed in human UC. Treatment of colitis mice with resveratrol significantly reduced DAI and SI and improved histologic alterations. Furthermore, a high dose of resveratrol appears to have better efficacy than a low dose of resveratrol. Resveratrol also improved the systemic symptoms and intestinal inflammation by improving the DAI and intestinal pathology in colitis mice. The observation that resveratrol affects the spleen function encourages us to further investigate the effect of resveratrol on immune responses.
Our results show that the spleens in colitis mice were significantly enlarged, whereas treatment with resveratrol substantially reduced the spleen size. It was thus confirmed that as an important immune organ, the spleen exhibited compensatory increases after DSS stimulation. Flow cytometry analysis showed that colitis mice had a significant decrease of Treg and increase of Th17 cells, suggesting that DSS-induced systemic and intestinal immune disorder is a key factor for the formation and progression of colitis. Previous studies have demonstrated that Th17 cells affect the innate and acquired immune responses and are involved in the pathogenesis of IBD immunity and prognosis through the release of cytokines, e.g., IL-17[5]. Treg/Th17 imbalance is frequently observed in a variety of autoimmune diseases. Extensive literature has reported that human IBD is closely correlated with the alteration of Treg and Th17 cells and their secreted cytokines. Therefore, Treg/Th17 imbalance is a potential target for the treatment of IBD[4,11,32].
Therapeutic efficacy of resveratrol on colitis mice can be achieved by the following mechanisms. First, anti-inflammatory cytokines are increased, while the proinflammatory cytokines are decreased after resveratrol treatment. Maintaining the balance of cytokines may play an important role in the treatment of UC. Cytokines can promote interaction between immune cells, stimulate proliferation of antigen-specific effector cells, and cause local or systemic inflammatory response, which may lead to the development of UC[33,34]. IL-10 can downregulate T cell- and macrophage-secreted IL-1β, IL-6, and tumor necrosis factor-α by inhibiting antigen-presenting cells, which ultimately inhibits T cell-mediated immune response and improves intestinal inflammation in UC[33]. Resveratrol achieves its central role in inhibiting proinflammatory cytokines and anti-inflammatory effects by increasing IL-10 levels. Second, our results demonstrate that resveratrol regulates Treg/Th17 in a dose-dependent manner. RHD regulates the balance of Treg/Th17 by both downregulating Th17 cells and upregulating Treg cells, while RLD regulates the balance of Treg/Th17 mainly through downregulation of Th17 cells. Furthermore, Treg/Th17 ratio achieved by RHD is closer to the physiologic level. Th17 cells belong to the CD4+ T-cell subset, and are characterized by secretion of IL-17. Th17 cells play an important role in the intestinal mucosal immune response and inflammatory process[35]. Treg cells can suppress the inflammatory response cascade and maintain the balance of the intestinal immune response by secreting and regulating anti-inflammatory cytokines, e.g., IL-10 and TGF-β[36,37]. Our results show that the Treg/Th17 ratio is decreased in colitis mice, which is consistent with human IBD[4]. Our results further demonstrate that resveratrol inhibits the expression of HIF-1α, mTOR, and STAT3, suggesting that resveratrol regulates Treg/Th17 balance mainly through inhibiting hypoxia-mTOR-HIF-1α-Th17 and IL-6-STAT3-HIF-1α-Th17 pathways. Studies have suggested that local intestinal tissue hypoxia in colitis activates mTOR and promotes translocation of HIF-1α into the nucleus, leading to the activation of Th17, degradation of Foxp3, and inhibition of Treg[10,38]. It was also shown that inflammation leads to increased IL-6 through the IL-6/STAT3 signaling pathway, which results in the activation of HIF-1α and influences the Treg/Th17 balance[39-41]. Our studies suggest that HIF-1α plays a central role in these two pathways involved in the regulation of Treg/Th17 balance. The HIF-1α-Th17 pathway is closely correlated with Th17 development, activation, and production of inflammatory cytokines such as IL-17. HIF-1α also leads to accelerated degradation of Foxp3 and downregulation of Treg. Hypoxia directly upregulates the expression of mTOR, which in turn promotes translocation of HIF-1α into the nucleus to activate Th17. Third, resveratrol itself has anti-inflammatory and antioxidant functions. Resveratrol promotes the rebalance of Treg/Th17 by inhibiting the production of leukocyte eicosanoid and inflammatory cytokines, which further prevents the inflammatory cascade and plays a very important role in the recovery of UC. At the same time, resveratrol can reduce and inhibit neutrophil and macrophage exudation, regulate intestinal immune disorders, and relieve intestinal endothelial cell swelling and increased permeability, which further reduces intestinal inflammation[42,43].
