Abstract
AIM: To investigate serum PC-594 fatty acid levels as a potential biomarker in North American pancreatic cancer (PaC) patients, and to compare its performance to CA19-9.
METHODS: Using tandem mass spectrometry, we evaluated serum PC-594 levels from 84 North American patients with confirmed PaC and 99 cancer-free control subjects. We determined CA19-9 levels by ELISA. Significance between PaC patients and controls, and association with clinical variables was determined by analysis of variance and t-tests. Diagnostic performance was evaluated by receiver-operator characteristic (ROC) curve analysis, and PC-594 correlation with age and CA19-9 determined by regression analysis.
RESULTS: Mean PC-594 levels were 3.7 times lower in PaC patients compared to controls (P < 0.0001). The mean level in PaC patient serum was 0.76 ± 0.07 μmol/L, and the mean level in control subjects was 2.79 ± 0.15 μmol/L. There was no correlation between PC-594 and age, disease stage or gender (P > 0.05). Using 1.25 μmol/L as a PC-594 threshold produced a relative risk (RR) of 9.4 (P < 0.0001, 95%CI: 5.0-17.7). The area under the receiver-operator characteristic curve (ROC-AUC) was 0.93 (95%CI: 0.91-0.95) for PC-594 and 0.85 (95%CI: 0.82-0.88) for CA19-9. Sensitivity at 90% specificity was 87% for PC-594 and 71% for CA19-9. Six PaC patients with CA19-9 above 35 U/mL showed normal PC-594 levels, while 24 PaC patients with normal CA19-9 showed low PC-594 levels. Eighty-five of the 99 control subjects (86%) showed normal levels of both markers.
CONCLUSION: PC-594 biomarker levels are significantly reduced in North American PaC patients, and showed superior diagnostic performance compared to CA19-9.
Keywords: Pancreatic cancer, Risk, Screening, Blood, Fatty acid, Biomarker, Metabolite, PC-594
Core tip: The incidence of pancreatic cancer (PaC) in the general population is too low to warrant screening by imaging or endoscopic ultrasound. In this paper, we provide further validation that the serum fatty acid metabolite PC-594 is a viable PaC biomarker by showing that it is significantly reduced in North American PaC patients compared to control subjects, and that its performance is superior to CA19-9. This reduction represents a near 10-fold increase for PaC risk, and a PaC incidence among PC-594 deficient subjects that exceeds the incidence of colorectal cancer considered sufficient to warrant colonoscopy-based screening. PC-594 therefore represents an opportunity to identify a subset of the general population with high PaC risk.
INTRODUCTION
PaC has the fourth highest mortality rate among all cancers in North America[1-3]. In Canada, there are 9.3/100000 new cases diagnosed per year, with 8.6/100000 deaths[1]. PaC is usually diagnosed at a late stage, well after the presentation of symptoms including jaundice, pain, and weight loss. By this time, conventional treatments, such as surgery and radiation, are generally ineffective. The median survival time of PaC is therefore only 4 mo[4], with a 5-year survival of less than 5% for stage III/IV[5]. In comparison, the 5-year survival for stage I is 30%[5]. Therefore, as with most cancers, improved early detection of PaC prior to metastatic disease would lead to better prognosis.
The current major challenge in the management of PaC is the identification of high-risk patients, since greater than 90% of cases are sporadic with no familial association[6]. Unlike colorectal cancer (CRC), for example, there are no PaC screening tests or screening guidelines for the average-risk population. This is due largely to the low incidence, lack of suitable molecular markers, and the limitations of medical imaging approaches such as endoscopic ultrasound (EUS) and computed tomography (CT) for screening the general population. Therefore, a blood-based test for identifying a subset of the population with PaC incidence sufficient to warrant further screening would represent a significant advancement in PaC management.
PC-594 is a novel circulating 36-carbon long-chain polyunsaturated fatty acid that has previously been implicated in Japanese PaC patients[7]. In the current study, we investigated whether serum PC-594 levels are affected in North American PaC patients and compared the results to CA19-9.
MATERIALS AND METHODS
Sample cohort
PaC (n = 84) and control (n = 99) serum samples were obtained from Conversant Bio (www.conversantbio.com). All patients signed informed consents, and samples were collected under ethics-approved protocols according to the requirements of Conversant Bio. Patients also signed Conversant Bio informed consents and pathology reports were provided for most cases. Serum was prepared off-the-clot using red-topped vacutainer tubes. Inclusion criterial for the controls was no current or prior diagnosis of any cancer including PaC. For PaC patients, a pathologist-confirmed diagnosis of pancreatic adenocarcinoma of any stage was required. Basic demographics are shown in Table 1.
Table 1.
