Abstract
AIM: To investigate the relationship between sense of coherence, psychological distress and health related quality of life in inflammatory bowel disease (IBD).
METHODS: This cross-sectional study enrolled a consecutive sample of 147 IBD (aged 45.1 ± 14.1 years; 57.1% female) patients recruited from a tertiary gastroenterology service. Sixty-four participants met diagnostic criteria for Crohn’s disease, while eighty-three patients had ulcerative colitis. Socio-demographic data (education, age, race, gender, gross monthly income and marital status), disease-related variables (illness activity, relapse rate in past 2 years, history of surgery and time since diagnosis), sense of coherence (Antonovsky’s SOC scale), psychological distress symptoms (Hospital Anxiety and Depression Scale) and health-related quality of life (HRQoL; WHOQOL-Bref) were assessed. Hierarchical multiple regression analyses were performed to identify factors that are independently associated with psychological distress and HRQoL in patients with IBD and to provide indications for possible moderating or mediating effects. In addition, formal moderation and mediation analyses (Sobel tests) were performed to confirm potential moderators/mediators of the relationship between SOC, psychological distress symptoms and HRQoL.
RESULTS: Lower SOC scores (std beta= -0.504; P < 0.001), female gender (std beta = 0.176; P = 0.021) and White race (std beta = 0.164; P = 0.033) were independently associated with higher levels of depressive symptoms, while lower levels of SOC (std beta = -0.438; P < 0.001) and higher relapse rate (std beta = 0.161; P = 0.033) were independently associated with more severe anxiety symptoms. A significant interaction between time since diagnosis and SOC was found with regard to the severity of depressive or anxiety symptoms, as the interaction term (time since diagnosis X SOC) had beta coefficients of -0.191 (P = 0.009) and -0.172 (P = 0.026), respectively. Lower levels of anxiety symptoms (std beta = -0.369; P < 0.001), higher levels of SOC (std beta = 0.231; P = 0.016) and non-White race (std beta = -0.229; P = 0.006), i.e., mixed-race, which represented the reference category, were independently associated with higher levels of overall HRQoL. Anxiety symptoms were the most potent independent correlate of most aspects of HRQoL. In addition, anxiety mediated the association between SOC and satisfaction with health, as well as its relationship with physical, mental, and social relations HRQoL. Depressive symptoms also mediated the association between SOC and mental HRQoL.
CONCLUSION: Our data indicated that SOC is an important construct, as it influences psychological distress and has significant albeit indirect effects on several HRQoL domains in IBD.
Keywords: Sense of coherence, Coping, Anxiety, Depression, Quality of life, Crohn’s disease, Ulcerative colitis
Core tip: Sense of coherence (SOC) has emerged as an important correlate of psychological distress and health-related quality of life (HRQoL) across several chronic diseases. The associations between SOC and both psychological distress and HRQoL in inflammatory bowel disease (IBD) remain unknown. Here, SOC was inversely associated with depressive and anxiety symptoms. Anxiety symptoms were strong independent correlates of most aspects of HRQoL. Anxiety mediated the associations of SOC with satisfaction with health, physical, mental, and social relations HRQoL. Thus, SOC should be considered an important construct influencing IBD-related distress and HRQoL. Future prospective studies should confirm these findings.
INTRODUCTION
Inflammatory bowel disease (IBD) refers to a group of chronic inflammatory disorders of the gastrointestinal tract. During the course of their illness, IBD patients experience several gastrointestinal manifestations, including, but not limited to, diarrhea, abdominal pain, malabsorption and weight loss. Crohn’s disease (CD) and ulcerative colitis (UC) are the most frequent phenotypes. Extra-intestinal involvement is also frequent in CD and UC[1]. The most frequent systemic manifestations include erythema nodosum, arthritis, uveitis and conjunctivitis[1,2]. The clinical course of IBD is variable, with periods of exacerbation and remission[3].
Patients with IBD often experience remarkable levels of psychological distress because of the complex interactions between biological mechanisms (e.g., inflammation, abnormalities in the gut-brain axis and shared genetic factors), personality aspects, and the challenging psychosocial adaptation process to these chronic diseases[4]. Compared with the general population, the prevalence of anxiety and depression is two to three times higher in patients with IBD, particularly among those individuals with active disease[5-7]. There is also some evidence from prospective studies that higher levels of psychological distress predict a higher relapse rate. Mittermaier et al[8] identified depression and anxiety symptoms as risk factors for early clinical recurrence and more severe disease. Conversely, in a prospective study[9], aggressive and/or active disease predicted the development of depression. IBD has a relapsing course, with unpredictable and potentially severe flares, which often require intensive treatment, hospitalization and significant modifications on the patients’ routines. These illness-related factors may contribute to psychosocial distress in IBD[10]. Moreover, evidence suggests that psychological distress leads to negative outcomes in chronic medical conditions and reduces the patients’ health-related quality of life (HRQoL) by amplifying symptom burden, decreasing treatment adherence and increasing disability[11]. Thus, HRQoL has emerged as an important outcome measure in IBD.
Several lines of evidence indicate that HRQoL is significantly impaired among IBD patients compared with general population norms[12-14]. Socio-demographic variables (e.g., female gender and lower education) may influence IBD patients’ HRQoL[15,16]. Clinical variables such as type of treatment, extra-intestinal manifestations and disease activity status (i.e., in relapse or remission)[16,17] have also been reported to influence HRQoL in IBD. However, considering that some IBD patients have impaired HRQoL despite being in clinical remission[18], other variables, notably psychosocial factors, appear to be important, including perceived psychological distress and personality variables[15,19-22].
Living with IBD is a severe psychological distress and it is likely that the patient’s predominant coping abilities may influence his or her psychological adaptation and the formation of HRQoL. Thus, individual differences may play a role in the patients’ psychological response and outcome, indicating that various psychological processes may play a major role in this respect. Sense of coherence is a measurable psychosocial concept that has attracted increasing attention regarding its contribution to diseases’ outcomes[23-25].
Sense of coherence (SOC) is a theoretical construct that aims to explain why some people, regardless of major stressful situations and severe hardships, fall ill and others do not[26,27]. SOC is defined as a global orientation that expresses the extent to which one has a pervasive, enduring, though dynamic, feeling of confidence, taking into consideration that, (1) the stimuli deriving from one’s internal and external environments in the course of living are structured, predictable and explicable (comprehensibility); (2) the resources are available to one to meet the demands posed by these stimuli (manageability); and (3) these demands are challenges worthy of investment and engagement (meaningfulness)[28]. SOC is hypothesized to develop during the first three decades of life, and although it was considered a stable disposition of personality[29], it is not static[30]. Studies have shown, for example, that SOC tends to increase with age[31,32] and that a disease’s progress or medical interventions may induce intrapersonal alterations of SOC[31,33-36].
Having the ability to use generalized resistance resources such as social support, childhood experiences, intelligence, coping strategies, family socialization, or physical characteristics appears to be centrally important in developing a strong SOC[31,37]. In turn, a strong SOC helps the person’s ability to mobilize his/her resistance resources as he/she seeks a solution or as he/she copes with a stressor[31,37,38], harmonizing the reciprocal relationship between SOC and generalized resistance resources[31,37].
SOC is the key construct of Antonovsky’s salutogenic theory, which represents a relatively new approach with regard to coping with a stressor (e.g., a chronic medical condition), because it focuses mainly on coping per se rather than on the stressor itself[28]. Antonovsky’s theory of SOC hypothesizes that an individual with a strong SOC maintains and enhances health through effective and flexible coping with stressors by adopting health-enhancing and avoiding unhealthy behaviors. By contrast, low levels of SOC characterize individuals who do not perceive stressors as predictable and explicable, have no confidence in their capacity to overcome health stressors and lack any motivation to face health difficulties or challenges[38].
A number of studies have shown that SOC seems to have an impact on HRQoL; the stronger the SOC the better the HRQoL[39]. However, whether SOC is associated with more favorable health outcomes is a matter of debate. Although studies have shown that a strong SOC predicts better health outcomes[40] and Eriksson’s and Lindström’s systematic review on this matter concluded that SOC seems to be a health promoting resource that strengthens resilience[27], others challenged the potential of SOC to predict health outcomes[41,42]. Flensborg-Madsen et al[43]’s recent review concluded that the SOC scale can only serve as a predictor for health that is measured by incorporating psychological aspects, while its capacity to predict physical health, as measured only by means of physical terms, is limited, indicating that further research is needed in this respect.
Studies investigating the role of SOC in patients with IBD are limited. A comparative study assessed SOC in patients with IBD and patients with Irritable Bowel Syndrome and found no significant differences[44]. Opheim et al[25] have recently explored associations between SOC and sociodemographic, disease-related and personal characteristics in patients with IBD and found a positive association between SOC and self-efficacy, together with an inverse association between SOC and fatigue. Finally, another study found no differences in physiological markers between IBD patients with low vs high levels of SOC[45]. To the best of our knowledge, however, no studies have investigated the role of this psychological construct and its interaction with psychological distress and disease’s parameters in the formation of IBD patients’ HRQoL. Our previous studies in rheumatological disorders showed that SOC is associated with psychological distress and HRQoL[46], while psychological distress mediates the relationship of SOC with physical HRQoL[47]. Prompted by these findings, the aim of the present study was to test the following hypotheses: Hypothesis 1, SOC is associated with psychological distress in IBD patients, independently of demographic and disease-related parameters; Hypothesis 2, Psychological distress in IBD patients is associated with HRQoL, independently of demographic and disease-related parameters. Hypothesis 3, SOC is associated with HRQOL; however, this association is mediated by symptoms of depression and anxiety.
MATERIALS AND METHODS
Participants
The sample consisted of 147 consecutively enrolled patients with a confirmed diagnosis of IBD. Patients were receiving regular outpatient care at the gastroenterology service of the Hospital Universitário Walter Cantídio (HUWC), Fortaleza, Brazil. Diagnosis of CD or UC was confirmed based on clinical, endoscopic, radiological and histopathological evidence, in accordance with standard diagnostic criteria[48,49]. Exclusion criteria were: unconfirmed diagnosis of IBD, current hospitalization, co-morbid psychotic or substance use disorders, and dementia and cognitive impairment, as assessed by the Brazilian Portuguese version of the Mini-Mental Status Examination[50]. All participants underwent a structured diagnostic interview (using the Brazilian Portuguese version of the Mini International Neuropsychiatric Interview)[51] with a research psychiatrist (THF).
The a priori estimated sample size for the intended multivariate analyses with a maximum of 15 predictors, a desired statistical power of 0.8, an anticipated medium effect size (f2 = 0.15), and a significance level (alpha) of 0.05 was approximately 140 patients. Out of 155 invited patients, 150 were eligible and 147 agreed to participate in the study (response rate: 98%). No statistically significant differences in major demographic variables were found between the participating and non-participating groups (data available upon request). All participants provided written informed consent. The ethics committee of the HUWC approved the study protocol.
