Abstract
Heartland virus (HRTV; Bunyaviridae: Phlebovirus) has recently emerged as a causative agent of human disease characterized by thrombocytopenia and leukopenia in the United States. The lone star tick (Amblyomma americanum L.) has been implicated as a vector. To identify candidate vertebrate amplification hosts associated with enzootic maintenance of the virus, sera and ticks were sampled from 160 mammals (8 species) and 139 birds (26 species) captured near 2 human case residences in Andrew and Nodaway Counties in northwest Missouri. HRTV-specific neutralizing antibodies were identified in northern raccoons (42.6%), horses (17.4%), white-tailed deer (14.3%), dogs (7.7%), and Virginia opossums (3.8%), but not in birds. Virus isolation attempts from sera and ticks failed to detect HRTV. The high antibody prevalence coupled with local abundance of white-tailed deer and raccoons identifies these species as candidate amplification hosts.
Introduction
Heartland virus (HRTV) is a bunyavirus (Bunyaviridae: Phlebovirus) originally isolated from two humans experiencing a persistent multifaceted syndrome including fatigue, fever, thrombocytopenia, and leukopenia in Andrew and Nodaway Counties, Missouri, in 2009.1 HRTV was identified subsequently as the etiologic agent of additional disease cases in a broader area of Missouri, and has contributed to one death in Tennessee.2,3 Although several phleboviruses have been isolated in the United States since the 1960s, such as Lone Star virus (LSV; 1967),4 Sunday Canyon virus (SCV; 1969),5 and Rio Grande virus (RGV; 1973),6 HRTV represents the first known autochthonous phleboviral pathogen of humans in North America.1 Entomologic investigations of the original case residences discovered HRTV-infected nymphal lone star ticks (Amblyomma americanum L.) but no infected mosquitoes.7 HRTV is genetically most closely related to a recently discovered tick-borne virus infecting humans in China known as severe fever with thrombocytopenia syndrome virus (SFTSV),1,8,9 which is believed to be acquired by larval ticks feeding on viremic domestic farm animals, transstadially passaged to the nymphal stage and transmitted to humans by blood-feeding nymphs.10 Because of the tick exposures reported by both of the original human cases in 2009,1 the detection of HRTV-infected nymphal ticks in 2012 from one of the human case residences and a nearby conservation area,7 and the tick-associated transmission of the closely related SFTS virus,11 HRTV was presumed to be maintained by ticks and nonhuman vertebrate amplification host(s). The lone star tick, the putative vector, is known to feed on a wide range of medium- and large-sized vertebrate hosts.12,13 In an initial study to identify domestic and wild animal populations exposed to HRTV, wild and domestic animals in proximity to the human case residences in northwest Missouri were examined for the presence of attached lone star ticks, and sera assayed for neutralizing antibodies against HRTV. Results of this serosurvey were used to identify candidate vertebrate reservoir host(s).
Methods
Viruses and cells.
An isolate of HRTV obtained from the serum of an acutely ill human patient in the fall of 20091 was used for all plaque reduction neutralization tests (PRNTs). The isolate was passaged once on Vero E6 cells (African green monkey kidney cell line), which produced well-defined plaques after 9–11 days of incubation at 37°C, 5% CO2 using a dual 0.5% agarose overlay procedure with neutral red as described previously14 with the modification that the secondary overlay was applied at seventh day. LSV strain TMA 1381 (isolated from a single nymphal Amblyomma americanum collected from a woodchuck in Kentucky in 1967)4 and SCV strain RML 52301-11 (isolated from a soft tick, Argas cooleyi, collected near a swallow colony in Randall County, TX, in 1969)5 were used to assess cross-neutralization potential.
Sample collection.