Clinical characteristics
| Controls, n | PaC, n | |
| All | 99 | 84 |
| Male | 46 | 45 |
| Female | 53 | 39 |
| Caucasian | 75 | 77 |
| African American | 23 | 6 |
| Other | 1 | 1 |
| Age (yr), median | 45 (19-85) | 64 (41-96) |
| TNM Stage | ||
| I/II | 11 | |
| III/IV | 23 | |
| Stage unknown | 50 | |
| Surgery | ||
| Sample collected prior to surgery | 38 | |
| Sample collected after surgery | 23 | |
| NA | 23 | |
| Chemo | ||
| Sample collected prior to chemo | 6 | |
| Sample collected after chemo | 17 | |
| Sample collected during chemo | 56 | |
| NA | 5 | |
| Radiation | ||
| Sample collected prior radiation | 47 | |
| Sample collected after radiation | 11 | |
| Sample collected during radiation | 3 | |
| NA | 23 |
NA: Not available.
PC-594 isolation
PC-594 was isolated from commercial human serum by batch extracting 60 mL of human serum (Seracare Lifesciences) with 120 mL methanol and 240 mL ethyl acetate. After centrifugation at 1300 rpm for 5 min, the supernatant was decanted and then partitioned with 240 mL hexane. The upper layer was separated from the aqueous phase using a 2 L separation funnel and the organic phase concentrated under vacuum using a rotary evaporator. PC-594 was purified from the residue (ca. 0.25 g) through different stages of liquid chromatographic separation as described below. The process was repeated for total 40 L of human serum. 10 g of pooled extract residue was then fractionated by flash column chromatographic separation on silica gel (Merck, 0.04-0.063 mm, 100 g), and eluted sequentially with a mixture of hexane:ethyl acetate (1:9, 4 L; 4:1, 4 L; 4:3, 4 L, and 0:1, 8 L). All fractions collected were submitted for LC/MS analysis using an Agilent 1200 HPLC coupled to an ABSciex QStar XL mass spectrometry system. Fractions eluted with hexane:ethyl acetate (4:1) containing PC-594 were combined and concentrated using a rotary evaporator under reduced pressure. The process was repeated until all extracts were fractionated. The obtained fraction (0.34 g) containing PC-594 as major component was further purified on a preparative LC system using a preparative SB C-18 column (Agilent XDB C-18 column, 21.2 mm × 150 mm, 5 μm), and eluted with a mixture of acetonitrile:water (75:25, in 35 min; flow rate: 25 mL/min). The fractions were monitored using a diode array detector (G1315D). Fractions containing PC-594 (tR: 16.9 min) were combined and concentrated under vacuum. Finally, the purer fraction (70 mg) was further purified by LC using a semi-preparative SB CN column (Agilent SB CN, 9.4 mm × 250 mm, 5 μm), eluted with mixture of hexane:ethyl acetate (1-35 min, 94:6; 36-50 in, 50:50; 51-75 min, 94:6; flow rate: 5 mL/min). The fraction was monitored using a diode array detector (Agilent G1315D). All fractions were finally analyzed by LC/MS on an Agilent 1200 HPLC coupled to the ABSciex QStar mass spectrometry system. Fractions containing similar purity of PC-594 were combined and analyzed using LC/MS analysis as well as NMR analysis. A fraction (0.3 mg) with purity greater than 98% was qualified as a reference standard for the quantitative analysis of PC-594 in human serum.
Tandem mass spectrometry
Samples were prepared for tandem MS by vortexing 20 μL of serum with 30 μL of 0.3% formic acid and 750 μL of ethyl acetate at 1500 RPM for 60 min. Samples were then centrifuged at 15400 RCF for 2 min. The upper organic fractions were transferred to new vials, and directly infused into an Ionics 3Q Molecular Analyzer tandem mass spectrometer under negative atmospheric pressure ionization using a Glison-271 liquid handler. 100 μL of sample was injected at a speed of 0.5 mL/min using mobile-phase solvent of 2% water in ethyl acetate. The mass spectrometer was set to MRM mode, with parent ion selection of 593.5 [M-H]- (m/z) in Q1 and 371.5 [M-H]- (m/z) in Q2. Acquisition time was 1.00 min with 190 scans. Other parameters were: drying gas: 100.0 °C, HSID: 200.0 °C, nebulizer gas: 300.0 °C, corona discharge: -4.0, probe temperature: 350.0 °C.
CA19-9 determination
Serum CA19-9 levels were determined by ELISA using a commercially-available CA19-9 ELISA kit from Affymetrix (manufactured by Panomics Inc.), according to the manufacturer’s instructions.
Statistical analysis
PC-594 concentrations were determined by extrapolation of peak areas from external standard curves of HPLC-purified PC-594 standard. CA19-9 concentrations were extrapolated from external standard curves according to the manufacturer’s instructions (Panomics, Inc). Univariate analysis and logistic regression were performed with Microsoft Excel and STATA version 13. ROC curves were performed using JROCFIT 1.0.2. (http://www.rad.jhmi.edu/jeng/javarad/roc/JROCFITi.html). Beeswarm scatter plots were performed with R 2.15.1. Differences were deemed significant if P values were less than 0.05. The statistical methods of this study were reviewed by Dr. Bassirou Chitou, a biostatistician from Phenomenome Discoveries, Inc.