In conclusion, resveratrol can decrease DAI and SI, improve histologic alteration, and achieve therapeutic efficacy in mice with colitis. Mechanistically, the therapeutic effect of resveratrol is achieved by reducing proinflammatory cytokines and increasing the anti-inflammatory cytokines, which ultimately leads to the rebalance of Treg/Th17 via inhibition of the HIF-1α-Th17 pathway. Our results also demonstrate that resveratrol inhibits inflammatory responses in a dose-dependent manner. These results suggest that resveratrol could be a potential new therapeutic for the treatment of UC.
COMMENTS
Background
Ulcerative colitis (UC) is an inflammatory bowel disease manifested by recurrent and chronic diarrhea, bloody and mucous stools, and abdominal pain. Resveratrol is a natural and biologically active polyphenol with a variety of anti-inflammatory and anti-oxidant functions, which are beneficial to human health.
Research frontiers
The etiology and pathogenesis of UC are not clear. This study defines the mechanism of resveratrol’s therapeutic effect in UC.
Innovations and breakthroughs
The results demonstrated that the therapeutic efficacy of resveratrol in inflammatory bowel disease is dose dependent and closely associated with the regulation of Treg/Th17 balance and the hypoxia inducible factor-1α/mammalian target of rapamycin signaling pathway.
Applications
Regulation of immune disorder in mice with colitis further demonstrated that resveratrol had an excellent therapeutic effect on UC, which provides a potential new treatment of UC.
Peer-review
The manuscript by Yao et al reports on the effects of resveratrol, a naturally occurring polyphenolic compound found in the skin of various berries, on signaling in cells of the immune system in the gut during UC. More specifically, they have studied the therapeutic effects of resveratrol using a well-established UC model (mice exposed to dextran sodium sulphate). They observed that resveratrol affected the Treg/Th17-balance of immune cells and regulated a number of cytokines and intracellular signal transducers. They concluded that resveratrol exerts an anti-ulcerative effect by reducing pro-inflammatory cytokines and increasing anti-inflammatory cytokines.
Footnotes
Supported by Outstanding Doctoral Thesis Support Project of Guangdong Province, No. 85514045; the Technical Research and Development Project of Shenzhen, No. JCYJ20130402092657774; and the Medical Research Foundation of Guangdong Province, No.B2013347.
Ethics approval: All experimental procedures were conducted in conformity with institutional guidelines for the care and use of laboratory animals in Southern Medical University, Guangdong, China, and conformed to the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985).
Institutional animal care and use committee: All animal experiments were reviewed and approved by the Guangdong Animal Medical Ethics Committee, China.
Conflict-of-interest: The authors declared that they have no conflicts of interest to this work.