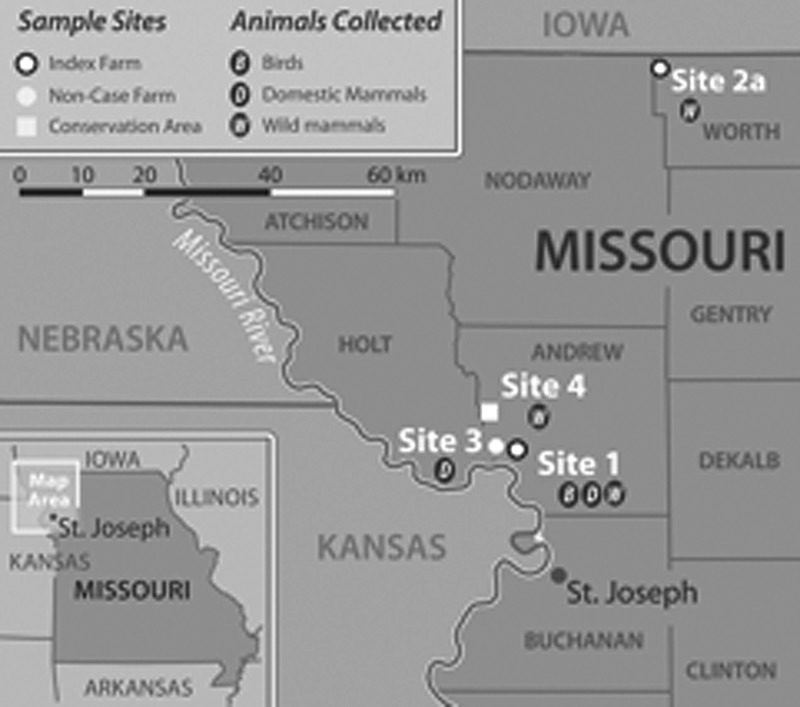
Serum samples were taken from wild birds, northern raccoons (Procyon lotor), Virginia opossums (Didelphis virginiana), white-tailed deer (Odocoileus virginianus), eastern fox squirrels (Sciurus niger), eastern cottontails (Sylvilagus floridanus), and domestic animals (horses [Equus caballus], dogs [Canis familiaris], and cats [Felis catus]). Wild birds were trapped with Japanese mist nets of varying sizes, banded and blood drawn by jugular venipuncture during the first 2 weeks of August 2012. Blood was placed in serum separator tubes, allowed to clot at ambient temperature for ∼15 minutes and centrifuged at 2,000 × g in the field prior to storage on dry ice. Wild turkeys were trapped with cannon nets during January 2013, and blood was drawn by brachial venipuncture. Deer sera were obtained from pooled blood in the body cavities of hunter-killed deer during January and February 2013. Northern raccoons, Virginia opossums, fox squirrels, and cottontails were trapped in August 2012 and summer of 2013 using Tomahawk traps (Tomahawk Live Traps, Hazelhurst, WI) and anesthetized with either isoflurane in customized anesthesia chambers similar to those described previously15 or by intramuscular injection of ketamine/xylazine (up to 60:12 mg/kg mixture for opossums, 15–20:3–4 mg/kg for raccoons and up to 38:7.6 mg/kg mixture for other species).16 Blood from these wild mammals was drawn from either the jugular or saphenous vein and placed into collection tubes containing ethylenediaminetetraacetic acid (EDTA) and/or serum separator tubes. These tubes were centrifuged in the field for separation of serum as described above and sera were transferred to cryovials for freezing and transport. Wild mammals were individually marked with ear tags and released at their locations of capture on recovery from anesthesia. Blood was drawn from horses in the field and from dogs and cats through the participation of local veterinary clinics in August 2012 by jugular or saphenous venipuncture, and serum or plasma was extracted in a similar manner as described for raccoons and opossums. All animals were screened for ticks and, if present, ticks were removed with forceps, placed in a cryovial and frozen on dry ice. See Figure 1 for a map of field collection sites.
Figure 1.
Map of approximate trapping and sample collection locations for birds, domestic mammals, and wild mammals.
Virus isolation.
All samples were assayed for virus isolation by plaque assay on Vero E6 cells as previously described.14 In brief, 100 μL serum (diluted 1:5 or 1:10) was added to confluent cell monolayers in 6-well plates, incubated for 60 minutes at 37°C, and a 3 mL agarose overlay was added to each well. Secondary overlay with neutral red was added on day 7 postinoculation and plates were observed for an additional 7 days to confirm the growth of any viral plaques.
Cross-neutralization between HRTV and LSV and SCV.
Cross-neutralization was evaluated for three native North American phleboviruses, HRTV, LSV, and SCV. Antisera to these viruses were provided by the Division of Vector-Borne Diseases, Centers for Disease Control and Prevention (CDC) arbovirus reference collection, and were composed of human convalescent serum obtained from one of the two original HRTV cases and mouse hyperimmune ascites fluid generated against LSV and SCV. The optimal threshold for serum neutralization was assessed at a series of thresholds (90%, 80%, and 70%) to identify the optimal range for maximum sensitivity without loss of specificity.