Measures
Participants were examined by experienced gastroenterologists and socio-demographic and medical data were collected from the patients’ medical records, using a standardized data-collection form. Relapse rate was estimated according to the number of relapses within the past 2 years and was coded as follows: “low” (i.e., 0-2 relapses in the last 2 years, reflecting a mean annual relapse rate of 1 or lower), “moderate” (i.e., 3-5 relapses in the last two years, reflecting a mean annual relapse rate of approximately 2) and “high” (i.e., 6 or more relapses in the last two years, reflecting a mean annual relapse rate of 3 or higher). Disease activity/severity was estimated based on relevant clinical variables, assessed by the treating gastroenterologist. For CD patients, disease activity was assessed using the Crohn’s disease activity index (CDAI), developed by Best and colleagues; values above 150 indicated an active disease[52]. For UC patients, disease severity was assessed according to the six criteria proposed by Truelove and Witts[53], adopted by the Brazilian Study Group of inflammatory bowel diseases[54]. A score of 6 or lower in the Truelove-Witts ulcerative colitis severity index (TWT) indicated “mild” disease severity, whereas scores above 6 indicated “moderate” or “severe” disease. To construct a common measure of disease activity/severity applicable to both diseases (i.e., CD and UC), we created a new categorical variable labeled “disease in remission”, coded as follows: “yes” (i.e., mild disease activity/severity; CDAI score < 150 or TWT score ≤ 6, accordingly) vs “no” (i.e., moderate or severe disease activity/severity; CDAI score ≥ 150 or TWT score > 6, accordingly). To assess SOC, psychological distress symptoms, and HRQoL the following self-report questionnaires were used:
Sense of coherence
The validated Brazilian Portuguese 29-item version of the SOC scale was used[55]. The SOC scale is regarded as a cross-culturally sensitive and valid scale for assessing long-term outcomes for various mental or physical illnesses[39]. Higher scores reflect higher levels of SOC, indicating stronger resources for coping with health-stressors. Cronbach’s alpha derived from the present sample was 0.75.
Psychological distress symptoms
Anxiety and depressive symptoms were assessed using the validated Brazilian Portuguese version of the Hospital Anxiety and Depression Scale (HADS)[56,57]. It comprises 14 items equally distributed between anxiety and depression subscales. Each item is rated on a 4-point Likert scale and a score of 8 or higher is considered to indicate clinically relevant anxiety or depressive symptoms, accordingly[56]. An extensive review of more than 700 studies employing HADS confirmed its sound psychometric properties, with adequate internal consistency and reliability, and adequate diagnostic accuracy for screening anxiety and depressive disorders (sensitivity and specificity of approximately 0.80) among several medical samples from diverse cultural backgrounds[58]. Cronbach’s alphas derived from the present sample were 0.83, 0.81 and 0.67 for the entire scale, and for the anxiety and depression subscales, respectively.
Health-related quality of life
The validated 26-item Brazilian Portuguese version[59] of the World Health Organization Quality of Life instrument-Abbreviated version (WHOQOL-Bref)[60] was used. The WHOQOL-Bref assesses overall HRQoL, satisfaction with general health, and physical, mental, social relations and environmental aspects of HRQoL. Each item is rated on a 5-point Likert scale and a higher score indicates better HRQoL. The WHOQOL-Bref is a reliable and valid measure of HRQoL when applied in IBD samples[61,62]. Cronbach’s alphas derived from the present sample were 0.86 for the entire instrument and 0.75, 0.64, 0.72 and 0.64 for the physical, mental, social relations and environment HRQoL, respectively.
Statistical analysis
The statistical methods of this study were reviewed by Professor Thomas Hyphantis, from the Department of Psychiatry, University of Ioannina, Greece, and Professor Andre F. Carvalho, from the Department of Clinical Medicine, Federal University of Ceará, Fortaleza, Brazil. Descriptive summary statistics for all variables were calculated (Table 1). Comparisons between CD and UC were performed by means of chi-square and two-tailed t-tests, as appropriate. Univariable associations between all independent variables and criterion variables (i.e., anxiety and depressive symptoms and each one of the WHOQOL-Bref domains) were carried out using two-tailed t-tests, one-way ANOVAs, or Pearson’s or Spearman’s correlations, as appropriate.
Table 1.
Socio-demographic profile, clinical variables, sense of coherence, anxiety and depression symptoms and health-related quality of life in inflammatory bowel disease patients and comparisons between Crohn’s disease and ulcerative colitis n (%)
| Total (n =147) | CD (n = 64) | UC (n = 83) | CD vs UC (P value) | |
| Demographic | ||||
| Sex | 0.8851 | |||
| Male | 63 (42.9) | 27 (42.2) | 36 (43.4) | |
| Female | 84 (57.1) | 37 (57.8) | 47 (56.6) | |
| Race | 0.2181 | |||
| Latin/Mixed-race | 96 (65.3) | 44 (68.8) | 52 (62.7) | |
| White | 36 (24.5) | 16 (25.0) | 20 (24.1) | |
| Black | 9 (6.1) | 3 (4.7) | 6 (7.2) | |
| Asian | 5 (3.4) | 0 (0) | 5 (6.0) | |
| Indian | 1 (0.7) | 1 (1.6) | 0 (0) | |
| Age | 45.1 ± 14.1 | 43.9 ± 14.9 | 46.1 ± 13.9 | 0.3502 |
| Years of education | 8.4 ± 4.9 | 8.1 ± 4.9 | 8.5 ± 5.1 | 0.6272 |
| Marital status | ||||
| With spouse/partner | 98 (66.7) | 46 (71.9) | 52 (62.7) | 0.2391 |
| Gross monthly Income (USD) | 482.9 ± 560.0 | 487.6 ± 665.2 | 479.3 ± 467.4 | 0.9302 |
| Clinical | ||||
| Time since diagnosis (mo) | 80.5 ± 71.8 | 79.7 ± 73.9 | 81.0 ± 70.6 | 0.9142 |
| Relapse rate | 0.0231 | |||
| Low (0-2 in 2 yr) | 53 (36.3) | 23 (36.5) | 30 (36.1) | |
| Moderate (3-5 in 2 yr) | 61 (41.8) | 20 (31.7) | 41 (49.4) | |
| High (≥ 6 in 2 yr) | 32 (21.9) | 20 (31.7) | 12 (14.5) | |
| Surgery for IBD | 26 (17.7) | 20 (31.3) | 6 (7.2) | < 0.0011 |
| CDAI, mean ± SD | NA | 64.75 ± 43.57 | NA | |
| TWT, mean ± SD | NA | NA | 7.53 ± 1.61 | |
| Disease in remission | 90 (61.2) | 62 (96.9) | 28 (33.7) | < 0.0011 |
| Psychological | ||||
| SOC | 138.4 ± 24.8 | 138.6 ± 23.2 | 138.2 ± 26.1 | 0.9232 |
| Anxiety symptoms (HADS-A) | 5.58 ± 4.30 | 6.00 ± 4.06 | 5.26 ± 4.47 | 0.3022 |
| Depressive symptoms (HADS-D) | 5.27 ± 3.78 | 5.00 ± 3.83 | 5.48 ± 3.74 | 0.4452 |
| HADS-A ≥ 8 (anxiety) | 37 (25.2) | 21 (32.8) | 16 (19.3) | 0.0611 |
| HADS-D ≥ 8 (depression) | 36 (24.5) | 15 (23.4) | 21 (25.3) | 0.7941 |
| HRQoL | ||||
| Overall HRQoL | 3.88 ± 0.81 | 3.94 ± 0.75 | 3.84 ± 0.85 | 0.4852 |
| Satisfaction with Health | 3.59 ± 0.96 | 3.34 ± 1.10 | 3.78 ± 0.80 | 0.0082 |
| Physical HRQoL | 3.48 ± 0.64 | 3.42 ± 0.60 | 3.54 ± 0.67 | 0.2722 |
| Mental HRQoL | 3.82 ± 0.67 | 3.68 ± 0.61 | 3.92 ± 0.71 | 0.0302 |
| Social Relations HRQoL | 3.90 ± 0.74 | 3.98 ± 0.74 | 3.83 ± 0.74 | 0.2342 |
| Environment HRQoL | 3.38 ± 0.53 | 3.41 ± 0.54 | 3.36 ± 0.53 | 0.5542 |
Data are presented as mean ± SD for continuous variables and as n (%) for categorical variables; remission was defined according to CDAI (< 150) and TWT (≤ 6) scores, accordingly;
χ2 test;
Two-tailed t-test. NA: Not applicable; CDAI: Crohn's disease activity index; TWT: Truelove-Witts ulcerative colitis severity index; HRQoL: Health-related quality of life; SOC: Sense of coherence; UC: Ulcerative Colitis; CD: Crohn’s disease.
To identify the variables most closely and independently associated with each criterion variable, a series of hierarchical multiple regression analyses were performed, with the anxiety and depressive symptoms as well as all WHOQOL-Bref domains as dependent variables. For anxiety and depressive symptoms, demographic variables were entered in step 1, disease-related parameters in step 2, and SOC scores in step 3. For the WHOQOL-Bref domains, demographic variables were entered in step 1, disease-related parameters in step 2, SOC scores in step 3, depressive symptoms in step 4 and anxiety symptoms in step 5. The magnitude of the R2 change at each step was used to determine the variance explained by each set of variables. Colinearity between independent variables was tested based on variance inflation factors (VIF) and tolerances for individual variables. All tolerance values were greater than 0.2 and all VIFs were less than 2, indicating that multicollinearity did not bias the regression models[63].
Moderation and mediation analyses: For a variable (M) to be considered as a moderator of the association between a predictor (X) and the outcome (Y), M must be a background characteristic that can affect the association between X and Y (i.e., the association between X and Y is significantly changed at different levels of M i.e., low vs high M)[64]. Scatterplots with lines of best fit for the two levels of M (i.e., low vs high) were plotted to probe the significant interaction effects and hierarchical multiple regression analyses were then performed to quantify the moderator effects. For this, the raw scores of M and X were standardized, i.e., converted to Z-scores, to produce an interaction term, i.e., (z)X*(z)M. To demonstrate moderation, the interaction term should be significantly associated with Y[63,65,66]. For mediation, Baron and Kenny[64] suggested that certain assumptions must be met. Specifically, the mediator (M) should be significantly associated with both the predictor (X) and the outcome (Y). In addition, an association between X and Y before controlling for M should be present alongside a significant decrease of the effect of X on Y (attenuation of the association) after controlling for M. If an initially significant association between X and Y is rendered non-significant after controlling for M, a complete mediation is present. Mediation analyses were performed using the Sobel test based on the “process” module developed by Hayes[67]. All analyses were carried out using the Statistical Package for Social Sciences (SPSS Inc., Chicago, IL), version 20.0. The statistical significance level was set at P < 0.05.