Data sharing: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: November 27, 2014
First decision: December 11, 2014
Article in press: March 12, 2015
P- Reviewer: Danielsen EM, Goetze TO S- Editor: Qi Y L- Editor: AmEditor E- Editor: Ma S
References
- 1.Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620–1633. doi: 10.1053/j.gastro.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Lichtenstein GR, Rutgeerts P. Importance of mucosal healing in ulcerative colitis. Inflamm Bowel Dis. 2010;16:338–346. doi: 10.1002/ibd.20997. [DOI] [PubMed] [Google Scholar]
- 3.Yao J, Wang JY, Lai MG, Li YX, Zhu HM, Shi RY, Mo J, Xun AY, Jia CH, Feng JL, et al. Treatment of mice with dextran sulfate sodium-induced colitis with human interleukin 10 secreted by transformed Bifidobacterium longum. Mol Pharm. 2011;8:488–497. doi: 10.1021/mp100331r. [DOI] [PubMed] [Google Scholar]
- 4.Eastaff-Leung N, Mabarrack N, Barbour A, Cummins A, Barry S. Foxp3+ regulatory T cells, Th17 effector cells, and cytokine environment in inflammatory bowel disease. J Clin Immunol. 2010;30:80–89. doi: 10.1007/s10875-009-9345-1. [DOI] [PubMed] [Google Scholar]
- 5.Raza A, Yousaf W, Giannella R, Shata MT. Th17 cells: interactions with predisposing factors in the immunopathogenesis of inflammatory bowel disease. Expert Rev Clin Immunol. 2012;8:161–168. doi: 10.1586/eci.11.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuss IJ. Is the Th1/Th2 paradigm of immune regulation applicable to IBD? Inflamm Bowel Dis. 2008;14 Suppl 2:S110–S112. doi: 10.1002/ibd.20683. [DOI] [PubMed] [Google Scholar]
- 7.Liu ZJ, Yadav PK, Su JL, Wang JS, Fei K. Potential role of Th17 cells in the pathogenesis of inflammatory bowel disease. World J Gastroenterol. 2009;15:5784–5788. doi: 10.3748/wjg.15.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogino H, Nakamura K, Ihara E, Akiho H, Takayanagi R. CD4+CD25+ regulatory T cells suppress Th17-responses in an experimental colitis model. Dig Dis Sci. 2011;56:376–386. doi: 10.1007/s10620-010-1286-2. [DOI] [PubMed] [Google Scholar]
- 9.Abraham C, Cho J. Interleukin-23/Th17 pathways and inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1090–1100. doi: 10.1002/ibd.20894. [DOI] [PubMed] [Google Scholar]
- 10.Nutsch K, Hsieh C. When T cells run out of breath: the HIF-1α story. Cell. 2011;146:673–674. doi: 10.1016/j.cell.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 11.Awasthi A, Murugaiyan G, Kuchroo VK. Interplay between effector Th17 and regulatory T cells. J Clin Immunol. 2008;28:660–670. doi: 10.1007/s10875-008-9239-7. [DOI] [PubMed] [Google Scholar]
- 12.Kleczynska W, Jakiela B, Plutecka H, Milewski M, Sanak M, Musial J. Imbalance between Th17 and regulatory T-cells in systemic lupus erythematosus. Folia Histochem Cytobiol. 2011;49:646–653. doi: 10.5603/fhc.2011.0088. [DOI] [PubMed] [Google Scholar]
- 13.Nistala K, Wedderburn LR. Th17 and regulatory T cells: rebalancing pro- and anti-inflammatory forces in autoimmune arthritis. Rheumatology (Oxford) 2009;48:602–606. doi: 10.1093/rheumatology/kep028. [DOI] [PubMed] [Google Scholar]