Plaque reduction neutralization testing.
Neutralization tests were performed with a human HRTV isolate described above. PRNT assays were performed by mixing equal volumes of 2-fold serially diluted sera with an equal volume of virus suspension containing approximately 100 plaque-forming units (PFU) of HRTV per 0.1 mL, and incubating at 37°C for 1 hour. The virus–serum mixtures were then adsorbed onto Vero E6 monolayers using 6-well cell culture dishes by incubating for 1 hour at 37°C. Cultures were then overlaid with 3 mL of 0.5% agarose in nutrient media followed by a secondary overlay containing neutral red at day 7 postinoculation as described above.14 Samples were initially screened at a 1:10 serum dilution against HRTV and any samples that demonstrated at least 50% neutralization against either virus were titrated in duplicate up to a 1:2,560 serum dilution using both HRTV and LSV. PRNT endpoints were calculated at the 70% neutralization threshold.
Tick processing and screening.
All ticks were identified, categorized by life stage, and either pooled or triturated individually. Supernatants were inoculated onto Vero E6 cells and assessed for cytopathic effects for 10–14 days. In addition, RNA was extracted from all tick homogenates, and quantitative reverse transcription polymerase chain reaction (qRT-PCR) for HRTV RNA was performed as described previously.7
Results
Cross-neutralization assessment between HRTV and LSV and SCV.
Empirical cross-neutralization between viruses and homologous and heterologous antisera was assessed at a series of thresholds. A 70% endpoint was determined to provide optimal sensitivity without sacrificing the capacity to serologically differentiate between viruses. Mouse immune sera generated against LSV and SCV demonstrated homologous reciprocal neutralizing titers (PRNT70) of ≥ 320 with a heterologous titer against HRTV of < 20. In contrast, human HRTV convalescent serum exhibited a homologous titer of 160 with heterologous neutralizing titers of 20 against both LSV and SCV (Table 1). These results indicated serological distinction (4-fold or greater difference in a two-way beta PRNT assay) between HRTV and two other potential domestic phleboviral infections from wild and domestic animal samples.
Table 1.
Cross-neutralization of HRTV, LSV, and SCV using a 70% neutralization threshold for a positive titer
| Virus | α-HRTV | α-LSV | α-SCV |
|---|---|---|---|
| HRTV | 160 | < 20 | < 20 |
| LSV | 20 | ≥ 640 | < 20 |
| SCV | 20 | < 20 | 320 |
HRTV = Heartland virus; LSV = Lone Star virus; SCV = Sunday Canyon virus.
Homologous virus/antisera comparisons are designated in bold.
Detection of phlebovirus/HRTV-neutralizing antibodies in vertebrate sera.
Serum and plasma samples from a total of 299 domestic and wild animals were screened for the presence of phlebovirus-neutralizing antibodies. Of them, 56 (15.4%) positive samples were identified (Table 2) demonstrating phlebovirus-neutralizing antibodies. Although none of 26 species of birds (139 specimens in total) (Table 3) demonstrated at least a 50% reduction in plaque formation at the 1/10 screening dilution against either HRTV or LSV, 35% of wild and domestic mammalian species (56 of 160 samples) were found to neutralize HRTV or LSV at this dilution. Mammals demonstrating exposure to phleboviruses included raccoon, white-tailed deer, horse, dog, and Virginia opossum (Table 2). White-tailed deer demonstrated the highest phlebovirus seroprevalence rate of the taxa assayed at 64%. All animals that screened positive were tested for cross-neutralization between HRTV and LSV to determine if the response was specific for either virus using a 4-fold difference as the criterion for specific neutralization. A large proportion of HRTV-positive deer and, to a lesser extent, raccoons also demonstrated neutralizing titers for LSV that were within the 4-fold threshold and were considered phlebovirus reactive with an indeterminate etiology. Of the phlebovirus-reactive samples, 29 raccoon, 2 deer, 5 horse, and single positive dog and opossum samples were confirmed HRTV reactive by this criterion. One raccoon was confirmed LSV positive; the infecting agent could not be distinguished for the remaining samples.
Table 2.