RESULTS
We determined PC-594 levels in the serum of North American PaC patients and control subjects using tandem MS (Table 1). The mean PC-594 levels were 0.76 ± 0.07 μmol/L in PaC patients and 2.79 ± 0.15 μmol/L in control subjects (Figure 1, P < 0.0001 PaC vs control subjects). ROC analysis produced an AUC of 0.93 (95%CI: 0.91-0.95; Figure 2A). Optimum sensitivity (86%) and specificity of (91%) was achieved at a PC-594 concentration of 1.25 μmol/L. The relationship between PC-594 concentration, specificity, and sensitivity is shown in Figure 2B. Using 1.25 μmol/L as a threshold resulted in a relative risk (RR) of 9.4 (P < 0.0001, 95%CI: 5.0-17.7).
Figure 1.
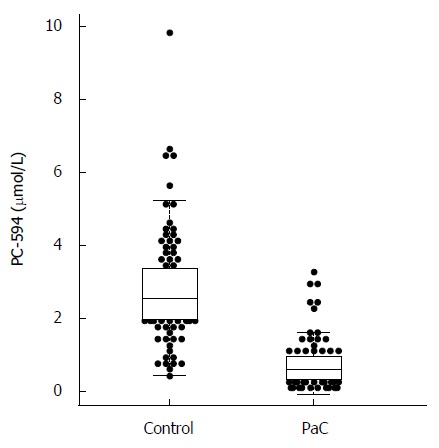
Beeswarm scatter plot of PC-594 levels in control and pancreatic cancer patient sera. Boxes indicate the 25th to 75th percentile, while the horizontal line in the box represents the median level. Whiskers indicate the 5th to 95th percentile.
Figure 2.
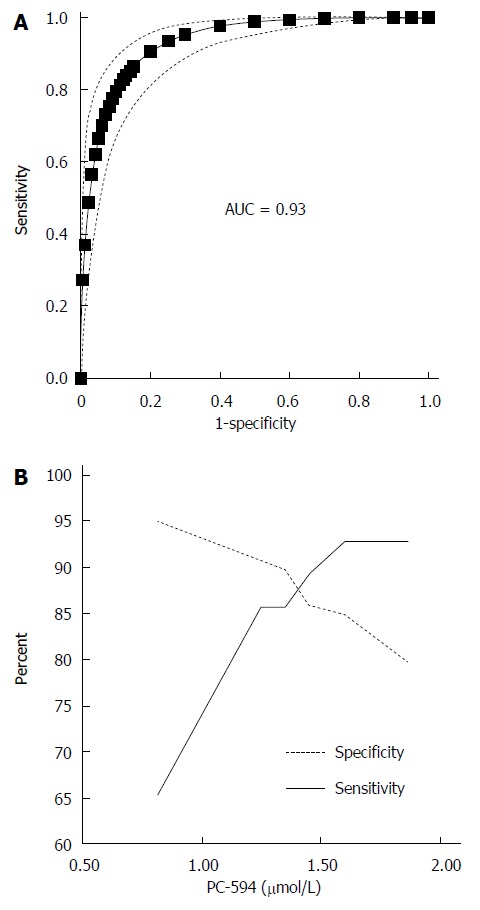
Receiver-operator characteristic analysis of PC-594. A: ROC curve based on PC-594 levels in PaC and control subjects, Dotted lines indicate the 95%CI; B: Line pots of sensitivity and specificity vs serum PC-594 concentration.
There were 34 PaC patients with available staging data; 11 stage I/II, and 23 stage III/IV. The mean PC-594 level among the stage I/II patients was 0.75 ± 0.18 μmol/L, while the stage III/IV patient mean was 0.56 ± 0.06 μmol/L. The difference between stage I/II and stage III/IV was not significant (P = 0.2; Figure 3A).
Figure 3.
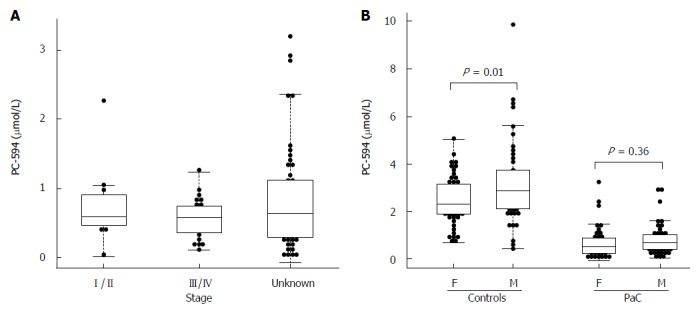
Beeswarm scatter plot of PC-594 levels by disease stage (A) and gender (B). Boxes indicate the 25th to 75th percentile, while the horizontal line in the box represents the median level. Whiskers indicate the 5th to 95th percentile. PaC: Pancreatic cancer.