- 14.Yao J, Wang JY, Liu L, Li YX, Xun AY, Zeng WS, Jia CH, Wei XX, Feng JL, Zhao L, et al. Anti-oxidant effects of resveratrol on mice with DSS-induced ulcerative colitis. Arch Med Res. 2010;41:288–294. doi: 10.1016/j.arcmed.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Trapp V, Parmakhtiar B, Papazian V, Willmott L, Fruehauf JP. Anti-angiogenic effects of resveratrol mediated by decreased VEGF and increased TSP1 expression in melanoma-endothelial cell co-culture. Angiogenesis. 2010;13:305–315. doi: 10.1007/s10456-010-9187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh UP, Singh NP, Singh B, Hofseth LJ, Taub DD, Price RL, Nagarkatti M, Nagarkatti PS. Role of resveratrol-induced CD11b(+) Gr-1(+) myeloid derived suppressor cells (MDSCs) in the reduction of CXCR3(+) T cells and amelioration of chronic colitis in IL-10(-/-) mice. Brain Behav Immun. 2012;26:72–82. doi: 10.1016/j.bbi.2011.07.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdallah DM, Ismael NR. Resveratrol abrogates adhesion molecules and protects against TNBS-induced ulcerative colitis in rats. Can J Physiol Pharmacol. 2011;89:811–818. doi: 10.1139/y11-080. [DOI] [PubMed] [Google Scholar]
- 18.Sands BE. Therapy of inflammatory bowel disease. Gastroenterology. 2000;118:S68–S82. doi: 10.1016/s0016-5085(00)70007-2. [DOI] [PubMed] [Google Scholar]
- 19.Katz JA. Advances in the medical therapy of inflammatory bowel disease. Curr Opin Gastroenterol. 2002;18:435–440. doi: 10.1097/00001574-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Aberra FN, Lichtenstein GR. Infliximab in ulcerative colitis. Gastroenterol Clin North Am. 2006;35:821–836. doi: 10.1016/j.gtc.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Feagan BG, Reinisch W, Rutgeerts P, Sandborn WJ, Yan S, Eisenberg D, Bala M, Johanns J, Olson A, Hanauer SB. The effects of infliximab therapy on health-related quality of life in ulcerative colitis patients. Am J Gastroenterol. 2007;102:794–802. doi: 10.1111/j.1572-0241.2007.01094.x. [DOI] [PubMed] [Google Scholar]
- 22.Nishimoto N, Nakahara H, Yoshio-Hoshino N, Mima T. Successful treatment of a patient with Takayasu arteritis using a humanized anti-interleukin-6 receptor antibody. Arthritis Rheum. 2008;58:1197–1200. doi: 10.1002/art.23373. [DOI] [PubMed] [Google Scholar]
- 23.Ardizzone S, Bianchi Porro G. Biologic therapy for inflammatory bowel disease. Drugs. 2005;65:2253–2286. doi: 10.2165/00003495-200565160-00002. [DOI] [PubMed] [Google Scholar]
- 24.Reddy JG, Loftus EV. Safety of infliximab and other biologic agents in the inflammatory bowel diseases. Gastroenterol Clin North Am. 2006;35:837–855. doi: 10.1016/j.gtc.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Elmali N, Baysal O, Harma A, Esenkaya I, Mizrak B. Effects of resveratrol in inflammatory arthritis. Inflammation. 2007;30:1–6. doi: 10.1007/s10753-006-9012-0. [DOI] [PubMed] [Google Scholar]
- 26.Ma ZH, Ma QY, Wang LC, Sha HC, Wu SL, Zhang M. Effect of resveratrol on peritoneal macrophages in rats with severe acute pancreatitis. Inflamm Res. 2005;54:522–527. doi: 10.1007/s00011-005-1388-z. [DOI] [PubMed] [Google Scholar]
- 27.Sun L, Nava GM, Stappenbeck TS. Host genetic susceptibility, dysbiosis, and viral triggers in inflammatory bowel disease. Curr Opin Gastroenterol. 2011;27:321–327. doi: 10.1097/MOG.0b013e32834661b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Zhao Y. Influence of Zuoguiyin on Antioxidation in Blood Thymus And Spleen Index of Senile Mice. Zhongguo Shiyan Fangjixue Zazhi. 2007;13:67–68. [Google Scholar]