Mammals tested for HRTV-neutralizing (PRNT70) antibodies
| Taxon | N | Number of Phlebo-neutralizing positive | % Positive* | Number of HRTV-neutralizing positive | % Positive† |
|---|---|---|---|---|---|
| Northern raccoon (Procyon lotor) | 68 | 40 | 58.8 | 29 | 42.6 |
| White-tailed deer (Odocoileus virginianus) | 14 | 9 | 64.3 | 2 | 14.3 |
| Horse (Equus ferus caballus) | 23 | 5 | 21.7 | 4 | 17.4 |
| Dog (Canis lupus familiaris) | 13 | 1 | 7.7 | 1 | 7.7 |
| Virginia opossum (Didelphis virginiana) | 26 | 1 | 3.8 | 1 | 3.8 |
| Eastern cottontail (Sylvilagus floridanus) | 10 | 0 | 0 | 0 | 0 |
| Fox squirrel (Sciurus niger) | 4 | 0 | 0 | 0 | 0 |
| Domestic cat (Felis catus) | 2 | 0 | 0 | 0 | 0 |
HRTV = Heartland virus; LSV = Lone Star virus; PRNT = plaque reduction neutralization test.
Demonstrated ≥ 1/10 PRNT50 titer upon initial screening with HRTV.
Demonstrated a ≥ 4-fold PRNT70 titer to HRTV compared with LSV.
Table 3.
Birds tested for HRTV-neutralizing (PRNT50) antibodies
| Bird species (common name) | Scientific name | Number sampled |
|---|---|---|
| Northern cardinal | Cardinalis cardinalis | 24 |
| Mourning dove | Zenaida macroura | 16 |
| American goldfinch | Spinus tristis | 14 |
| House sparrow | Passer domesticus | 12 |
| Black-capped chickadee | Poecile atricapillus | 11 |
| Indigo bunting | Passerina cyanea | 7 |
| Tufted titmouse | Baeolophus bicolor | 7 |
| Wild turkey | Meleagris gallopavo | 6 |
| Downy woodpecker | Picoides pubescens | 5 |
| Eastern wood pewee | Contopus virens | 5 |
| Red-eyed vireo | Vireo olivaceus | 5 |
| Carolina wren | Thryothorus ludovicianus | 4 |
| Summer tanager | Piranga rubra | 4 |
| White-breasted nuthatch | Sitta carolinensis | 4 |
| Hairy woodpecker | Picoides villosus | 3 |
| Baltimore oriole | Icterus galbula | 2 |
| Chipping sparrow | Spizella passerina | 1 |
| Eastern bluebird | Sialia sialis | 1 |
| Eastern towhee | Pipilo erythrophthalmus | 1 |
| Field sparrow | Spizella pusilla | 1 |
| Gray catbird | Dumetella carolinensis | 1 |
| House wren | Troglodytes aedon | 1 |
| Least flycatcher | Empidonax minimus | 1 |
| Rose-breasted grosbeak | Pheucticus ludovicianus | 1 |
| Red-bellied woodpecker | Melanerpes carolinus | 1 |
| Yellow-bellied flycatcher | Empidonax flaviventris | 1 |
HRTV = Heartland virus; PRNT = plaque reduction neutralization test.
Screening of samples for evidence of HRTV infection.
All ticks that were collected from vertebrate hosts at the time of sampling and subsequently tested were determined to be negative for HRTV RNA by qRT-PCR and infectious virus by plaque assay. A total of 180 tick pools containing larvae, nymphs, or adults were tested. Ticks removed from raccoons accounted for 136 samples, with 66 (49%) identified as Amblyomma americanum, 55 (40%) as Dermacentor variabilis, and the remaining 15 (11%) various Ixodes spp. Twenty-nine ticks were identified from opossums, with only 1 (3%) Amblyomma americanum and 28 (97%) Dermacentor variabilis. Ticks removed and tested from three horses and two rabbits were all Amblyomma americanum. Ticks from deer included 53 Dermacentor albipictus and 2 Ixodes spp.
HRTV was not detected in any of the serum samples from mammals or birds by plaque assay. One serum sample from a Virginia opossum was positive for West Nile virus (Flaviviridae: Flavivirus) by plaque assay,17 and Mermet virus (Bunyaviridae: Orthobunyavirus) was isolated from serum of a tufted titmouse (Baeolophus bicolor).
