To the editor:
Myeloproliferative neoplasms (MPNs) show a familial aggregation whereby first-degree relatives of MPN patients have a 5 to 7 times higher risk of developing MPNs.1,2 MPN patients have a reduced life expectancy as compared with the general population,3 but there is limited information on the impact of familiality on survival. Therefore, we conducted a population-based study comparing survival in familial vs sporadic MPN patients.
Patients diagnosed with MPNs between 1958 and 2005 were identified through the Swedish Cancer Register and through our national MPN network.1 From the Multigenerational Register, which started in 1937, we obtained information on all first-degree relatives of MPN patients. Familial MPN was defined as having ≥1 first-degree relative with any MPN. Time of death was obtained from the Cause of Death Register, with follow-up to 2007. Cox regression was used to estimate risk of death from any cause. The study was approved by the Stockholm Regional Ethics Review Board. Informed consent was waived, because we had no contact with study patients and the data used for analyses did not contain any personal identifiers.
A total of 8122 patients diagnosed with an MPN and 25 781 linkable first-degree relatives were included. Median age at diagnosis was 65 years and 49% were males. In total, 92 MPN patients had a first-degree relative with an MPN, of which 82 patients were parent/offspring and 14 were siblings; hence, 4 patients had both a sibling and a parent/offspring with an MPN.
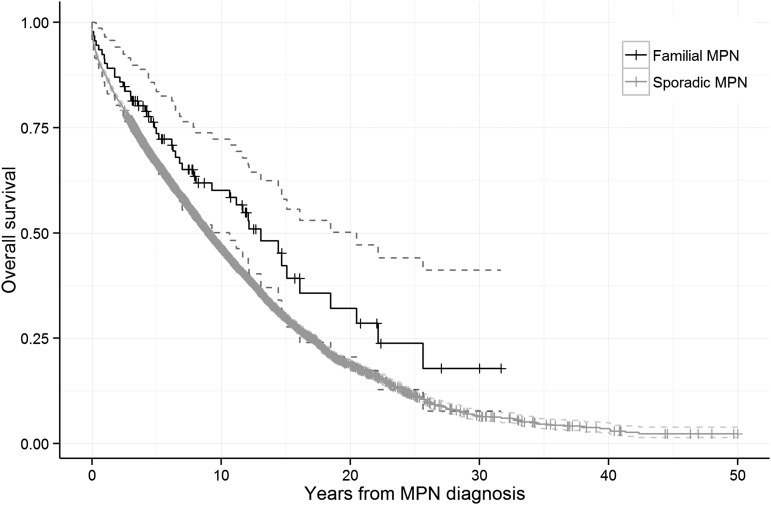
We found no difference in survival when comparing familial to sporadic MPN patients (hazard ratio [HR], 1.1; 95% confidence interval [CI], 0.9-1.5; P = .39; Figure 1). In stratified analyses, there was no significant difference in outcome in males (HR, 1.2; 95% CI, 0.7-1.9) and females (HR, 1.1; 95% CI, 0.7-1.8), in patients aged <70 years (HR, 0.8; 95% CI, 0.5-1.2) and ≥70 years (HR, 1.2; 95% CI, 0.7-2.1), or in patients diagnosed during different calendar periods (data not shown) when comparing familial vs sporadic MPN patients. Moreover, familial MPN patients had a higher mortality compared with their non-MPN relatives (HR, 1.3; 95% CI, 1.2-1.4). In analyses stratified by MPN subtype, the HRs of death from any cause in familial patients with polycythemia vera, essential thrombocythemia, and primary myelofibrosis were 1.2 (95% CI, 0.8-1.9), 1.0 (95% CI, 0.4-2.4), and 0.4 (95% CI, 0.03-5.1), respectively, when compared with sporadic MPN patients of the corresponding subtype. Our novel findings on similar survival in familial and sporadic MPN patients are consistent with earlier reports on comparable clinical presentation and rate of thrombosis in familial and sporadic MPN patients.2,4 These findings do not suggest that familial MPNs are associated with a more aggressive disease course, which is important prognostic information for patients with a family history of MPNs.
Figure 1.
Survival in patients with sporadic and familial MPNs. The HR of death from any cause was 1.1 (95% CI, 0.9-1.5; P = .39) when familial MPN patients were compared with sporadic MPN patients.
In familial MPNs, patients most likely inherit a predisposition to acquire somatic MPN-associated mutations. In fact, the JAK2V617F mutation and mutations in CALR and MPL are found in similar frequencies in familial and sporadic MPNs.4-6 The 46/1 haplotype and 2 common germline variants in the JAK2 locus and variants in the TERT locus have been correlated with an increased risk of developing MPNs on a population-based level, but this does not fully explain familial clustering of MPNs.7-9 In addition, germline mutation in RBBP6 may be enriched in patients with familial MPNs.10 Even though the underlying hereditary mechanisms of MPNs are largely unknown, familial and sporadic MPNs are most likely driven by the same acquired somatic mutations, which is in line with our results on similar overall survival in familial and sporadic MPNs observed in our own and earlier studies.4
Our study has several strengths, including the register-based design, which minimizes information and recall bias that may arise when information is gathered from living relatives. Limitations pertain primarily to limitations of the registers (ie, lack of detailed clinical and laboratory information and only inclusion of first-degree relatives and patients born after 1936).