- 29.Hirata I, Yasumoto S, Toshina K, Inoue T, Nishikawa T, Murano N, Murano M, Wang FY, Katsu K. Evaluation of the effect of pyrrolidine dithiocarbamate in suppressing inflammation in mice with dextran sodium sulfate-induced colitis. World J Gastroenterol. 2007;13:1666–1671. doi: 10.3748/wjg.v13.i11.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scharl M, Rogler G. Inflammatory bowel disease pathogenesis: what is new? Curr Opin Gastroenterol. 2012;28:301–309. doi: 10.1097/MOG.0b013e328353e61e. [DOI] [PubMed] [Google Scholar]
- 31.Stephani J, Radulovic K, Niess JH. Gut microbiota, probiotics and inflammatory bowel disease. Arch Immunol Ther Exp (Warsz) 2011;59:161–177. doi: 10.1007/s00005-011-0122-5. [DOI] [PubMed] [Google Scholar]
- 32.Hundorfean G, Neurath MF, Mudter J. Functional relevance of T helper 17 (Th17) cells and the IL-17 cytokine family in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:180–186. doi: 10.1002/ibd.21677. [DOI] [PubMed] [Google Scholar]
- 33.Ko JK, Auyeung KK. Inflammatory bowel disease: etiology, pathogenesis and current therapy. Curr Pharm Des. 2014;20:1082–1096. doi: 10.2174/13816128113199990416. [DOI] [PubMed] [Google Scholar]
- 34.Szkaradkiewicz A, Marciniak R, Chudzicka-Strugała I, Wasilewska A, Drews M, Majewski P, Karpiński T, Zwoździak B. Proinflammatory cytokines and IL-10 in inflammatory bowel disease and colorectal cancer patients. Arch Immunol Ther Exp (Warsz) 2009;57:291–294. doi: 10.1007/s00005-009-0031-z. [DOI] [PubMed] [Google Scholar]
- 35.Lohr J, Knoechel B, Caretto D, Abbas AK. Balance of Th1 and Th17 effector and peripheral regulatory T cells. Microbes Infect. 2009;11:589–593. doi: 10.1016/j.micinf.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta S. Immune homeostasis: regulatory T cells (Treg) and molecules. J Clin Immunol. 2008;28:617–618. doi: 10.1007/s10875-008-9259-3. [DOI] [PubMed] [Google Scholar]
- 37.Unutmaz D, Pulendran B. The gut feeling of Treg cells: IL-10 is the silver lining during colitis. Nat Immunol. 2009;10:1141–1143. doi: 10.1038/ni1109-1141. [DOI] [PubMed] [Google Scholar]
- 38.Tseng WP, Yang SN, Lai CH, Tang CH. Hypoxia induces BMP-2 expression via ILK, Akt, mTOR, and HIF-1 pathways in osteoblasts. J Cell Physiol. 2010;223:810–818. doi: 10.1002/jcp.22104. [DOI] [PubMed] [Google Scholar]
- 39.Mitsuyama K, Matsumoto S, Masuda J, Yamasakii H, Kuwaki K, Takedatsu H, Sata M. Therapeutic strategies for targeting the IL-6/STAT3 cytokine signaling pathway in inflammatory bowel disease. Anticancer Res. 2007;27:3749–3756. [PubMed] [Google Scholar]
- 40.Carey R, Jurickova I, Ballard E, Bonkowski E, Han X, Xu H, Denson LA. Activation of an IL-6: STAT3-dependent transcriptome in pediatric-onset inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:446–457. doi: 10.1002/ibd.20342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jung JE, Kim HS, Lee CS, Shin YJ, Kim YN, Kang GH, Kim TY, Juhnn YS, Kim SJ, Park JW, et al. STAT3 inhibits the degradation of HIF-1alpha by pVHL-mediated ubiquitination. Exp Mol Med. 2008;40:479–485. doi: 10.3858/emm.2008.40.5.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martín AR, Villegas I, Sánchez-Hidalgo M, de la Lastra CA. The effects of resveratrol, a phytoalexin derived from red wines, on chronic inflammation induced in an experimentally induced colitis model. Br J Pharmacol. 2006;147:873–885. doi: 10.1038/sj.bjp.0706469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahal HS, Mukherjee T. Scavenging of reactive oxygen radicals by resveratrol: antioxidant effect. Res Chem Intermediat. 2006;32:59–71. [Google Scholar]