Discussion
HRTV was found to be serologically distinguishable from two other known tick-borne phleboviruses of North America, SCV and LSV. Known high-titer antisera produced in mice against LSV neutralized LSV at more than a 4-fold (≥ 32-fold) higher dilution than HRTV or SCV. Similarly, hyperimmune ascites fluid generated from SCV inoculation exhibited neutralization at ≥ 16-fold higher dilutions for SCV compared with either HRTV or LSV. Reciprocal comparisons using the HRTV convalescent human serum demonstrated 8-fold higher homologous titer against HRTV than against either LSV or SCV (Table 1). These data indicate serological distinction of HRTV from alternative domestic phleboviruses by using the herein described PRNT70 assay. The low neutralizing activity that the human HRTV antisera exhibited against LSV and SCV could be the result of a previous exposure to LSV, SCV, or another phlebovirus or could indicate a low cross-neutralization of HRTV antisera with the alternative phleboviruses.
The vertebrate serosurvey results presented herein have revealed a broad range of mammalian species, both wild and domestic, that have been exposed to phleboviruses and specifically to HRTV in close proximity to the initially described HRTV case–patient residences. The catholic feeding behavior of Amblyomma americanum is consistent with these findings. Although different life stages of Amblyomma americanum have been associated with feeding on avian hosts,12,18 no avian serum assayed in this study demonstrated any evidence of phlebovirus antibodies, indicating the possible refractoriness of avian hosts to HRTV infection. However, few ticks were detected on the 139 birds handled for this study, and the majority of these were Ixodes spp. (data not shown). In addition, the use of mist nets resulted in a sampling of a great range of passeriform species (Table 3) that, unlike ground-foraging columbiform or galliform birds, could have a lower exposure rate to ticks. In contrast, large numbers of Amblyomma americanum and Dermacentor variabilis were removed from raccoons and opossums. Ticks removed from deer in winter were primarily Dermacentor albipictus. The coincidence of high seroprevalence for HRTV infection and massive tick infestations of these mammals further implicates tick species as an important natural vector of HRTV. The highest phlebovirus-neutralizing antibody rate was observed in white-tailed deer, a species that has been documented to serve as a host for all life stages of Amblyomma americanum.12 Raccoon was also identified as a potential HRTV amplifier with a high incidence of exposure to Amblyomma americanum and subsequently demonstrated a high seroprevalence for HRTV infection determined by detection of HRTV-neutralizing antibody. The presence of neutralizing antibodies does not provide direct evidence of vertebrate reservoir capacity of any given animal species and is merely indicative that these hosts were exposed and mounted an immune response sufficient for detection of the host neutralizing antibody response. Nevertheless, the high seroprevalence in raccoons compared with opossums could be due to the feeding preferences of the tick vector, considering that Amblyomma americanum was found to parasitize raccoons more frequently than opossums in this study.
Despite screening of larvae, nymphal, and adult stage lone star ticks in the study sites adjacent to the index human cases used for the vertebrate survey presented herein, only nymphal ticks have been reported to be infected with HRTV.7 This finding indicates that ticks were either infected as larvae and the infection was passed transstadially to the nymphal stage or transstadial passage of virus to the nymphal stage resulted from transovarial infection through the egg of an infected adult female. Nevertheless, the lack of viral isolations from larvae or adult Amblyomma americanum denotes the likelihood of the former manner of transmission. Evidence of transovarial transmission of Rio Grande virus, a woodrat-associated phlebovirus isolated originally in Texas, in phlebotamine sand flies could support this potential infection route of HRTV in ticks.19 Another possibility is that HRTV could be transmitted horizontally from infected nymphal ticks co-feeding with noninfected nymphs, as observed in experimental infections with Thogoto virus.20 Elucidation of these infection mechanism(s) could provide valuable information on not only the vector transmission cycle of HRTV, but could also further incriminate vertebrate species' involvement in the transmission cycle as different life stages of Amblyomma americanum have different feeding preferences on various mammalian hosts. For instance, Amblyomma americanum adults typically feed on white-tailed deer,12,21 whereas larval and nymphal lone star ticks feed on deer in addition to a vast number of small mammals and birds.12,18,22,23
On the basis of the elevated exposure rates of HRTV among white-tailed deer, horse, and raccoon, future experimental infection studies with these taxa are warranted to assess the magnitude and duration of viremia that could implicate them as competent reservoir hosts. Previously published studies in domestic animals in proximity to human SFTSV infections in Shandong Province, China, demonstrated up to 95% seropositivity in goats,24,25 as well as detectable antibody titers in sheep, cattle, dogs, pigs, and chickens.26 An epidemiological study further implicated goat ownership and exposure to Haemaphysalis longicornis ticks as risk factors for SFTSV infection in Jiangsu Province, China.10 Goats were not assessed for HRTV-neutralizing antibodies in this study as this species of domesticated animal was absent in our study sites.