RESULTS
Participants’ characteristics and comparisons between CD and UC
The sample comprised 147 patients, 64 with CD (43.5%) and 83 with UC (56.5%). Table 1 presents the demographic, clinical and psychological profile of the sample. The median age was 46 years and 84 (57.1%) were female. Most patients (66.7%) were living with a spouse/partner and belonged to the mulatto race (65.3%). The median years of formal education received was 9.5 years and the median gross monthly income was 276 USD. The median time since IBD diagnosis was 57.5 mo, and the majority (82.3%) had not undergone surgery for IBD. A remarkable proportion (41.8%) had a moderate relapse rate (i.e., 3-5 relapses in the last 2 years) and were in remission at the time of the study (61.2%), according to the corresponding disease activity/severity indices. Figure 1 illustrates the distribution of patients by relapse rate alongside with current disease activity status (in remission or not). χ2 analysis revealed that disease activity/severity at the time of the study was not associated with previous relapse rate, either in CD (P = 0.109) or UC (P = 0.316), indicating that the patients status with respect to disease activity/severity at the time of the study was independent of their previous relapse rates. Regarding the prevalence of psychological distress symptoms, a quarter of IBD patients presented HADS scores indicative of clinically significant anxiety (25.2%) or depressive (24.5%) symptoms (Table 1).
Figure 1.
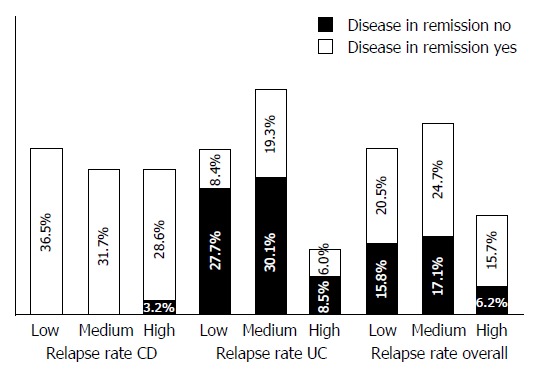
Distribution of patients by relapse rate together with the disease activity/severity status at the time of the study (in remission or not).
As also shown in Table 1, CD patients had a similar demographic profile to UC patients. However, the proportion of the patients with a higher relapse rate was higher for CD compared with UC patients (P = 0.023), and more CD patients had undergone surgery than their UC counterparts (P < 0.001). On the other hand, more CD than UC patients were in remission at the time of the study (P < 0.001).
Regarding psychological distress, although the mean scores on HADS-A and HADS-D were comparable between UC and CD, more CD than UC patients scored above the cut-off indicative of clinically significant levels of anxiety (32.8% vs 19.3%); however, this difference marginally failed to reach statistical significance (P = 0.061). Finally, most HRQoL domains were similar in CD and UC patients, with the exception of “satisfaction with health” (P = 0.008) and mental HRQoL (P = 0.03), where CD patients scored significantly lower than their UC counterparts (Table 1).
Variables associated with anxiety and depressive symptoms
As shown in Table 2, univariable analyses revealed that time since diagnosis and SOC correlated with anxiety symptoms, while gender, age, time since diagnosis and SOC correlated with depressive symptoms. Of note, SOC was not associated with relapse rate (r = 0.067, P = 0.424) or with disease activity/severity at the time of the study (for patients in remission SOC = 138.32 ± 25.73 and for those with active disease SOC = 138.48 ± 24.30, P = 0.969) in the total sample, and the same applied for CD and UC patient samples (data available upon request).
Table 2.
Univariable associations of demographic, clinical and psychological variables with anxiety, depression and health-related quality of life (n =147)
| Anxiety symptoms | Depressive symptoms | Overall HRQoL | Satisfaction with Health | Physical HRQoL | Mental HRQoL | Social Relations HRQoL | Environment HRQoL | |
| Sex1 | ||||||||
| Male | 5.01 ± 4.17 | 4.40 ± 3.2a | 4.02 ± 0.66 | 3.65 ± 0.99 | 3.65 ± 0.59b | 3.93 ± 0.47 | 3.96 ± 0.71 | 3.40 ± 0.54 |
| Female | 6.01 ± 4.37 | 5.93 ± 4.0 | 3.79 ± 0.89 | 3.55 ± 0.95 | 3.36 ± 0.65 | 3.73 ± 0.78 | 3.85 ± 0.77 | 3.36 ± 0.53 |
| Race2 | ||||||||
| Latin/Mixed-race | 5.40 ± 4.00 | 5.02 ± 3.62 | 3.95 ± 0.81 | 3.63 ± 0.93 | 3.50 ± 0.62 | 3.84 ± 0.67 | 3.92 ± 0.73 | 3.39 ± 0.55 |
| White | 5.80 ± 4.35 | 5.86 ± 3.98 | 3.66 ± 0.72 | 3.43 ± 1.01 | 3.47 ± 0.65 | 3.77 ± 0.72 | 3.70 ± 0.83 | 3.39 ± 0.48 |
| Other | 6.19 ± 5.91 | 5.50 ± 4.35 | 4.00 ± 0.89 | 3.75 ± 1.06 | 3.39 ± 0.77 | 3.75 ± 0.60 | 4.17 ± 0.58 | 3.29 ± 0.54 |
| Age3 | 0.125 | 0.188a | -0.181a | 0.081 | -0.196a | -0.036 | -0.244d | -0.078 |
| Education (yr)3 | 0.016 | -0.156 | 0.166a | 0.006 | 0.132 | 0.038 | 0.166a | 0.212a |
| Marital status1 | ||||||||
| Living alone | 5.40 ± 4.52 | 5.39 ± 4.30 | 3.86 ± 0.89 | 3.45 ± 0.96 | 3.47 ± 0.70 | 3.85 ± 0.82 | 3.87 ± 0.70 | 3.35 ± 0.59 |
| Living with spouse | 5.67 ± 4.21 | 5.21 ± 3.51 | 3.90 ± 0.77 | 3.66 ± 0.96 | 3.49 ± 0.62 | 3.80 ± 0.59 | 3.91 ± 0.77 | 3.39 ± 0.50 |
| Income3 | -0.022 | -0.123 | 0.045 | -0.060 | 0.107 | 0.087 | 0.116 | 0.252b |
| Disease1 | ||||||||
| CD | 6.00 ± 4.06 | 5.00 ± 3.83 | 3.94 ± 0.75 | 3.34 ± 1.10b | 3.42 ± 0.60 | 3.68 ± 0.61a | 3.98 ± 0.74 | 3.41 ± 0.54 |
| UC | 5.26 ± 4.47 | 5.48 ± 3.74 | 3.84 ± 0.85 | 3.78 ± 0.80 | 3.54 ± 0.67 | 3.92 ± 0.71 | 3.83 ± 0.74 | 3.36 ± 0.53 |
| Time since diagnosis3 | 0.274d | 0.259b | -0.1116 | -0.097 | -0.177a | -0.217b | -0.202a | 0.019 |
| Surgery for IBD1 | ||||||||
| No | 5.56 ± 4.38 | 5.50 ± 3.73 | 3.84 ± 0.82 | 3.59 ± 0.97 | 3.48 ± 0.63 | 3.82 ± 0.66 | 3.87 ± 0.73 | 3.34 ± 0.52 |
| Yes | 5.69 ± 4.00 | 4.23 ± 3.88 | 4.08 ± 0.74 | 3.62 ± 0.94 | 3.50 ± 0.70 | 3.81 ± 0.73 | 4.03 ± 0.80 | 3.56 ± 0.58 |
| Relapse rate4 | 0.141 | 0.057 | -0.040 | -0.077 | -0.228b | -0.136 | 0.009 | -0.212a |
| Disease activity1 | ||||||||
| In Remission | 5.71 ± 4.60 | 5.13 ± 3.97 | 3.90 ± 0.81 | 3.52 ± 1.03 | 3.50 ± 0.62 | 3.77 ± 0.63 | 3.95 ± 0.78 | 3.43 ± 0.57 |
| Active | 5.39 ± 3.81 | 5.49 ± 3.48 | 3.86 ± 0.81 | 3.70 ± 0.84 | 3.47 ± 0.68 | 3.89 ± 0.74 | 3.81 ± 0.69 | 3.29 ± 0.47 |
| SOC5 | -0.398d | -0.458d | 0.271d | 0.232b | 0.313d | 0.341d | 0.266b | 0.266b |
| Anxiety symptoms3 | N/A | 0.551d | -0.424d | -0.431d | -0.567d | -0.503d | -0.500d | -0.341d |
| Depressive symptoms3 | 0.551d | N/A | -0.327d | -0.272d | -0.463d | -0.473d | -0.459d | -0.375d |
Two-tailed t-test;
One-way ANOVA;
Pearson correlation coefficient;
Spearman correlation coefficient);
Sense of Coherence.
P < 0.01,
P < 0.01,
P < 0.05 vs control. IBD: Inflammatory bowel disease; CD: Crohn's disease; UC: Ulcerative colitis; NA: Not applicable; HRQoL: Health-related quality of life.
Hierarchical regression analysis performed with anxiety symptoms as the dependent variable (Table 3) showed that the demographic (model 1) and clinical (model 2) variables explained 6.2% of the variance in the HADS-A scale (P = 0.011); time since diagnosis and relapse rate were the significant independent correlates of anxiety symptoms. Addition of SOC scores increased the variance explained by 17.4% (P < 0.001); however, time since diagnosis was no longer significant, indicating that SOC might interact with disease duration in the formation of anxiety symptoms (model 3). In the final model, lower levels of SOC (P < 0.001) and higher relapse rates (P = 0.033) were the variables independently associated with higher levels of anxiety.
Table 3.
Hierarchical Regression analyses to assess the variables independently associated with depressive and anxiety symptoms, as measured by the Hospital Anxiety and Depression scale (n =147)
|
Depressive symptoms |
Anxiety symptoms |
|||||
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |
| Sex (female) | 0.151 | 0.130 | 0.176a | 0.093 | 0.038 | 0.078 |
| Race (white) | 0.091 | 0.064 | 0.164a | 0.004 | -0.047 | 0.040 |
| Race (other) | 0.052 | 0.029 | 0.060 | 0.021 | 0.023 | 0.050 |
| Age | 0.162 | 0.099 | 0.165 | 0.158 | 0.120 | 0.178 |
| Education | -0.055 | -0.085 | -0.087 | 0.135 | 0.124 | 0.122 |
| Living with spouse | -0.063 | -0.030 | 0.018 | -0.009 | 0.008 | 0.049 |
| Income | -0.098 | -0.108 | -0.007 | -0.061 | -0.096 | -0.008 |
| Disease (CD = 1, UC = 2) | - | 0.046 | 0.052 | - | -0.106 | -0.101 |
| Time since diagnosis | - | 0.235b | 0.100 | - | 0.282b | 0.165 |
| Surgery for IBD | - | -0.137 | -0.129 | - | -0.057 | -0.050 |
| Relapse rate | - | 0.057 | 0.039 | - | 0.177a | 0.161a |
| Disease in Remission | - | 0.044 | 0.048 | - | -0.030 | -0.027 |
| SOC score | - | - | -0.504d | - | - | -0.438d |
| Regression statistics | ||||||
| Adjusted R2 | 0.047 | 0.080 | 0.314 | -0.011 | 0.062 | 0.236 |
| Incremental adjusted R2 | 0.047 | 0.033 | 0.234 | -0.011 | 0.073 | 0.174 |
| Significance of F change | 0.058 | 0.088 | < 0.001 | 0.599 | 0.011 | < 0.001 |
Values shown are standardized beta regression coefficients.