We also investigated possible associations between PC-594 and gender, age, race, and treatment status. There was no mean PC-594 difference between male and female PaC patients (P = 0.36), however, there was a slightly lower mean PC-594 level in female control subjects compared to males (P = 0.01; Figure 3B). When all samples were grouped together, there was no mean difference between male and female subjects (P = 0.2). There was also no association with age, as determined by regression analysis, in either the control subjects (R = 0.08, F = 0.6, P = 0.4; Figure 4A) or PaC patients (R = 0.2, F = 3.5, P = 0.06; Figure 4B). Mean PC-594 levels by age group are shown in Figure 4C. There was also no difference in PC-594 levels between subjects grouped based on age less than 50, 50-64, or 65 and older (P > 0.5).
Figure 4.
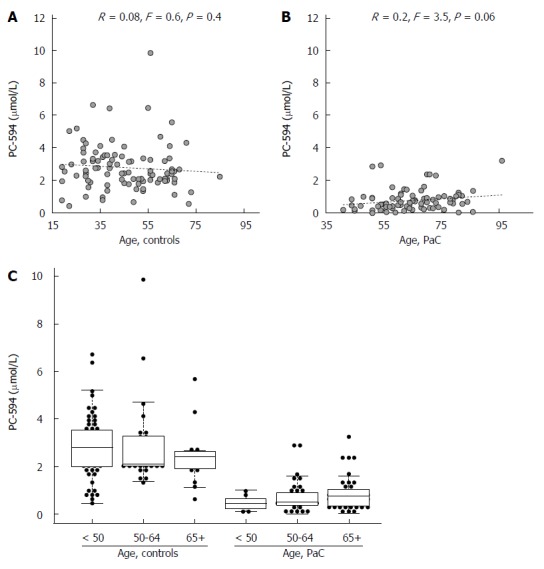
Age analysis. Scatter plots of PC-594 level vs age for controls (A) and PaC patients (B); Beeswarm scatter plot of PC-594 levels by age in controls and PaC patients (C). Boxes indicate the 25th to 75th percentile, while the horizontal line in the box represents the median level. Whiskers indicate the 5th to 95th percentile.
Likewise, we observed no significant difference in PC-594 level between African Americans and Caucasians in the control group (P = 0.18; Table 2), and no effect of surgery, chemo, or radiation therapy relative to the time of sample collection (Table 2).
Table 2.
t-test results of ethnicity and treatment status on PC-594 levels
| Comparison | P value |
| African American (23) vs Caucasian (75) | 0.18 |
| Surgery pre (38) vs post (23) | 0.20 |
| Chemo pre (6) vs post (17) | 0.40 |
| Chemo post (17) vs active (56) | 0.06 |
| Radiation pre (47) vs post (11) | 0.10 |
We next determined CA19-9 levels and compared the results to PC-594 (Figure 5A). 54 of the 84 PaC patients had CA19-9 levels greater than 35 U/mL (64% sensitivity), the concentration above which prognosis is generally considered poor[8]. This resulted in an ROC-AUC of 0.85 (95%CI: 0.82-0.88; Figure 5B). There was, however, a significant inverse correlation between CA19-9 levels and PC-594 (R = 0.48, F = 46 and P < 0.0001, Figure 6A). There was no difference in CA19-9 levels between stage I/II and III/IV patients (162 ± 72 vs 130 ± 43, respectively, P = 0.7).
Figure 5.
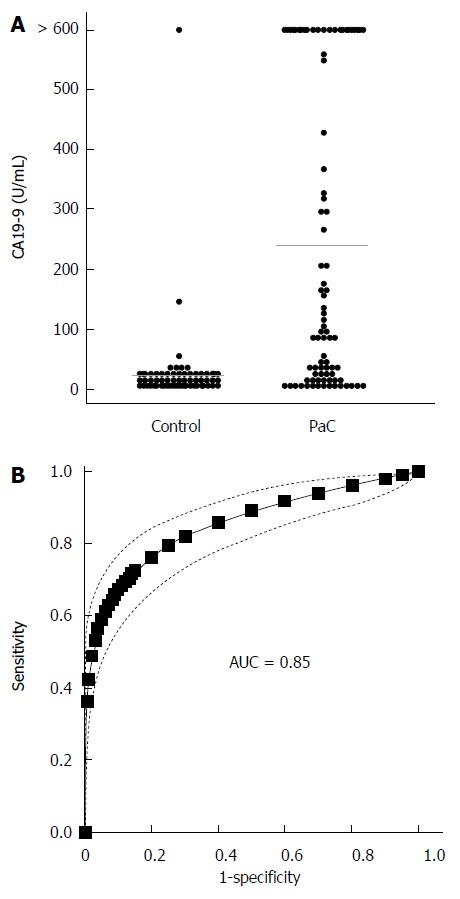
CA19-9 levels. A: Beeswarm scatter plot of CA19-9 levels in controls and PaC patients. Grey lines indicate median levels; B: ROC curve based on CA19-9 levels; Dotted lines indicate the 95%CI.
Figure 6.