In summary, we found survival to be similar in familial and sporadic MPN patients. With increasing knowledge on the genetic basis of MPNs, screening of family members of MPN patients with the aim of identifying patients early in the disease course may soon be possible. Early detection and preventive measures may decrease the rate of thromboembolic complications. We recommend that family history of MPNs should be incorporated in the clinical workup of MPN patients. However, until we gain more insight regarding the potential genetic and clinical differences, clinical management should not differ between familial and sporadic MPN patients.
Authorship
Acknowledgments: This work was supported by Blodcancerfonden; the regional agreement on medical training and clinical research between Stockholm County Council and Karolinska Institutet; the Swedish Cancer Society; the Karolinska Institutet Foundations; the Adolf H. Lundin Charitable Foundation; the Intramural Research Program, National Cancer Institute, National Institutes of Health; the University of Iceland Research Fund; the Icelandic Centre for Research; Landspitali University Hospital Research Fund; and a Marie Curie Career Integration Grant.
Contribution: M.H., S.H.L., and S.Y.K. designed the study; S.Y.K., M.B., J.S., and O.L. gathered the data; S.H.L. performed the statistical analysis; all authors analyzed and interpreted the data; M.H. and S.Y.K. wrote the paper; and all authors approved the final manuscript.
Conflict-of-interest disclosure: The authors have no conflicts of interest to disclose.
Correspondence: Malin Hultcrantz, Division of Hematology, Department of Medicine, Karolinska University Hospital Solna, SE-171 76, Stockholm, Sweden; e-mail: malin.hultcrantz@ki.se.
References
- 1.Landgren O, Goldin LR, Kristinsson SY, Helgadottir EA, Samuelsson J, Björkholm M. Increased risks of polycythemia vera, essential thrombocythemia, and myelofibrosis among 24,577 first-degree relatives of 11,039 patients with myeloproliferative neoplasms in Sweden. Blood. 2008;112(6):2199–2204. doi: 10.1182/blood-2008-03-143602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rumi E, Passamonti F, Della Porta MG, et al. Familial chronic myeloproliferative disorders: clinical phenotype and evidence of disease anticipation. J Clin Oncol. 2007;25(35):5630–5635. doi: 10.1200/JCO.2007.12.6896. [DOI] [PubMed] [Google Scholar]
- 3.Hultcrantz M, Kristinsson SY, Andersson TM, et al. Patterns of survival among patients with myeloproliferative neoplasms diagnosed in Sweden from 1973 to 2008: a population-based study. J Clin Oncol. 2012;30(24):2995–3001. doi: 10.1200/JCO.2012.42.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malak S, Labopin M, Saint-Martin C, Bellanne-Chantelot C, Najman A French Group of Familial Myeloproliferative Disorders. Long term follow up of 93 families with myeloproliferative neoplasms: life expectancy and implications of JAK2V617F in the occurrence of complications. Blood Cells Mol Dis. 2012;49(3-4):170–176. doi: 10.1016/j.bcmd.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Lundberg P, Nienhold R, Ambrosetti A, Cervantes F, Pérez-Encinas MM, Skoda RC. Somatic mutations in calreticulin can be found in pedigrees with familial predisposition to myeloproliferative neoplasms. Blood. 2014;123(17):2744–2745. doi: 10.1182/blood-2014-01-550863. [DOI] [PubMed] [Google Scholar]
- 6.Rumi E, Harutyunyan AS, Pietra D, et al. Associazione Italiana per la Ricerca sul Cancro Gruppo Italiano Malattie Mieloproliferative Investigators. CALR exon 9 mutations are somatically acquired events in familial cases of essential thrombocythemia or primary myelofibrosis. Blood. 2014;123(15):2416–2419. doi: 10.1182/blood-2014-01-550434. [DOI] [PubMed] [Google Scholar]
- 7.Jones AV, Chase A, Silver RT, et al. JAK2 haplotype is a major risk factor for the development of myeloproliferative neoplasms. Nat Genet. 2009;41(4):446–449. doi: 10.1038/ng.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olcaydu D, Rumi E, Harutyunyan A, et al. The role of the JAK2 GGCC haplotype and the TET2 gene in familial myeloproliferative neoplasms. Haematologica. 2011;96(3):367–374. doi: 10.3324/haematol.2010.034488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oddsson A, Kristinsson SY, Helgason H, et al. The germline sequence variant rs2736100_C in TERT associates with myeloproliferative neoplasms. Leukemia. 2014;28(6):1371–1374. doi: 10.1038/leu.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giambruno R, Krendl C, Stukalov A, et al. Germline RBBP6 mutations in myeloproliferative neoplasms [abstract]. Blood. 2013;122(21) doi: 10.1182/blood-2015-09-668673. Abstract 267. [DOI] [PMC free article] [PubMed] [Google Scholar]