P < 0.01,
P < 0.01,
P < 0.05 vs control.
Similar analysis with depressive symptoms as the criterion variable (Table 3) showed that the demographic and the clinical variables explained 8% of the variance in HADS-D (model 2); time since diagnosis was the only significant independent correlate of depressive symptoms. Addition of SOC score increased the variance explained by 23.4% (P < 0.001); again, time since diagnosis was no longer significant, indicating that SOC might interact with disease duration in the formation of depressive symptoms as well (model 3). In the final model, female gender (P = 0.021), white race (P = 0.033) and lower levels of SOC (P < 0.001) were the variables most closely independently associated with higher levels of depressive symptoms.
Moderation analysis
Given that the previous analyses raised the possibility of an interaction effect of SOC in the relationship of time since diagnosis with anxiety and depressive symptoms (Table 3), moderation analyses were performed to formally test and quantify the moderator effects[63]. As shown in Figure 2A, the slope of the relationship between depressive symptoms and time since diagnosis is steeper for people with low rather than high SOC scores (Pearson correlation coefficients: 0.315 and 0.106, respectively). Similarly, as shown in Figure 2B, the slope of the relationship between anxiety symptoms and time since diagnosis is steeper for people with low rather than high SOC scores (Pearson correlation coefficients: 0.335 and 0.039, respectively).
Figure 2.
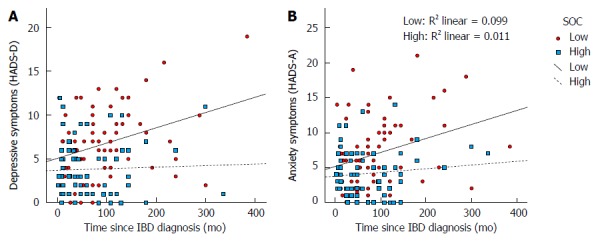
Scatterplots showing lines of best fit for low (solid line) and high (dashed line) sense of coherence patients in relationship with depressive symptoms (A) and anxiety symptoms (B) with time since inflammatory bowel disease diagnosis. Sense of coherence (SOC) was dichotomized based on its median. IBD: Inflammatory bowel disease.
Further hierarchical linear regression analyses (Table 4) confirmed that SOC moderated the relationship between depressive or anxiety symptoms and time since diagnosis, as the interaction term (time since diagnosis × SOC) had beta coefficients of -0.191 (P = 0.009) and -0.172 (P = 0.026), for depressive and anxiety symptoms as dependent variables, respectively (Tables 4). These data indicated that, in people with IBD, time since diagnosis is significantly associated with higher levels of anxiety and depressive symptoms only in patients with lower SOC scores.
Table 4.
Hierarchical multiple regression analyses to assess whether sense of coherence is a moderator of the relationship between time since diagnosis and psychological distress symptoms (i.e., depressive and anxiety symptoms) (n = 147)
|
Depressive symptoms |
Anxiety symptoms |
|||||
| model 1 | model 2 | model 3 | model 1 | model 2 | model 3 | |
| (z) Time since diagnosis | 0.235b | 0.100 | 0.068 | 0.282b | 0.165 | 0.135 |
| (z) SOC | - | -0.504d | -0.500d | - | -0.438d | -0.434d |
| (z) Time since diagnosis × SOC | - | - | -0.191d | - | - | -0.172d |
| Adjusted R2 of the model | 0.080 | 0.314 | 0.344 | 0.062 | 0.236 | 0.260 |
| Significance of F change | 0.026 | < 0.001 | 0.009 | 0.056 | < 0.001 | 0.026 |
Values shown are standardized beta regression coefficients adjusted for sex, race, age, education, marital status, income, disease type, surgery for Inflammatory bowel disease, relapse rate, and disease in remission.
P < 0.01,
P < 0.01 vs control.
Association of psychological distress and SOC with health-related quality of life
Univariable analyses shown in Table 2 revealed that anxiety and depressive symptoms, as well as SOC scores, were significantly correlated with all aspects of HRQoL, along with several demographic and clinical variables.
Hierarchical multiple regression analysis performed with overall HRQoL as the dependent variable showed that, in the final step (Table 5, model 5), lower levels of anxiety (P < 0.001) and higher levels of SOC (P = 0.016) were independently associated with higher levels of overall HRQoL. The relationship of SOC with overall HRQoL held even after the inclusion of depressive and anxiety symptoms. Notably, depressive symptoms were only weakly associated with overall HRQoL.
Table 5.
Hierarchical multiple regression analyses to assess variables independently associated with overall health-related quality of life and satisfaction with health (n = 147)
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
| Overall HRQoL | Satisfaction with Health | |||||||||
| Sex (female) | -0.119 | -0.120 | -0.152 | -0.136 | -0.135 | -0.101 | -0.080 | -0.102 | -0.062 | -0.061 |
| Race (white) | -0.153 | -0.162 | -0.233b | -0.218a | -0.229b | -0.101 | -0.096 | -0.146 | -0.109 | -0.121 |
| Race (other) | 0.017 | 0.033 | 0.011 | 0.016 | 0.025 | 0.017 | -0.021 | -0.036 | -0.023 | -0.013 |
| Age | -0.125 | -0.115 | -0.163 | -0.148 | -0.108 | 0.143 | 0.128 | 0.095 | 0.132 | 0.176 |
| Education | -0.103 | 0.127 | 0.129 | 0.121 | 0.180 | 0.105 | 0.081 | 0.082 | 0.063 | 0.127 |
| Living with spouse | 0.060 | 0.042 | 0.008 | 0.010 | 0.025 | 0.087 | 0.095 | 0.071 | 0.075 | 0.092 |
| Income | 0.013 | 0.001 | -0.071 | -0.072 | -0.074 | -0.125 | -0.120 | -0.170 | -0.172 | -0.174a |
| Disease (CD = 1, UC = 2) | - | -0.096 | -0.100 | -0.096 | -0.141 | - | 0.326b | 0.323b | 0.335b | 0.285b |
| Time since diagnosis | - | -0.051 | 0.045 | 0.054 | 0.099 | - | -0.092 | -0.024 | -0.002 | 0.048 |
| Surgery for IBD | - | 0.117 | 0.111 | 0.099 | 0.101 | - | 0.121 | 0.117 | 0.087 | 0.089 |
| Relapse rate | - | -0.074 | -0.061 | -0.057 | -0.004 | - | -0.041 | -0.032 | -0.023 | 0.036 |
| Disease in Remission | - | -0.099 | -0.101 | -0.097 | -0.114 | - | 0.099 | 0.097 | 0.108 | 0.089 |
| SOC score | - | - | 0.359d | 0.314b | 0.231a | - | - | 0.251b | 0.137 | 0.045 |
| Depressive symptoms | - | - | - | -0.090 | 0.067 | - | - | - | -0.227a | -0.052 |
| Anxiety symptoms | - | - | - | - | -0.369d | - | - | - | - | -0.409d |
| Regression Statistics | ||||||||||
| Adjusted R2 | 0.036 | 0.029 | 0.144 | 0.143 | 0.224 | -0.007 | 0.035 | 0.087 | 0.116 | 0.218 |
| Incremental adjusted R2 | 0.036 | -0.007 | 0.115 | -0.001 | 0.081 | -0.007 | 0.042 | 0.042 | 0.029 | 0.102 |
| Significance of F change | 0.102 | 0.539 | < 0.001 | 0.360 | < 0.001 | 0.547 | 0.058 | 0.004 | 0.025 | < 0.001 |
| Physical HRQoL | Mental HRQoL | |||||||||
| Sex (female) | -0.187a | -0.147 | -0.179a | -0.127 | -0.126 | -0.150 | -0.104 | -0.137 | -0.075 | -0.074 |
| Race (white) | -0.007 | 0.017 | -0.054 | -0.005 | -0.017 | -0.041 | -0.001 | -0.073 | -0.015 | -0.023 |
| Race (other) | -0.042 | -0.069 | -0.091 | -0.073 | -0.064 | -0.034 | -0.048 | -0.071 | -0.050 | -0.043 |
| Age | -0.180 | -0.187 | -0.234a | -0.185a | -0.145 | -0.030 | -0.008 | -0.057 | 0.002 | 0.032 |
| Education | 0.002 | -0.006 | -0.004 | -0.029 | 0.031 | -0.041 | -0.045 | -0.043 | -0.074 | -0.031 |
| Living with spouse | 0.068 | 0.059 | 0.025 | 0.030 | 0.046 | -0.018 | -0.020 | -0.055 | -0.049 | -0.038 |
| Income | 0.086 | 0.099 | 0.028 | 0.026 | 0.024 | 0.082 | 0.114 | 0.040 | 0.038 | 0.036 |
| Disease (CD = 1, UC = 2) | - | 0.172 | 0.168 | 0.183 | 0.136 | - | 0.205 | 0.201 | 0.219a | 0.186 |
| Time since diagnosis | - | -0.140 | -0.045 | -0.015 | 0.031 | - | -0.231a | -0.133 | -0.097 | -0.064 |
| Surgery for IBD | - | 0.060 | 0.055 | 0.016 | 0.018 | - | 0.061 | 0.055 | 0.009 | 0.010 |
| Relapse rate | - | -0.239b | -0.226b | -0.214b | -0.160a | - | -0.090 | -0.076 | -0.063 | -0.023 |
| Disease in Remission | - | 0.112 | 0.110 | 0.124 | 0.106 | - | 0.038 | 0.036 | 0.053 | 0.040 |
| SOC score | - | - | 0.356d | 0.208a | 0.123 | - | - | 0.368d | 0.188a | 0.128 |
| Depressive symptoms | - | - | - | -0.295b | -0.134 | - | - | - | -0.357d | -0.241a |
| Anxiety symptoms | - | - | - | - | -0.378d | - | - | - | - | -0.270b |
| Regression Statistics | ||||||||||
| Adjusted R2 | 0.047 | 0.105 | 0.219 | 0.273 | 0.360 | -0.012 | 0.039 | 0.159 | 0.241 | 0.282 |
| Incremental adjusted R2 | 0.047 | 0.058 | 0.114 | 0.054 | 0.087 | -0.012 | 0.051 | 0.120 | 0.082 | 0.041 |
| Significance of F change | 0.059 | 0.020 | < 0.001 | 0.001 | < 0.001 | 0.631 | 0.037 | < 0.001 | < 0.001 | 0.004 |
| Social relations HRQoL | Environmental HRQoL | |||||||||
| Sex (female) | -0.003 | 0.015 | -0.012 | 0.042 | 0.043 | 0.008 | 0.013 | -0.015 | 0.029 | 0.029 |
| Race (white) | -0.109 | -0.085 | -0.146 | -0.095 | -0.106 | 0.007 | -0.001 | -0.062 | -0.021 | -0.026 |
| Race (other) | 0.110 | 0.137 | -0.118 | 0.136 | 0.146a | -0.052 | -0.068 | -0.087 | -0.072 | -0.068 |
| Age | -0.279b | -0.233a | -0.274b | -0.222a | -0.181a | -0.140 | -0.154 | -0.195a | -0.154 | -0.137 |
| Education | -0.008 | 0.022 | 0.023 | -0.004 | 0.057 | 0.005 | 0.006 | 0.007 | -0.014 | 0.012 |
| Living with spouse | 0.063 | 0.037 | 0.008 | 0.013 | 0.029 | 0.095 | 0.076 | 0.046 | 0.050 | 0.057 |
| Income | 0.157 | 0.169 | 0.107 | 0.105 | 0.103 | 0.264b | 0.245b | 0.183a | 0.181a | 0.181a |
| Disease (CD = 1, UC = 2) | - | -0.136 | -0.139 | -0.123 | -0.170 | - | 0.024 | 0.020 | 0.033 | 0.013 |
| Time since diagnosis | - | -0.162 | -0.080 | -0.048 | -0.002 | - | -0.003 | 0.080 | 0.105 | 0.125 |
| Surgery for IBD | - | 0.046 | 0.042 | 0.001 | 0.003 | - | 0.083 | 0.078 | 0.046 | 0.047 |
| Relapse rate | - | -0.082 | -0.071 | -0.059 | -0.004 | - | -0.214a | -0.203a | -0.194a | -0.170a |
| Disease in Remission | - | -0.067 | -0.069 | -0.054 | -0.072 | - | 0.101 | 0.099 | 0.111 | 0.103 |
| SOC score | - | - | 0.309d | 0.152 | 0.067 | - | - | 0.310d | 0.185 | 0.148 |
| Depressive symptoms | - | - | - | -0.311b | -0.148 | - | - | - | -0.248a | -0.178 |
| Anxiety symptoms | - | - | - | - | -0.383d | - | - | - | - | -0.164 |
| Regression Statistics | ||||||||||
| Adjusted R2 | 0.077 | 0.084 | 0.167 | 0.228 | 0.317 | 0.045 | 0.069 | 0.153 | 0.189 | 0.200 |
| Incremental adjusted R2 | 0.077 | 0.007 | 0.083 | 0.061 | 0.089 | 0.045 | 0.024 | 0.084 | 0.036 | 0.011 |
| Significance of F change | 0.012 | 0.303 | < 0.001 | 0.001 | < 0.001 | 0.066 | 0.136 | < 0.001 | 0.010 | 0.097 |
Values shown are standardized beta regression coefficients.