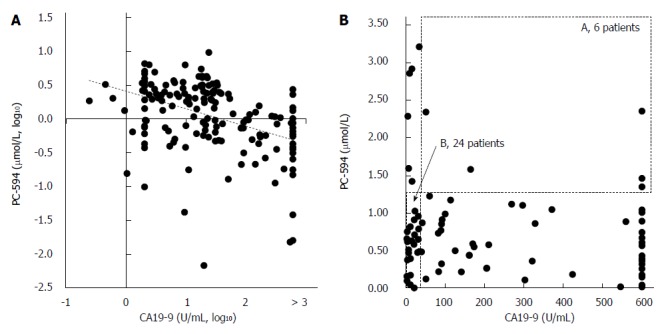
Correlation between CA19-9 and PC-594. Scatter plots of PC-594 vs CA19-9 concentrations (A, log10; B, unlogged). In B, the dotted box A represents CA19-9 above 35 U/mL and PC-594 levels above 1.25 (elevated CA19-9 and normal PC-594), while the dotted box B represents CA19-9 levels below 35 U/mL and PC-594 levels below 1.25 (normal CA19-9 and abnormal PC-594).
To determine if the combination of CA19-9 and PC-594 resulted in improved discrimination, we calculated the number of patients with abnormal levels of either one or both markers. There were only six PaC patients with normal PC-594 levels who had CA19-9 above 35 U/mL, while 24 PaC patients with normal CA19-9 showed low PC-594 levels (Figure 6B and Table 3). Interestingly, there were only six PaC patients who had normal levels of both markers, meaning that 78 of the 84 patients (93%) had an abnormal level of at least one of the markers. For controls, 85 of the 99 subjects (86%) showed normal levels of both markers (Table 3, bottom row). The additional discrimination afforded by CA19-9 in this study therefore resulted in a 7% improvement in sensitivity, but at the expense of 5% specificity (86 vs 91).
Table 3.
Interaction between CA19-9 and PC-594 n (%)
| CA19-9 (U/mL) | PC-594 (μmol/L) | PaC | Control |
| Abnormal | 54 (64) | 5 (5) | |
| Abnormal | 72 (86) | 9 (9) | |
| Abnormal | Abnormal | 48 (57) | 0 (0) |
| Abnormal | Normal | 6 (7) | 5 (5) |
| Normal | Abnormal | 24 (29) | 9 (9) |
| Normal | Normal | 6 (7) | 85 (86) |
Abnormal CA19-9: Greater than 35 U/mL. PaC: Pancreatic cancer.
DISCUSSION
Our results indicate that PC-594 is significantly reduced in the serum of North American PaC patients, with 86% of cases and 9% of controls showing levels below 1.25 μmol/L (86% sensitivity and 91% specificity). This is similar to previously published results showing a sensitivity of 81% at 95% specificity[7]. Our findings herein provide further evidence that PC-594 is a robust marker of PaC in both North American and Japanese patients, and we conclude from this that PC-594 reduction in PaC is independent of geography or ethnicity. This was further corroborated by the lack of difference between African American and Caucasian control PC-594 levels.
The lack of association between PC-594 levels and disease stage in North American patients suggests the possibility that PC-594 becomes reduced prior to the development of the disease, as the levels should have been lower among advanced cases if the tumor was causing the reduction. Since PaC is rarely diagnosed at an early stage, detecting a PC-594 deficiency might offer a new approach for early detection, or for detecting future increased risk of PaC among the general population. Since prognosis is better with early detection, identification and surveillance of high-risk subjects should result in reduced mortality.
In addition to the lack of stage effect, the lack of association between PC-594 and treatment status at the time of collection further underscores that the reduction is not likely due to the tumor. We also have data from Japanese patients (manuscript in preparation) showing that intra-subject PC-594 levels do not change following surgery. Ultimately, a longitudinal study will be required to confirm whether PC-594 deficient subjects who are asymptomatic show higher future PaC incidence rates.
Our data suggest that PC-594 reduction is a significant risk factor for PaC. PC-594 deficient subjects in this study showed a RR of 9.4, which represents a projected PaC incidence in a PC-594 deficient population that is approximately two to three times higher than the general population incidence of CRC[1]. Given that the incidence of CRC at age 50 is sufficient to warrant endoscopy-based screening, an appropriate follow-up schema for the PC-594 deficient subpopulation should be considered.
This study is the first report examining the correlation between PC-594 and CA19-9 we are aware of. CA19-9 is the only marker routinely used in the management of PaC, primarily for prognosis[9,10]. Due to the high false-negative rate of CA19-9 (PaC patients with normal CA19-9 levels), it is not recommended for average-risk screening by the American Society for Clinical Oncology[11]. Furthermore, elevated levels are often reported in other conditions such as obstruction[8]. In this study, only 64% of the PaC patients showed abnormally high CA19-9 levels, which is consistent with other reports (see Ballehaninna et al[12] for review). Overall, PC-594 showed superior performance (AUC of 0.93 vs 0.85 for CA19-9), with sensitivity of 86% at 91% specificity. Adding CA19-9 to PC-594 by way of a simple Boolean approach increased sensitivity by 7%, but at a loss of 5% specificity. It is probably not feasible to combine the markers for risk-based stratification, as the 5% reduction in specificity (or increase in false positive rate) would require over a third more follow-up screening procedures. Since we do not know for certain whether the control distributions across different ethnicities are similar, the 1.25 μmol/L optimal cut-off reported here could be optimal for the United States population, while different cut-offs may be required for other geographic or ethnic populations.