Bunyaviruses have a tripartite genome consisting of small, medium, and large genomic segments.8 Coinfection of arthropods with more than one bunyavirus can result in reassortment of the genetic segments. Specifically, reassortment of phlebovirus segments has been described recently with the small segments of different SFTSV strains.27 This could have implications on the serological assays performed in this study as there was some evidence that LSV and HRTV were co-circulating in the two study sites. The close genetic identity of LSV with HRTV8 indicates the likely compatibility of reassortant viruses and the potential that such reassortants could confound some serological assessments of HRTV and LSV host associations. In this serosurvey, only one of the samples expressed 4-fold or higher neutralization of LSV compared with HRTV; however, all sera samples were screened initially against HRTV virus and this would fail to identify some weakly reactive LSV-specific infections as none of the HRTV-negative samples were screened initially against LSV virus. In addition, unknown amplification hosts that were not surveyed in this study, either due to species-targeted sampling approaches or mortality following natural infection, could play a role in enzootic maintenance. These data serve to provide the first indications of potential reservoir hosts for the natural transmission cycle of HRTV and serve to initially target certain hosts for experimental infection studies that could identify animal models for HRTV infection and transmission as well as competent reservoir host(s).
Although the object of this serosurvey was in part to identify candidate amplification hosts, based on detection of exposure between HRTV and specific animal populations, the results also provide a guide for targeting sentinels that can be used to monitor for HRTV activity in northwest Missouri or beyond. Raccoons appear to be the most efficient sentinels for detection of HRTV-specific antibodies, whereas the low seroprevalence in opossums detracts from this species' utility as a sentinel. Deer blood may be obtainable from hunting activities in the fall and winter, and raccoons can be easily trapped. Domestic animals were a less sensitive indicator for local transmission activity because of their lower seroprevalence. Nonetheless, because of their close association with human populations, blood from horses and dogs can be readily obtained from veterinary practices and may be useful in certain circumstances, especially when the animals have been housed in defined locations. Animals that travel with their owners would present uncertainty for interpreting the location of seroconversion and hence reduce their utility as sentinels for local transmission.
To conclude, we have shown that a variety of domestic and wild mammals have been exposed to HRTV infection in northwest Missouri where the index human cases resided. Raccoons and potentially deer represent efficient wildlife sentinels, whereas horses and dogs may be useful peridomestic indicator species. More field data and transmission and infection studies utilizing Amblyomma americanum as vectors will be required to conclusively implicate any of these vertebrate hosts as important amplifiers.
ACKNOWLEDGMENTS
We thank the residents of Andrew and Nodaway Counties, Missouri, who graciously allowed the sampling of wild and domestic animals on their properties. We also thank the Missouri Department of Health and Senior Services, the Andrew County Health Department, the Nodaway County Health Center, the Missouri Department of Conservation and participating veterinary clinics for their respective assistance. We specifically thank David Ashley, Department of Biology, Missouri Western State University (MWSU), for accommodating lab space for specimen processing and Luke Miller and Nathan Hubbard (USDA-APHIS-Wildlife Services) for assisting with mammalian field collections. Finally, we extend gratitude to Jason Velez for generating Vero E6 plates for the neutralization assays performed.
Footnotes
Financial support: Funding for Angela Bosco-Lauth was provided by a fellowship from the American Society of Microbiology (ASM).
Authors' addresses: Angela M. Bosco-Lauth, Nicholas A. Panella, Kristen L. Burkhalter, Marvin S. Godsey, Harry M. Savage, Nicholas Komar, and Aaron C. Brault, Division of Vector-Borne Diseases, Arboviral Disease Branch, Centers for Disease Control and Prevention, Fort Collins, CO, E-mails: mopargal@rams.colostate.edu, nap4@cdc.gov, ktb3@cdc.gov, mjg9@cdc.gov, hms1@cdc.gov, nck6@cdc.gov, and abrault@cdc.gov. J. Jeffrey Root and Tom Gidlewski, U.S. Department of Agriculture, Wildlife Services, National Wildlife Research Center, Fort Collins, CO, E-mails: Jeff.Root@aphis.usda.gov and Thomas.Gidlewski@aphis.usda.gov. R. Ryan Lash, Jessica R. Harmon, and William L. Nicholson, Division of Vector-Borne Diseases, Rickettsial Diseases Branch, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: rlash@cdc.gov, jharmon@cdc.gov, and wnicholson@cdc.gov.