P < 0.01,
P < 0.01,
P < 0.05 vs control. IBD: Inflammatory bowel disease; CD: Crohn's disease; UC: Ulcerative colitis; HRQoL: Health-related quality of life.
Similar analysis with “satisfaction with health” as the dependent variable revealed that having CD was associated with poorer satisfaction with general health in all steps of this analysis (Table 5, models 2-5, P = 0.008). The addition of SOC in model 3 increased the variance explained by 4.2% (P = 0.004), but addition of depressive symptoms rendered the relationship of SOC with “satisfaction with health” non-significant, indicating that depressive symptoms might mediate the relationship of SOC with “satisfaction with health”. Addition of anxiety symptoms further increased the variance by 10.2% (model 5, P < 0.001) and rendered the previously significant relationship of depressive symptoms non-significant. In the final step, lower levels of anxiety symptoms (P < 0.001), having UC (P = 0.008) and lower income (P = 0.048) were the variables independently associated with higher levels of “satisfaction with health”.
The same patterns regarding the relationship of psychological distress and SOC with HRQoL domains emerged in the hierarchical analyses performed with the other four WHOQOL-Bref components as dependent variables. SOC was initially associated with physical, mental, social relations and environment HRQoL, but these relationships became non-significant after inclusion of psychological distress symptoms (Table 5). In the final models, lower relapse rate (P = 0.024) and lower levels of anxiety symptoms (P < 0.001) were the important independent correlates of better physical HRQoL (Table 5). Lower levels of anxiety (P = 0.004) and lower levels of depressive symptoms (P = 0.015) were the significant independent correlates of better mental HRQoL (Table 5). Younger age (P = 0.047), race other than Latin or White (P = 0.049) and lower levels of anxiety symptoms (P < 0.001) were the important independent correlates of better social relations HRQoL (Table 5). Finally, higher income (P = 0.043) and lower relapse rate (P = 0.031) were the variables independently associated with better environmental HRQoL (Table 5).
Mediation analysis
SOC was associated with all HRQoL domains and this association was either significantly attenuated or became non-significant following the inclusion of psychological distress symptoms (suggesting that anxiety/depressive symptoms may mediate the effects of SOC upon certain domains of HRQoL); therefore, we assessed any indirect (i.e., mediator) effects through formal mediation analyses. Six separately produced mediation analyses were performed (Table 6).
Table 6.
Mediation analysis (Sobel test) to assess whether the effect of sense of coherence on health-related quality of life domains is mediated by depressive and/or anxiety symptoms
|
Specific indirect effects |
|||
| via depressive symptoms | via anxiety symptoms | ||
| Direct effect | Indirect effect; Z | Indirect effect; Z | |
| Overall HRQoL | 0.008a | -0.001; -0.648 | 0.005; 3.112b |
| Satisfaction with Health | 0.002 | 0.001; 0.503 | 0.007; 3.325d |
| Physical HRQoL | 0.003 | 0.002; 1.399 | 0.004; 3.372d |
| Mental HRQoL | 0.004 | 0.003; 2.290a | 0.003; 2.546a |
| Social relations HRQoL | 0.002 | 0.002; 1.488 | 0.005; 3.328d |
| Environment HRQoL | 0.003 | 0.002; 1.650 | 0.001; 1.578 |
Direct effect represents the independent effect of the predictor on the outcome after controlling for potential mediators; predictor: SOC; mediators: anxiety symptoms, depressive symptoms; control variables: sex, race, age, education, marital status, income, disease type, time since IBD diagnosis, surgery for IBD, relapse rate, and disease in remission.
P < 0.01,
P < 0.01,
P < 0.05 vs control. HRQoL: Health-related quality of life.
In a preliminary step, we investigated whether the assumptions suggested by Baron and Kenny[64] were met for a mediation to be present. As shown in univariate and multivariate analyses, all associations between outcome variables (HRQoL domains) with both the predictor (SOC) and the potential mediators (anxiety and depressive symptoms), as well as between the predictor and the mediators, were significant, thus supporting the applicability of the mediation analyses.
As shown in Table 6, anxiety symptoms significantly (and fully) mediated the effects of SOC upon certain HRQoL domains, namely satisfaction with health, physical, mental, and social relations HRQoL. In addition, anxiety symptoms partially mediated the effect of SOC on overall HRQoL: a significant indirect effect combined with a significant direct effect of SOC on overall HRQoL was found. Conversely, depressive symptoms did not mediate the effects of SOC upon most HRQoL dimensions, namely physical, social relations and environment HRQoL, with the exception of mental HRQoL, where depressive symptoms fully mediated the effect of SOC on mental HRQoL (Table 6).
DISCUSSION
The results of the present study showed that lower levels of SOC are associated with higher levels of anxiety and depressive symptoms, independent of demographic and disease-related variables, confirming our first hypothesis. The relapse rate also correlated with anxiety symptoms, while time since diagnosis was associated with anxiety and depressive symptoms only in patients with lower SOC scores. Anxiety symptoms were the most important independent correlate of most aspects of HRQoL, mediating the effects of SOC and depressive symptoms upon physical and social relations HRQoL, and satisfaction with general health. Anxiety and depressive symptoms were also independent correlates of mental HRQoL, while SOC was an independent correlate of overall HRQoL. These findings confirmed our second and third hypotheses. To the best of our knowledge, this is the first study demonstrating the relationship of SOC with psychological distress and HRQoL in IBD.
Our study confirmed that psychological distress symptoms are frequent in IBD patients. We found a prevalence of clinically relevant anxiety or depressive symptoms of 25.2% and 24.5%, respectively, while 15.6% had a positive screen for both anxiety and depression. Studies using the same instrument (HADS) have reported a prevalence of clinically relevant anxiety and/or depressive symptoms of up to 43% in IBD patients[68], while studies using the BDI-PC reported a prevalence of 25% for depression[69]. Regarding the clinical variables studied, we found here that anxiety symptoms were independently associated with relapse rate, in line with previous findings[8,70]. By contrast, depressive symptoms were not found to be strongly associated with relapse rate or illness severity. Instead, they were associated with time since diagnosis, although our moderation analysis showed that the relationship of both anxiety and depressive symptoms with time since diagnosis holds only for those patients with lower capacities to cope with the illness, i.e., those with lower SOC scores. These findings suggested that acute or sub-acute phenomena, such as relapses, are associated with anxiety, whereas chronic mild stress, such as having a long-term disabling condition for several years, is rather associated with depressive symptomatology, especially for IBD patients with limited coping resources.
Our first main new finding is that SOC was independently associated with psychological distress, i.e., anxiety and depressive symptoms. Opheim et al[25] assessed SOC in IBD patients using the short 13-item version of SOC scale and, similar to the findings of our study, found no association between SOC and disease activity measures. They also found that SOC was inversely associated with the Five-item Fatigue Severity Scale (FSS-5)[71], and that higher SOC scores were associated with better self-efficacy, but a measure of psychological distress was lacking. The relationship of SOC with psychological distress has been studied in other chronic medical conditions, such as myocardial infarction[72], chronic obstructive pulmonary disease (COPD)[73], systemic sclerosis[74], rheumatoid arthritis[46] and colorectal cancer[23], where significant inverse associations between SOC and psychological distress symptoms have been demonstrated. Our findings demonstrate that the same applies to patients with IBD. Furthermore, the present findings provide support for previous suggestions that medical interventions should not only aim at alleviating the disease, but also at improving health by strengthening a person’s SOC and by considering person-centered medicine[30]. Therefore, it is essential for gastroenterologists to be able to identify patients with difficulties in coping with IBD. Notwithstanding that our preliminary results deserve replication in prospective studies, the SOC scale could be a useful and time-efficient method to detect psychologically vulnerable IBD patients and refer them for psycho-educational and/or psychotherapeutic interventions aiming to prevent the development of psychopathology.