This latter point is one of the most challenging aspects of PaC screening programs - what to do with asymptomatic high-risk subjects? Unlike CRC, where high-risk subjects can undergo colonoscopy, there are currently no recommended guidelines for managing high-risk PaC patients. Since PC-594 deficiency is a risk factor similar to age, family history, smoking, or other factors[13], it could be used as an additional tool in the clinic to aid in identifying high risk subjects. Stratifying the general population into those with a PC-594 deficiency, followed by assessment of other risk factors and potentially medical imaging, is a logical clinical path for monitoring risk. We also previously showed, using decision curve analysis, that there is a significant net clinical benefit in using PC-594 to identify a subset of subjects over imaging everyone[7]. Medical imaging in the form of either EUS[14,15], magnetic resonance (MR)[16-18], CT[19,20] or positron emission tomography (PET)[21] are all viable approaches; however, the invasiveness (of EUS), cost, available resources, or radiation dosage are all factors that must be considered for wide-spread adoption. However, recent advances in transabdominal ultrasound (TUS) are showing promise for the detection of various pancreatic lesions and carcinomas, and could represent a cost-effective and non-invasive follow-up approach for monitoring high-risk subjects[21-24]. We are currently establishing a clinical protocol to evaluate the potential of TUS as a follow-up modality in PC-594 deficient subjects.
What role PC-594 plays in the body is currently unknown. PC-594 was originally identified as a 594 Da member and the top PaC discriminating metabolite among a group of 36-carbon containing polyunsaturated fatty acids in the blood using a high-resolution metabolomics approach[7]. The molecular formula of PC-594 is C36H66O6, and it contains multiple unsaturations and hydroxylations. The 36-carbon fatty acids belong to a family of metabolites known as gastric tract acids (GTAs), some of which have been shown to exhibit pro-apoptotic and anti-inflammatory activities, the latter mediated through the inhibition of NFkappaB[25]. GTAs have not been detected in the blood of other species and thus appear to be specific to human serum. We have previously eluded to a possible association with microbiome metabolism, and are currently investigating whether GTAs are produced by microbes[25]. Our current hypothesis is that PC-594 is involved in protecting the body against inflammation and subsequent early proto-oncogenic events. Based on our data to date, we speculate that a decline in PC-594 with age in a subset of the population occurs to a point below which PC-594 can no longer counteract the accumulation of chronic inflammation over time, creating a microenvironment favorable for the subsequent development of cancer. Our current studies are focused on purifying and synthesizing sufficient quantities of PC-594 from human serum, investigating its biological activity, and determining its origin within the body. Whether a PC-594 reduction is implicated in non-cancerous inflammatory conditions is a question we are currently addressing through a comprehensive survey of various inflammatory pancreatic pseudocysts, and true cysts such as intraductal papillary mucinous neoplasms (IPMNs). Our previous data hints towards an association between IPMN and a PC-594 deficiency, which is intriguing given that IPMN can be a precursor for PaC[7].
The main limitation of our study was the retrospective case-control design. Although we took care in selecting samples collected and handled under stringent protocols, there is always the possibility of bias. However, we’ve now observed this PC-594 reduction in multiple independent, geographically disparate, and ethnically diverse populations using different sample collection protocols. Accordingly, the likelihood of bias due to any of these factors is minimal. A second limitation was that we did not have access to staging data on all patients and detailed medical histories on control subjects. This limited the confidence with which we could report an association with stage, and we could not definitively exclude the possibility that any of the controls had increased risk due to family history or other pancreatic conditions. A third limitation was a difference between the mean ages of the cases and controls; however, the lack of correlation with age in each cohort significantly reduced the likelihood of bias due to age. Collectively, the lack of association between PC-594 and other clinical parameters in the study including age, gender, stage, treatment, and ethnicity provided further confidence that the PC-594 reduction was associated with PaC, and no other factors.
In conclusion, our findings confirm that PC-594 reduction in PaC is independent of ethnicity or geography, and shows superior performance compared to CA19-9. A PC-594 deficiency represents a risk for PaC that exceeds the risk of CRC among the general population, thus necessitating further prospective validation and the establishment of appropriate follow-up screening guidelines.
ACKNOWLEDGMENTS
We would like to acknowledge the staff at Conversant Bio for their help in retrieving and organizing clinical data, and Ms. Alix Hayden for her careful review of the manuscript.
COMMENTS
Background
There are currently no tests for identifying subjects among the general population who might be at risk for pancreatic cancer. The purpose of this paper was to provide further validation of an association between a new blood-based metabolic marker, called PC-594, and pancreatic cancer, and to compare the findings to the prognostic marker CA19.9.