References
- 1.McMullan LK, Folk SM, Kelly AJ, MacNeil A, Goldsmith CS, Metcalfe MG, Batten BC, Albarino CG, Zaki SR, Rollin PE, Nicholson WL, Nichol ST. A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med. 2012;367:834–841. doi: 10.1056/NEJMoa1203378. [DOI] [PubMed] [Google Scholar]
- 2.Muehlenbachs A, Fata CR, Lambert AJ, Paddock CD, Velez JO, Blau DM, Staples JE, Karlekar MB, Bhatnagar J, Nasci RS, Zaki SR. Heartland virus-associated death in Tennessee. Clin Infect Dis. 2014;59:845–850. doi: 10.1093/cid/ciu434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pastula DM, Turabelidze G, Yates KF, Jones TF, Lambert AJ, Panella AJ, Kosoy OI, Velez JO, Fisher M, Staples E. Notes from the field: Heartland virus disease—United States, 2012–2013. MMWR. 2014;63:270–271. [PMC free article] [PubMed] [Google Scholar]
- 4.Kokernot RH, Calisher CH, Stannard LJ, Hayes J. Arbovirus studies in the Ohio-Mississippi Basin, 1964–1967. VII. Lone Star virus, a hitherto unknown agent isolated from the tick Amblyomma americanum (Linn) Am J Trop Med Hyg. 1969;18:789–795. [PubMed] [Google Scholar]
- 5.Yunker CE, Clifford CM, Thomas LA, Keirans JE, Casals J, George JE, Parker JC. Sunday Canyon virus, a new ungrouped agent from the tick Argas (A.) cooleyi in Texas. Acta Virol. 1977;21:36–44. [PubMed] [Google Scholar]
- 6.Calisher CH, McLean RG, Smith GC, Szmyd DM, Muth DJ, Lazuick JS. Rio Grande—a new phlebotomus fever group virus from south Texas. Am J Trop Med Hyg. 1977;26:997–1002. doi: 10.4269/ajtmh.1977.26.997. [DOI] [PubMed] [Google Scholar]
- 7.Savage HM, Godsey MS, Jr, Lambert A, Panella NA, Burkhalter KL, Harmon JR, Lash RR, Ashley DC, Nicholson WL. First detection of Heartland virus (Bunyaviridae: Phlebovirus) from field collected arthropods. Am J Trop Med Hyg. 2013;89:445–452. doi: 10.4269/ajtmh.13-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swei A, Russell BJ, Naccache SN, Kabre B, Veeraraghavan N, Pilgard MA, Johnson BJ, Chiu CY. The genome sequence of Lone Star virus, a highly divergent bunyavirus found in the Amblyomma americanum tick. PLoS ONE. 2013;8:e62083. doi: 10.1371/journal.pone.0062083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD, Sun YL, Zhang L, Zhang QF, Popov VL, Li C, Qu J, Li Q, Zhang YP, Hai R, Wu W, Wang Q, Zhan FX, Wang XJ, Kan B, Wang SW, Wan KL, Jing HQ, Lu JX, Yin WW, Zhou H, Guan XH, Liu JF, Bi ZQ, Liu GH, Ren J, Wang H, Zhao Z, Song JD, He JR, Wan T, Zhang JS, Fu XP, Sun LN, Dong XP, Feng ZJ, Yang WZ, Hong T, Zhang Y, Walker DH, Wang Y, Li DX. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med. 2011;364:1523–1532. doi: 10.1056/NEJMoa1010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang S, Bao C, Zhou M, Hu J, Tang F, Guo X, Jiao Y, Zhang W, Luo P, Li L, Zhu K, Tan W, Lu Q, Ge H, Chen A. Seroprevalence and risk factors for severe fever with thrombocytopenia syndrome virus infection in Jiangsu Province, China, 2011. Am J Trop Med Hyg. 2014;90:256–259. doi: 10.4269/ajtmh.13-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang XL, Wang XJ, Li JD, Ding SJ, Zhang QF, Qu J, Zhang S, Li C, Wu W, Jiang M, Liang MF, Bi ZQ, Li DX. Isolation, identification and characterization of SFTS bunyavirus from ticks collected on the surface of domestic animals. Bing Du Xue Bao. 2012;28:252–257. [PubMed] [Google Scholar]