Our second new finding is that SOC was independently associated with overall HRQoL. Moreover, SOC was also associated with all other aspects of HRQoL, but these relationships were mediated by psychological distress, mostly anxiety symptoms. Although the relationship of SOC with health outcomes has been challenged[41,42], especially its potential to predict physical health[43], its relationship with HRQoL is well documented, and evidence suggests that a strong SOC predicts better HRQoL[39]. However, extremely limited numbers of studies have investigated the association of SOC with HRQoL in patients with IBD. In line with the findings of the present study, in their study with 113 patients who had undergone a resection of the sigmoid colon or rectum including 53 IBD patients, Siassi et al[75] found that SOC was longitudinally associated with emotional HRQoL and, to a lesser extent, with the disease-specific Gastrointestinal Quality of Life Index (GLQI)[76]. The authors pointed out that the effect of personality on HRQoL by far exceeded the influence of common clinical variables. Our findings further confirmed the results of the aforementioned study by addressing the association of SOC and HRQoL in a larger and more homogeneous sample of IBD patients, suggesting that SOC may be considered to better determine the complex interactions between psychological distress and HRQoL in IBD.
Notably, anxiety symptoms were the major independent correlate of most aspects of HRQoL. Several studies have shown the association of psychological distress with HRQoL in IBD[77-80]. A prospective study indicated that anxiety is associated with impaired HRQoL[12], while Hyphantis et al[19] reported that psychological distress was strongly associated with impaired HRQoL in IBD, but somatization mediated this relationship. Our findings regarding the association of anxiety with HRQoL indicated that, apart from the early recognition and treatment of depression, clinicians should pay closer attention to IBD patients’ anxiety symptoms. Accordingly, IBD patients experience significant anxious cognitions related to the worry and fear of bowel symptoms in the social context[81].
Some limitations of this study should be addressed. First, the cross-sectional design did not allow inferences concerning causality to be drawn. Second, the drawback of using only self-reported measures for independent psychological predictors and HRQoL means that we could not refute the criticism that an underlying response style might have led to our results. Third, our sample was drawn from a specialized outpatient Gastroenterology department in a tertiary university hospital; therefore it may not be representative. In addition, our sample comprised patients with a rather “stable” disease, as 61.2% of the patients were in remission, a percentage that increased to 96.9% in CD patients. The low rate of active disease at the time of the study, particularly in CD patients, might partly be accounted for by the exclusion criteria applied, according to which, patients hospitalized at the time of the study were excluded. The large number of patients in remission at the time of the study may also reflect successful clinical management, raising an important consideration regarding how effectively these patients are being treated for their disease. We should also acknowledge that, as the disease activity/severity indices (CDAI/TWT) were categorized cumulatively to obtain a comparable index applicable to both types of IBD, the contribution of current disease state (i.e., in remission or not) to psychological distress and HRQoL should be interpreted with caution, because it was extracted from different scoring systems, although established cut-offs were used. Finally, the application of SOC as a “salutogenic index” is not uniformly supported by all studies, and some studies have recommended caution in the application of SOC to different populations[82,83].
On the other hand, the strengths of this study include the high response rate (98%), the use of widely available validated instruments and the fact that our findings are in accordance to previous results derived from similar studies. In addition, the concurrent assessment of disease severity both retrospectively (number of relapses in the past 2 years) and currently (by means of disease-specific activity measures such as CDAI and TWT) represent an additional strength of this study, given that ‘disease activity’ varies across studies, resulting in some controversy. More specifically, disease activity is often assessed retrospectively based on self-reporting of previous relapses[84] or a current self-reported perception of disease activity[78]. Thus, we offered discrimination between current (disease in remission, assessed by objective measures) and previous disease activity.
The present study might have important clinical implications. Psychological distress symptoms were found to be quite prevalent among patients with IBD and were inversely related to HRQoL. Despite the high rates of anxiety and/or depressive symptoms in patients with IBD, appropriate treatment remains an unmet need for the majority of the patients[68]. Given the deleterious effects of psychological distress symptoms in patients with IBD, timely recognition and management of both depressive and anxiety symptoms is imperative. Notably, anxiety symptoms emerged as the most potent correlate of impaired HRQoL, often rendering the association between depressive symptoms and certain domains of HRQoL non-significant. Moreover, anxiety was the psychological distress symptom that was associated with a higher relapse rate. Therefore, in support of previous findings, this study highlighted the importance of assessing and discussing anxiety, apart from depression, with IBD patients within the medical setting. However, whether psychological interventions have an impact on the treatment of IBD is a matter of debate. A recent systematic review found no evidence for efficacy of psychological therapy in adult patients with IBD in general, while psychological interventions in adolescents may be beneficial, suggesting that further research is needed to assess the efficacy of these therapies in subgroups identified as being in need of psychological interventions, and to identify what type of therapy may be most useful[85]. In the present study, SOC emerged as an important concept for IBD patients, as higher levels of SOC were independently associated with lower levels of psychological distress symptoms and higher levels of overall HRQoL; SOC was also found to buffer the negative impact of longer disease duration on psychological well-being. Therefore, our findings suggest that treatment strategies focusing on enhancing SOC, especially in those IBD patients with lower levels of SOC, might result in alleviating psychological distress, and in turn enhancing HRQoL, or might directly exert a positive effect on overall HRQoL, an hypothesis that remains to be confirmed in future longitudinal studies. Evidence suggests that SOC can be strengthened through personal activity and care[86], psychodynamic psychotherapy[87] or group-based cognitive behavioral therapy[88], which have been shown to enhance SOC and promote HRQoL in people with chronic medical conditions[88].
COMMENTS
Background
Patients with inflammatory bowel disease (IBD) often experience remarkable levels of psychological distress, leading to negative outcomes and reducing the patients’ quality of life by amplifying symptom burden, decreasing treatment adherence and increasing disability. Living with IBD is a severe psychological distress and it is likely that the patient’s predominant coping abilities may influence his or her psychological adaptation and the formation of quality of life. Studies in other chronic medical illnesses have shown that the patients’ capacities to cope with health-stressors are important determinants of quality of life.
Research frontiers
Sense of coherence (SOC) is a theoretical construct that aims to explain why some people, regardless of major stressful situations and severe hardships, fall ill and others do not. It is assumed that an individual with a strong SOC maintains and enhances health through effective and flexible coping with stressors by adopting health-enhancing and avoiding unhealthy behaviors. The current research hotspot is how SOC influences psychological distress and quality of life in patients with IBD, while simultaneously interacting with disease-related parameters.
Innovations and breakthroughs
Previous studies in IBD found that SOC was associated with self-efficacy and fatigue. Here, we confirmed that lower levels of SOC are associated with higher levels of anxiety and depressive symptoms, and impaired overall quality of life. We also found that time since diagnosis is only associated with anxiety and depressive symptoms in patients with lower levels of SOC, and that anxiety symptoms are the most important independent correlate of quality of life.
Applications
The results of the present study highlighted the importance of assessing and discussing anxiety, apart from depression, with IBD patients within the medical setting. In addition, treatment strategies focusing on enhancing SOC, especially in those IBD patients with lower levels of SOC, might result in alleviating psychological distress and enhancing quality of life. Therefore, it is essential for gastroenterologists to be able to identify patients with difficulties in coping with IBD, and the SOC scale could be a useful and time-efficient method to detect psychologically vulnerable IBD patients and refer them for psycho-educational and/or psychotherapeutic interventions: studies have shown that SOC can be strengthened through personal activity and care, psychodynamic psychotherapy or group-based cognitive behavioral therapy.
Terminology
SOC is defined as a global orientation that expresses the extent to which one has a pervasive, enduring, though dynamic, feeling of confidence, taking into consideration that (1) the stimuli deriving from one’s internal and external environments in the course of living are structured, predictable and explicable (comprehensibility); (2) the resources are available to one to meet the demands posed by these stimuli (manageability); and (3) these demands are challenges worthy of investment and engagement (meaningfulness).
Peer-review
This paper focuses on the influence of psychological factors, including Sense of Coherence, psychological distress and Health-Related Quality of Life in IBD, as a means of assessing how individual psychological experiences potentially contribute to disease penetration or persistence in IBD. The SOC is a health-promoting model reflecting a person’s worldview and responsiveness to stress situations like IBD, and may be important in adaptation to such chronic illnesses. Without doubt the topic of the manuscript is interesting and a contemporary issue in the IBD community.
Footnotes
Ethics approval: The study was reviewed and approved by the Hospital Universitario Walter Cantidio, Federal University of Ceará, Fortaleza, CE, Brazil Institutional Review Board, No. 001.02.12.
Informed consent: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest: The authors have no competing interests to report.