Research frontiers
The current hotspot for pancreatic cancer screening is to identify a subset of the population which has an increased risk high enough to warrant further screening. The challenge is that there is currently no screening test available, and performing imaging such as endoscopic ultrasound (EUS), magnetic resonance imaging (MRI) or computed tomography (CT) scans on the general population is not practical. What the community needs is a minimally-invasive test that would increase the incidence of pancreatic cancer to a point that warrants further medical evaluation, similar to that of colorectal cancer where at age 50 the incidence is considered high enough to recommend that everyone go for screening colonoscopy. Such an approach would represent a major breakthrough in the early detection of pancreatic cancer.
Innovations and breakthroughs
The only other biomarker relevant in the pancreatic cancer space is CA19-9. Even though CA19-9 is not recommended for screening, it is still the clinical gold standard biomarker when it comes to pancreatic cancer patient management, and is often used as a prognostic aid. Our data shows that PC-594 is superior to CA19-9 at identifying pancreatic cancer. 8.6 out of every 10 pancreatic cancer patients showed a PC-594 metabolic deficiency, while only 6.4 out of every 10 patients showed elevated CA19-9 levels.
Applications
PC-594 could be used among the general population to identify a subset of people with increased pancreatic cancer risk who should consider further evaluation.
Terminology
PC-594 is the name of a long-chain fatty acid metabolite in the blood, which has low levels in pancreatic cancer patients. CA19.9 (cancer antigen 19.9) is a protein that can appears in the blood when a person has advanced stage pancreatic cancer. Medical imaging methods to detect pancreatic cancer include EUS, an invasive procedure where patients are sedated and an endoscope with ultrasound capabilities is used to image and/or biopsy the pancreas. MRI and CT are non-invasive imaging methods.
Peer-review
General comments by the reviewers prior to revisions included: This manuscript describes PC-594 as a pancreatic cancer serum biomarker. Its outcome is very interesting and relevant for the field. However, authors should clarify how the novel data of the present paper compared to the previous one, and discuss more how to show the clinical significance of PC-594 in the future prospective study from this point of view.
Footnotes
Ethics approval: All human biospecimens distributed by Conversant Bio (601 Genome Way Suite 1200, Huntsville, Alabama 35806) were collected, processed, and distributed in full ethical and regulatory compliance with the Sites from which human biospecimens are collected. This includes independent ethical review, Institutional Review Board approval (where appropriate), independent regulatory review, and Conversant Bio ethical review for all of Conversant Bio’s collection Sites. All tissues were obtained within applicable laws.
Informed consent: Samples for this study were obtained commercially from Conversant Bio (601 Genome Way Suite 1200, Huntsville, Alabama 35806). Conversant Bio warrants that a separate patient consent form containing language substantially the same as that which is set forth in the consent form was signed by each patient from whom recipient party received human biospecimens. All patients signed informed consents, and samples were collected under ethics-approved protocols according to the requirements of Conversant Bio.
Conflict-of-interest: All authors of this work received salaries from Phenomenome Discoveries, Inc. during the duration of the project. Dayan Goodenowe is a shareholder of Phenomenome Discoveries, Inc. Shawn Ritchie is listed as inventor relating to the use of PC-594 for identifying pancreatic cancer risk, PCT/CA/2010/001565.
Data sharing: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 8, 2015
First decision: January 22, 2015
Article in press: March 19, 2015
P- Reviewer: Hosseini M, Merino G, Nishiyama M S- Editor: Qi Y L- Editor: A E- Editor: Ma S
References
- 1.Canadian Cancer Statistics (www. cancer.ca) Toronto: Canadian Cancer Society; 2014. [Google Scholar]
- 2.Surveillance, Epidemiology, and End Results Program (seer. cancer.gov) United States: National Cancer Institute; 2011. [Google Scholar]
- 3.Flook R, van Zanten SV. Pancreatic cancer in Canada: incidence and mortality trends from 1992 to 2005. Can J Gastroenterol. 2009;23:546–550. doi: 10.1155/2009/986531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma C, Eltawil KM, Renfrew PD, Walsh MJ, Molinari M. Advances in diagnosis, treatment and palliation of pancreatic carcinoma: 1990-2010. World J Gastroenterol. 2011;17:867–897. doi: 10.3748/wjg.v17.i7.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bilimoria KY, Bentrem DJ, Ko CY, Ritchey J, Stewart AK, Winchester DP, Talamonti MS. Validation of the 6th edition AJCC Pancreatic Cancer Staging System: report from the National Cancer Database. Cancer. 2007;110:738–744. doi: 10.1002/cncr.22852. [DOI] [PubMed] [Google Scholar]