- 12.Allan BF, Goessling LS, Storch GA, Thach RE. Blood meal analysis to identify reservoir hosts for Amblyomma americanum ticks. Emerg Infect Dis. 2010;16:433–440. doi: 10.3201/eid1603.090911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Childs JE, Paddock CD. The ascendancy of Amblyomma americanum as a vector of pathogens affecting humans in the United States. Annu Rev Entomol. 2003;48:307–337. doi: 10.1146/annurev.ento.48.091801.112728. [DOI] [PubMed] [Google Scholar]
- 14.Brault AC, Langevin SA, Bowen RA, Panella NA, Biggerstaff BJ, Miller BR, Komar N. Differential virulence of West Nile strains for American crows. Emerg Infect Dis. 2004;10:2161–2168. doi: 10.3201/eid1012.040486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bentler KT, Gossett DN, Root JJ. A novel anesthesia induction system for raccoons. Wildl Soc Bull. 2012;36:807–812. [Google Scholar]
- 16.Belant JL. Field immobilization of raccoons with ketamine hydrochloride and xylazine hydrochloride. Acta Theriol (Warsz) 1995;40:327–330. [Google Scholar]
- 17.Bosco-Lauth A, Harmon JR, Lash RR, Weiss S, Langevin S, Savage HM, Godsey MS, Jr., Burkhalter K, Root JJ, Gidlewski T, Nicholson WL, Brault AC, Komar N. West Nile virus isolated from a Virginia opossum (Didelphis virginiana) in northwestern Missouri, USA, 2012. J Wildl Dis. 2014;56:976–978. doi: 10.7589/2013-11-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tugwell P, Lancaster JL. Results of a tick-host study in northwest Arkansas. J Kans Entomol Soc. 1962;35:202–211. [Google Scholar]
- 19.Endris RG, Tesh RB, Young DG. Transovarial transmission of Rio Grande virus (Bunyaviridae: Phlebovirus) by the sand fly, Lutzomyia anthophora. Am J Trop Med Hyg. 1983;32:862–864. doi: 10.4269/ajtmh.1983.32.862. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Nuttall PA. Intra-stadial tick-borne Thogoto virus (Orthomyxoviridae) transmission: accelerated arbovirus transmission triggered by host death. Parasitology. 2001;122:439–446. doi: 10.1017/s0031182001007478. [DOI] [PubMed] [Google Scholar]
- 21.Cooley RA, Kohls GM. The genus Amblyomma (Ixodidae) in the United States. J Parasitol. 1941;30:77–111. [Google Scholar]
- 22.Clymer BC, Howell DE, Hair JA. Animal hosts of economically important ticks in eastern-central Oklahoma. Ann Entomol Soc Am. 1970;63:612–614. [Google Scholar]
- 23.Koch HG. Suitability of birds and mammals as hosts for immature stages of the lone star tick, Amblyomma americanum (Acari: Ixodidae) J Med Entomol. 1981;18:93–98. doi: 10.1093/jmedent/18.2.93. [DOI] [PubMed] [Google Scholar]
- 24.Zhao L, Zhai S, Wen H, Cui F, Chi Y, Wang L, Xue F, Wang Q, Wang Z, Zhang S, Song Y, Du J, Yu XJ. Severe fever with thrombocytopenia syndrome virus, Shandong Province, China. Emerg Infect Dis. 2012;18:963–965. doi: 10.3201/eid1806.111345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui F, Cao HX, Wang L, Zhang SF, Ding SJ, Yu XJ, Yu H. Clinical and epidemiological study on severe fever with thrombocytopenia syndrome in Yiyuan County, Shandong Province, China. Am J Trop Med Hyg. 2013;88:510–512. doi: 10.4269/ajtmh.11-0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niu G, Li J, Liang M, Jiang X, Jiang M, Yin H, Wang Z, Li C, Zhang Q, Jin C, Wang X, Ding S, Xing Z, Wang S, Bi Z, Li D. Severe fever with thrombocytopenia syndrome virus among domesticated animals, China. Emerg Infect Dis. 2013;19:756–763. doi: 10.3201/eid1905.120245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding NZ, Luo ZF, Niu DD, Ji W, Kang XH, Cai SS, Xu DS, Wang QW, He CQ. Identification of two severe fever with thrombocytopenia syndrome virus strains originating from reassortment. Virus Res. 2013;178:543–546. doi: 10.1016/j.virusres.2013.09.017. [DOI] [PubMed] [Google Scholar]