Data sharing: The technical appendix, statistical code, and dataset are available from the corresponding author at tyfantis@cc.uoi.gr. Participants’ consent was not obtained but the presented data are anonymized and the risk of identification is low.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: November 30, 2014
First decision: December 26, 2014
Article in press: February 5, 2015
P- Reviewer: Alexander JS, Jahnel J S- Editor: Qi Y L- Editor: Stewart G E- Editor: Wang CH
References
- 1.Levine JS, Burakoff R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Hepatol (N Y) 2011;7:235–241. [PMC free article] [PubMed] [Google Scholar]
- 2.Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380:1590–1605. doi: 10.1016/S0140-6736(12)60026-9. [DOI] [PubMed] [Google Scholar]
- 3.Simian D, Quijada MI, Lubascher J, Acuña R, Quera R. [Treatment of inflammatory bowel disease with infliximab: experience in 25 patients] Rev Med Chil. 2013;141:1158–1165. doi: 10.4067/S0034-98872013000900008. [DOI] [PubMed] [Google Scholar]
- 4.Filipovic BR, Filipovic BF. Psychiatric comorbidity in the treatment of patients with inflammatory bowel disease. World J Gastroenterol. 2014;20:3552–3563. doi: 10.3748/wjg.v20.i13.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graff LA, Walker JR, Bernstein CN. Depression and anxiety in inflammatory bowel disease: a review of comorbidity and management. Inflamm Bowel Dis. 2009;15:1105–1118. doi: 10.1002/ibd.20873. [DOI] [PubMed] [Google Scholar]
- 6.Fuller-Thomson E, Sulman J. Depression and inflammatory bowel disease: findings from two nationally representative Canadian surveys. Inflamm Bowel Dis. 2006;12:697–707. doi: 10.1097/00054725-200608000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Häuser W, Janke KH, Klump B, Hinz A. Anxiety and depression in patients with inflammatory bowel disease: comparisons with chronic liver disease patients and the general population. Inflamm Bowel Dis. 2011;17:621–632. doi: 10.1002/ibd.21346. [DOI] [PubMed] [Google Scholar]
- 8.Mittermaier C, Dejaco C, Waldhoer T, Oefferlbauer-Ernst A, Miehsler W, Beier M, Tillinger W, Gangl A, Moser G. Impact of depressive mood on relapse in patients with inflammatory bowel disease: a prospective 18-month follow-up study. Psychosom Med. 2004;66:79–84. doi: 10.1097/01.psy.0000106907.24881.f2. [DOI] [PubMed] [Google Scholar]
- 9.Panara AJ, Yarur AJ, Rieders B, Proksell S, Deshpande AR, Abreu MT, Sussman DA. The incidence and risk factors for developing depression after being diagnosed with inflammatory bowel disease: a cohort study. Aliment Pharmacol Ther. 2014;39:802–810. doi: 10.1111/apt.12669. [DOI] [PubMed] [Google Scholar]
- 10.Greenley RN, Hommel KA, Nebel J, Raboin T, Li SH, Simpson P, Mackner L. A meta-analytic review of the psychosocial adjustment of youth with inflammatory bowel disease. J Pediatr Psychol. 2010;35:857–869. doi: 10.1093/jpepsy/jsp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katon W, Ciechanowski P. Impact of major depression on chronic medical illness. J Psychosom Res. 2002;53:859–863. doi: 10.1016/s0022-3999(02)00313-6. [DOI] [PubMed] [Google Scholar]
- 12.Iglesias-Rey M, Barreiro-de Acosta M, Caamaño-Isorna F, Rodríguez IV, Ferreiro R, Lindkvist B, González AL, Dominguez-Munoz JE. Psychological factors are associated with changes in the health-related quality of life in inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:92–102. doi: 10.1097/01.MIB.0000436955.78220.bc. [DOI] [PubMed] [Google Scholar]
- 13.Lix LM, Graff LA, Walker JR, Clara I, Rawsthorne P, Rogala L, Miller N, Ediger J, Pretorius T, Bernstein CN. Longitudinal study of quality of life and psychological functioning for active, fluctuating, and inactive disease patterns in inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:1575–1584. doi: 10.1002/ibd.20511. [DOI] [PubMed] [Google Scholar]
- 14.Petrak F, Hardt J, Clement T, Börner N, Egle UT, Hoffmann SO. Impaired health-related quality of life in inflammatory bowel diseases: psychosocial impact and coping styles in a national German sample. Scand J Gastroenterol. 2001;36:375–382. doi: 10.1080/003655201300051171. [DOI] [PubMed] [Google Scholar]
- 15.Sainsbury A, Heatley RV. Review article: psychosocial factors in the quality of life of patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2005;21:499–508. doi: 10.1111/j.1365-2036.2005.02380.x. [DOI] [PubMed] [Google Scholar]
- 16.Casellas F, López-Vivancos J, Casado A, Malagelada JR. Factors affecting health related quality of life of patients with inflammatory bowel disease. Qual Life Res. 2002;11:775–781. doi: 10.1023/a:1020841601110. [DOI] [PubMed] [Google Scholar]
- 17.Casellas F, Arenas JI, Baudet JS, Fábregas S, García N, Gelabert J, Medina C, Ochotorena I, Papo M, Rodrigo L, et al. Impairment of health-related quality of life in patients with inflammatory bowel disease: a Spanish multicenter study. Inflamm Bowel Dis. 2005;11:488–496. doi: 10.1097/01.mib.0000159661.55028.56. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka M, Kazuma K. Ulcerative colitis: factors affecting difficulties of life and psychological well being of patients in remission. J Clin Nurs. 2005;14:65–73. doi: 10.1111/j.1365-2702.2004.00955.x. [DOI] [PubMed] [Google Scholar]
- 19.Hyphantis TN, Tomenson B, Bai M, Tsianos E, Mavreas V, Creed F. Psychological distress, somatization, and defense mechanisms associated with quality of life in inflammatory bowel disease patients. Digest Dis Sci. 2010;55:724–732. doi: 10.1007/s10620-009-0762-z. [DOI] [PubMed] [Google Scholar]
- 20.Vidal A, Gómez-Gil E, Sans M, Portella MJ, Salamero M, Piqué JM, Panés J. Health-related quality of life in inflammatory bowel disease patients: the role of psychopathology and personality. Inflamm Bowel Dis. 2008;14:977–983. doi: 10.1002/ibd.20388. [DOI] [PubMed] [Google Scholar]
- 21.Boye B, Jahnsen J, Mokleby K, Leganger S, Jantschek G, Jantschek I, Kunzendorf S, Benninghoven D, Wilhelmsen I, Sharpe M, et al. The INSPIRE study: are different personality traits related to disease-specific quality of life (IBDQ) in distressed patients with ulcerative colitis and Crohn’s disease? Inflamm Bowel Dis. 2008;14:680–686. doi: 10.1002/ibd.20367. [DOI] [PubMed] [Google Scholar]
- 22.Moreno-Jiménez B, López Blanco B, Rodríguez-Muñoz A, Garrosa Hernández E. The influence of personality factors on health-related quality of life of patients with inflammatory bowel disease. J Psychosom Res. 2007;62:39–46. doi: 10.1016/j.jpsychores.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 23.Sales PM, Carvalho AF, McIntyre RS, Pavlidis N, Hyphantis TN. Psychosocial predictors of health outcomes in colorectal cancer: a comprehensive review. Cancer Treat Rev. 2014;40:800–809. doi: 10.1016/j.ctrv.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Chumbler NR, Kroenke K, Outcalt S, Bair MJ, Krebs E, Wu J, Yu Z. Association between sense of coherence and health-related quality of life among primary care patients with chronic musculoskeletal pain. Health Qual Life Outcomes. 2013;11:216. doi: 10.1186/1477-7525-11-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Opheim R, Fagermoen MS, Jelsness-Jørgensen LP, Bernklev T, Moum B. Sense of coherence in patients with inflammatory bowel disease. Gastroenterol Res Pract. 2014;2014:989038. doi: 10.1155/2014/989038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geyer S. Some conceptual considerations on the sense of coherence. Soc Sci Med. 1997;44:1771–1779. doi: 10.1016/s0277-9536(96)00286-9. [DOI] [PubMed] [Google Scholar]
- 27.Eriksson M, Lindström B. Antonovsky’s sense of coherence scale and the relation with health: a systematic review. J Epidemiol Community Health. 2006;60:376–381. doi: 10.1136/jech.2005.041616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antonovsky A. Unraveling the mystery of health: how people manage stress and stay well. 1st ed. San Francisco: Jossey-Bass; 1987. [Google Scholar]
- 29.Sagy S, Antonovsky A, Adler I. Explaining life satisfaction in later life: The sense of coherence model and activity theory. Behavior, Health and Aging. 1990;1:11–25. [Google Scholar]
- 30.Alivia M, Guadagni P, Roberti di Sarsina P. Towards salutogenesis in the development of personalised and preventive healthcare. EPMA J. 2011;2:381–384. doi: 10.1007/s13167-011-0131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moons P, Norekvål TM. Is sense of coherence a pathway for improving the quality of life of patients who grow up with chronic diseases? A hypothesis. Eur J Cardiovasc Nurs. 2006;5:16–20. doi: 10.1016/j.ejcnurse.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 32.Lindström B, Eriksson M. Salutogenesis. J Epidemiol Community Health. 2005;59:440–442. doi: 10.1136/jech.2005.034777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schnyder U, Büchi S, Sensky T, Klaghofer R. Antonovsky’s sense of coherence: trait or state? Psychother Psychosom. 2000;69:296–302. doi: 10.1159/000012411. [DOI] [PubMed] [Google Scholar]
- 34.Karlsson I, Berglin E, Larsson PA. Sense of coherence: quality of life before and after coronary artery bypass surgery--a longitudinal study. J Adv Nurs. 2000;31:1383–1392. doi: 10.1046/j.1365-2648.2000.01408.x. [DOI] [PubMed] [Google Scholar]
- 35.Forsberg A, Bäckman L, Svensson E. Liver transplant recipients’ ability to cope during the first 12 months after transplantation--a prospective study. Scand J Caring Sci. 2002;16:345–352. doi: 10.1046/j.1471-6712.2002.00100.x. [DOI] [PubMed] [Google Scholar]
- 36.Snekkevik H, Anke AG, Stanghelle JK, Fugl-Meyer AR. Is sense of coherence stable after multiple trauma? Clin Rehabil. 2003;17:443–453. doi: 10.1191/0269215503cr630oa. [DOI] [PubMed] [Google Scholar]
- 37.Wolff AC, Ratner PA. Stress, social support, and sense of coherence. West J Nurs Res. 1999;21:182–197. doi: 10.1177/01939459922043820. [DOI] [PubMed] [Google Scholar]
- 38.Antonovsky A. Unraveling the mystery of health: how people manage stress and stay well. California Antonovsky: Jossey-Bass; 1987. [Google Scholar]
- 39.Eriksson M, Lindström B. Antonovsky’s sense of coherence scale and its relation with quality of life: a systematic review. J Epidemiol Community Health. 2007;61:938–944. doi: 10.1136/jech.2006.056028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suominen S, Helenius H, Blomberg H, Uutela A, Koskenvuo M. Sense of coherence as a predictor of subjective state of health: results of 4 years of follow-up of adults. J Psychosom Res. 2001;50:77–86. doi: 10.1016/s0022-3999(00)00216-6. [DOI] [PubMed] [Google Scholar]
- 41.Coe R, Romeis J, Hall M. Sense of coherence and survival in the chronically ill elderly. A five year follow-up. In: McCubbin H, Thompson E, Thompson A, editors. Sense of Coherence and Resiliency Stress, Coping and Health. Thousand Oaks: Sage; 1998. pp. 265–275. [Google Scholar]
- 42.Atroshi I, Andersson IH, Gummesson C, Leden I, Odenbring S, Ornstein E. Primary care patients with musculoskeletal pain. Value of health-status and sense-of-coherence measures in predicting long-term work disability. Scand J Rheumatol. 2002;31:239–244. doi: 10.1080/030097402320318440. [DOI] [PubMed] [Google Scholar]