- 6.Permuth-Wey J, Egan KM. Family history is a significant risk factor for pancreatic cancer: results from a systematic review and meta-analysis. Fam Cancer. 2009;8:109–117. doi: 10.1007/s10689-008-9214-8. [DOI] [PubMed] [Google Scholar]
- 7.Ritchie SA, Akita H, Takemasa I, Eguchi H, Pastural E, Nagano H, Monden M, Doki Y, Mori M, Jin W, et al. Metabolic system alterations in pancreatic cancer patient serum: potential for early detection. BMC Cancer. 2013;13:416. doi: 10.1186/1471-2407-13-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kau SY, Shyr YM, Su CH, Wu CW, Lui WY. Diagnostic and prognostic values of CA 19-9 and CEA in periampullary cancers. J Am Coll Surg. 1999;188:415–420. doi: 10.1016/s1072-7515(98)00326-3. [DOI] [PubMed] [Google Scholar]
- 9.Humphris JL, Chang DK, Johns AL, Scarlett CJ, Pajic M, Jones MD, Colvin EK, Nagrial A, Chin VT, Chantrill LA, et al. The prognostic and predictive value of serum CA19.9 in pancreatic cancer. Ann Oncol. 2012;23:1713–1722. doi: 10.1093/annonc/mdr561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin LK, Wei L, Trolli E, Bekaii-Saab T. Elevated baseline CA19-9 levels correlate with adverse prognosis in patients with early- or advanced-stage pancreas cancer. Med Oncol. 2012;29:3101–3107. doi: 10.1007/s12032-012-0278-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Locker GY, Hamilton S, Harris J, Jessup JM, Kemeny N, Macdonald JS, Somerfield MR, Hayes DF, Bast RC. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24:5313–5327. doi: 10.1200/JCO.2006.08.2644. [DOI] [PubMed] [Google Scholar]
- 12.Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J Gastrointest Oncol. 2012;3:105–119. doi: 10.3978/j.issn.2078-6891.2011.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becker AE, Hernandez YG, Frucht H, Lucas AL. Pancreatic ductal adenocarcinoma: risk factors, screening, and early detection. World J Gastroenterol. 2014;20:11182–11198. doi: 10.3748/wjg.v20.i32.11182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bournet B, Gayral M, Torrisani J, Selves J, Cordelier P, Buscail L. Role of endoscopic ultrasound in the molecular diagnosis of pancreatic cancer. World J Gastroenterol. 2014;20:10758–10768. doi: 10.3748/wjg.v20.i31.10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helmstaedter L, Riemann JF. Pancreatic cancer--EUS and early diagnosis. Langenbecks Arch Surg. 2008;393:923–927. doi: 10.1007/s00423-007-0275-1. [DOI] [PubMed] [Google Scholar]
- 16.Yao X, Kuang T, Wu L, Feng H, Liu H, Cheng W, Rao S, Wang H, Zeng M. Optimization of MR diffusion-weighted imaging acquisitions for pancreatic cancer at 3.0T. Magn Reson Imaging. 2014;32:875–879. doi: 10.1016/j.mri.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 17.O'Neill E, Hammond N, Miller FH. MR imaging of the pancreas. Radiol Clin North Am. 2014;52:757–777. doi: 10.1016/j.rcl.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Yao X, Zeng M, Wang H, Fei S, Rao S, Ji Y. Metabolite detection of pancreatic carcinoma by in vivo proton MR spectroscopy at 3T: initial results. Radiol Med. 2012;117:780–788. doi: 10.1007/s11547-011-0757-7. [DOI] [PubMed] [Google Scholar]
- 19.Holm J, Loizou L, Albiin N, Kartalis N, Leidner B, Sundin A. Low tube voltage CT for improved detection of pancreatic cancer: detection threshold for small, simulated lesions. BMC Med Imaging. 2012;12:20. doi: 10.1186/1471-2342-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeshita K, Kutomi K, Haruyama T, Watanabe A, Furui S, Fukushima J, Asano T. Imaging of early pancreatic cancer on multidetector row helical computed tomography. Br J Radiol. 2010;83:823–830. doi: 10.1259/bjr/80905803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee ES, Lee JM. Imaging diagnosis of pancreatic cancer: a state-of-the-art review. World J Gastroenterol. 2014;20:7864–7877. doi: 10.3748/wjg.v20.i24.7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beyer-Enke SA, Hocke M, Ignee A, Braden B, Dietrich CF. Contrast enhanced transabdominal ultrasound in the characterisation of pancreatic lesions with cystic appearance. JOP. 2010;11:427–433. [PubMed] [Google Scholar]
- 23.Dimcevski G, Erchinger FG, Havre R, Gilja OH. Ultrasonography in diagnosing chronic pancreatitis: new aspects. World J Gastroenterol. 2013;19:7247–7257. doi: 10.3748/wjg.v19.i42.7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan Z, Li Y, Yan K, Wu W, Yin S, Yang W, Xing B, Li X, Zhang X. Application of contrast-enhanced ultrasound in the diagnosis of solid pancreatic lesions--a comparison of conventional ultrasound and contrast-enhanced CT. Eur J Radiol. 2013;82:1385–1390. doi: 10.1016/j.ejrad.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 25.Ritchie SA, Jayasinghe D, Davies GF, Ahiahonu P, Ma H, Goodenowe DB. Human serum-derived hydroxy long-chain fatty acids exhibit anti-inflammatory and anti-proliferative activity. J Exp Clin Cancer Res. 2011;30:59. doi: 10.1186/1756-9966-30-59. [DOI] [PMC free article] [PubMed] [Google Scholar]