- 43.Flensborg-Madsen T, Ventegodt S, Merrick J. Sense of coherence and physical health. A review of previous findings. ScientificWorldJournal. 2005;5:665–673. doi: 10.1100/tsw.2005.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bengtsson M, Sjöberg K, Candamio M, Lerman A, Ohlsson B. Anxiety in close relationships is higher and self-esteem lower in patients with irritable bowel syndrome compared to patients with inflammatory bowel disease. Eur J Intern Med. 2013;24:266–272. doi: 10.1016/j.ejim.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 45.Kuroki T, Ohta A, Sherriff-Tadano R, Matsuura E, Takashima T, Iwakiri R, Fujimoto K. Imbalance in the stress-adaptation system in patients with inflammatory bowel disease. Biol Res Nurs. 2011;13:391–398. doi: 10.1177/1099800410388638. [DOI] [PubMed] [Google Scholar]
- 46.Goulia P, Voulgari PV, Tsifetaki N, Andreoulakis E, Drosos AA, Carvalho AF, Hyphantis T. Sense of coherence and self-sacrificing defense style as predictors of psychological distress and quality of life in rheumatoid arthritis: a 5-year prospective study. Rheumatol Int. 2015;35:691–700. doi: 10.1007/s00296-014-3134-8. [DOI] [PubMed] [Google Scholar]
- 47.Hyphantis T, Palieraki K, Voulgari PV, Tsifetaki N, Drosos AA. Coping with health-stressors and defence styles associated with health-related quality of life in patients with systemic lupus erythematosus. Lupus. 2011;20:893–903. doi: 10.1177/0961203311398264. [DOI] [PubMed] [Google Scholar]
- 48.Dignass A, Eliakim R, Magro F, Maaser C, Chowers Y, Geboes K, Mantzaris G, Reinisch W, Colombel JF, Vermeire S, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohns Colitis. 2012;6:965–990. doi: 10.1016/j.crohns.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 49.Van Assche G, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M, Ochsenkühn T, Orchard T, Rogler G, Louis E, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Definitions and diagnosis. J Crohns Colitis. 2010;4:7–27. doi: 10.1016/j.crohns.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 50.Bertolucci PH, Brucki SM, Campacci SR, Juliano Y. [The Mini-Mental State Examination in a general population: impact of educational status] Arq Neuropsiquiatr. 1994;52:1–7. [PubMed] [Google Scholar]
- 51.de Azevedo Marques JM, Zuardi AW. Validity and applicability of the Mini International Neuropsychiatric Interview administered by family medicine residents in primary health care in Brazil. Gen Hosp Psychiatry. 2008;30:303–310. doi: 10.1016/j.genhosppsych.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 52.Best WR, Becktel JM, Singleton JW, Kern F. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology. 1976;70:439–444. [PubMed] [Google Scholar]
- 53.Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. 1955;2:1041–1048. doi: 10.1136/bmj.2.4947.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brazilian Study Group of Inflammatory Bowel Diseases. Consensus guidelines for the management of inflammatory bowel disease. Arq Gastroenterol. 2010;47:313–325. doi: 10.1590/s0004-28032010000300019. [DOI] [PubMed] [Google Scholar]
- 55.Schmidt DR, Dantas RA. Analysis of validity and reliability of the adapted portuguese version of Antonovsky’s Sense of Coherence Questionnaire among nursing professionals. Rev Lat Am Enfermagem. 2011;19:42–49. doi: 10.1590/s0104-11692011000100007. [DOI] [PubMed] [Google Scholar]
- 56.Botega NJ, Bio MR, Zomignani MA, Garcia C, Pereira WA. [Mood disorders among inpatients in ambulatory and validation of the anxiety and depression scale HAD] Rev Saude Publica. 1995;29:355–363. doi: 10.1590/s0034-89101995000500004. [DOI] [PubMed] [Google Scholar]
- 57.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 58.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 59.Fleck MP, Louzada S, Xavier M, Chachamovich E, Vieira G, Santos L, Pinzon V. [Application of the Portuguese version of the abbreviated instrument of quality life WHOQOL-bref] Rev Saude Publica. 2000;34:178–183. doi: 10.1590/s0034-89102000000200012. [DOI] [PubMed] [Google Scholar]
- 60.Development of the World Health Organization WHOQOL-BREF quality of life assessment. The WHOQOL Group. Psychol Med. 1998;28:551–558. doi: 10.1017/s0033291798006667. [DOI] [PubMed] [Google Scholar]
- 61.Singh P, Agnihotri A, Pathak MK, Shirazi A, Tiwari RP, Sreenivas V, Sagar R, Makharia GK. Psychiatric, somatic and other functional gastrointestinal disorders in patients with irritable bowel syndrome at a tertiary care center. J Neurogastroenterol Motil. 2012;18:324–331. doi: 10.5056/jnm.2012.18.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cesaretti IU, Santos VL, Vianna LA. [Quality of life of the colostomized person with or without use of methods of bowel control] Rev Bras Enferm. 2010;63:16–21. doi: 10.1590/s0034-71672010000100003. [DOI] [PubMed] [Google Scholar]
- 63.Miles J, Shevlin M. Applying Regression and Correlation: A Guide for Students and Researchers. London: SAGE Publications; 2001. [Google Scholar]
- 64.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 65.Kraemer HC, Frank E, Kupfer DJ. Moderators of treatment outcomes: clinical, research, and policy importance. JAMA. 2006;296:1286–1289. doi: 10.1001/jama.296.10.1286. [DOI] [PubMed] [Google Scholar]
- 66.Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry. 2002;59:877–883. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- 67.Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. New York: Guilford Publications; 2013. [Google Scholar]
- 68.Bennebroek Evertsz’ F, Thijssens NA, Stokkers PC, Grootenhuis MA, Bockting CL, Nieuwkerk PT, Sprangers MA. Do Inflammatory Bowel Disease patients with anxiety and depressive symptoms receive the care they need? J Crohns Colitis. 2012;6:68–76. doi: 10.1016/j.crohns.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 69.Zhang CK, Hewett J, Hemming J, Grant T, Zhao H, Abraham C, Oikonomou I, Kanakia M, Cho JH, Proctor DD. The influence of depression on quality of life in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:1732–1739. doi: 10.1097/MIB.0b013e318281f395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nahon S, Lahmek P, Durance C, Olympie A, Lesgourgues B, Colombel JF, Gendre JP. Risk factors of anxiety and depression in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2086–2091. doi: 10.1002/ibd.22888. [DOI] [PubMed] [Google Scholar]
- 71.Mills R, Young C, Nicholas R, Pallant J, Tennant A. Rasch analysis of the Fatigue Severity Scale in multiple sclerosis. Mult Scler. 2009;15:81–87. doi: 10.1177/1352458508096215. [DOI] [PubMed] [Google Scholar]
- 72.Benyamini Y, Roziner I, Goldbourt U, Drory Y, Gerber Y. Depression and anxiety following myocardial infarction and their inverse associations with future health behaviors and quality of life. Ann Behav Med. 2013;46:310–321. doi: 10.1007/s12160-013-9509-3. [DOI] [PubMed] [Google Scholar]
- 73.Tselebis A, Bratis D, Pachi A, Moussas G, Karkanias A, Harikiopoulou M, Theodorakopoulou E, Kosmas E, Ilias I, Siafakas N, et al. [Chronic obstructive pulmonary disease: sense of coherence and family support versus anxiety and depression] Psychiatriki. 2013;24:109–116. [PubMed] [Google Scholar]
- 74.Hyphantis TN, Tsifetaki N, Pappa C, Voulgari PV, Siafaka V, Bai M, Alamanos Y, Drosos AA, Mavreas V. Clinical features and personality traits associated with psychological distress in systemic sclerosis patients. J Psychosom Res. 2007;62:47–56. doi: 10.1016/j.jpsychores.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 75.Siassi M, Weiss M, Hohenberger W, Lösel F, Matzel K. Personality rather than clinical variables determines quality of life after major colorectal surgery. Dis Colon Rectum. 2009;52:662–668. doi: 10.1007/DCR.0b013e31819ecf2e. [DOI] [PubMed] [Google Scholar]
- 76.Eypasch E, Williams JI, Wood-Dauphinee S, Ure BM, Schmülling C, Neugebauer E, Troidl H. Gastrointestinal Quality of Life Index: development, validation and application of a new instrument. Br J Surg. 1995;82:216–222. doi: 10.1002/bjs.1800820229. [DOI] [PubMed] [Google Scholar]
- 77.Turnbull GK, Vallis TM. Quality of life in inflammatory bowel disease: the interaction of disease activity with psychosocial function. Am J Gastroenterol. 1995;90:1450–1454. [PubMed] [Google Scholar]
- 78.Larsson K, Lööf L, Rönnblom A, Nordin K. Quality of life for patients with exacerbation in inflammatory bowel disease and how they cope with disease activity. J Psychosom Res. 2008;64:139–148. doi: 10.1016/j.jpsychores.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 79.Janke KH, Klump B, Gregor M, Meisner C, Haeuser W. Determinants of life satisfaction in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:272–286. doi: 10.1097/01.mib.0000160809.38611.f7. [DOI] [PubMed] [Google Scholar]
- 80.Guthrie E, Jackson J, Shaffer J, Thompson D, Tomenson B, Creed F. Psychological disorder and severity of inflammatory bowel disease predict health-related quality of life in ulcerative colitis and Crohn’s disease. Am J Gastroenterol. 2002;97:1994–1999. doi: 10.1111/j.1572-0241.2002.05842.x. [DOI] [PubMed] [Google Scholar]
- 81.Devlen J, Beusterien K, Yen L, Ahmed A, Cheifetz AS, Moss AC. The burden of inflammatory bowel disease: a patient-reported qualitative analysis and development of a conceptual model. Inflamm Bowel Dis. 2014;20:545–552. doi: 10.1097/01.MIB.0000440983.86659.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Faresjo T, Karalis I, Prinsback E, Kroon K, Lionis C. Sense of coherence in Crete and Sweden: key findings and messages from a comparative study. Eur J Gen Pract. 2009;15:95–98. doi: 10.1080/13814780903064489. [DOI] [PubMed] [Google Scholar]
- 83.Stein JA, Lee JW, Jones PS. Assessing cross-cultural differences through use of multiple-group invariance analyses. J Pers Assess. 2006;87:249–258. doi: 10.1207/s15327752jpa8703_05. [DOI] [PubMed] [Google Scholar]
- 84.Graff LA, Walker JR, Lix L, Clara I, Rawsthorne P, Rogala L, Miller N, Jakul L, McPhail C, Ediger J, et al. The relationship of inflammatory bowel disease type and activity to psychological functioning and quality of life. Clin Gastroenterol Hepatol. 2006;4:1491–1501. doi: 10.1016/j.cgh.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 85.Timmer A, Preiss JC, Motschall E, Rücker G, Jantschek G, Moser G. Psychological interventions for treatment of inflammatory bowel disease. Cochrane Database Syst Rev. 2011;(2):CD006913. doi: 10.1002/14651858.CD006913.pub2. [DOI] [PubMed] [Google Scholar]
- 86.Bäärnhielm S. Restructuring illness meaning through the clinical encounter: a process of disruption and coherence. Cult Med Psychiatry. 2004;28:41–65. doi: 10.1023/b:medi.0000018097.31002.79. [DOI] [PubMed] [Google Scholar]
- 87.Sandell R, Blomberg J, Lazar A, Carlsson J, Broberg J, Schubert J. Varieties of long-term outcome among patients in psychoanalysis and long-term psychotherapy. A review of findings in the Stockholm Outcome of Psychoanalysis and Psychotherapy Project (STOPP) Int J Psychoanal. 2000;81(Pt 5):921–942. doi: 10.1516/0020757001600291. [DOI] [PubMed] [Google Scholar]
- 88.Graziano F, Calandri E, Borghi M, Bonino S. The effects of a group-based cognitive behavioral therapy on people with multiple sclerosis: a randomized controlled trial. Clin Rehabil. 2014;28:264–274. doi: 10.1177/0269215513501525. [DOI] [PubMed] [Google Scholar]


