Abstract
Histoplasmosis is a common endemic human mycoses acquired mostly in the Americas. We reviewed 23 cases of histoplasmosis in Israeli travelers; 22 had traveled to Central or South America and one to North America. Fourteen cases had been exposed to bat habitats and were symptomatic, presenting ≤ 3 months after their return. Asymptomatic patients (N = 9) were diagnosed during the evaluation of incidental radiological findings or because a travel partner had been suspected of Histoplasma infection, 16–120 months after their return. Serological testing was positive in 75% of symptomatic cases but only 22% of asymptomatic cases. Histoplasmosis should be considered in travelers returning from the Americas with respiratory or febrile illness within weeks of return, particularly if exposed to bat habitats. Travel history is essential in patients presenting with pulmonary nodules, even years after travel to endemic countries.
Introduction
Histoplasmosis is an endemic mycosis caused by the thermally dimorphic Histoplasma capsulatum. The fungus can be found in low levels in soil worldwide; however, the majority of infections are acquired in the Americas. H. capsulatum is highly endemic in the United States in the Ohio and Mississippi River valleys and in Central and South America. Historically, histoplasmin skin testing in residents of these regions showed that many were positive, indicating current or past infection with H. capsulatum.1
Infection is acquired by inhalation of conidia. The clinical presentation of histoplasmosis is highly dependent on two major factors: the size of the inoculum and the immune status of the host.2 In some respects, the relationship between histoplasmosis infection and immune competence is analogous to primary tuberculosis; infants, elderly, and immunocompromised patients are likely to develop a (rapidly) progressive pulmonary and systemic infection that is often fatal, but in most immunocompetent adults, the primary infection is self-limited, and indeed is often not diagnosed in endemic regions.3
Reports of histoplasmosis in travelers are scarce, and when recognized, are mostly small outbreaks of respiratory illness occurring after visits to caves, mines, and so forth.4 However, other sources of outbreaks may occur as observed with one large outbreak in which the source of infection was apparently a hotel in Acapulco, Mexico.5 A survey of Spanish travelers returning from Central and South America found that 20% had a positive histoplasmin skin test (versus none of a control group with no history of travel to endemic countries), but only 19% of these had any symptoms.6 Prolonged travel, sleeping outdoors, and travel to Central (rather than South) America were associated with infection.
In this report, we summarize our experience with sporadic cases of histoplasmosis in returning Israeli travelers.
Methods
We retrospectively analyzed all cases of histoplasmosis referred to the Center for Geographic Medicine at the Chaim Sheba Medical Center, Ramat Gan, Israel, between 2000 and 2012. The study was approved by the Institutional Review Board of the hospital.
Diagnostic criteria and case definitions.
All “cases” had a history of travel to an area endemic for H. capsulatum. “Proven histoplasmosis” was diagnosed in symptomatic patients who also had positive serology, urinary antigen, culture, or histopathological evidence of histoplasmosis. “Probable histoplasmosis” was diagnosed in patients who, in addition to travel to an endemic location, had 1) radiological findings highly suggestive of Histoplasma infection (multiple calcified pulmonary nodules); or 2) radiological findings consistent with Histoplasma infection (one or more pulmonary nodules, with or without calcification) and no alternate diagnosis considered more likely than histoplasmosis, and/or a travel partner with proven Histoplasma infection.
Serology.
Serological testing was performed at the Fungal Serology Laboratory, Mycotic Diseases Branch, Centers for Disease Control and Prevention, Atlanta, Georgia. Serum specimens were tested for antibodies to H. capsulatum by both complement fixation (CF) tests with histoplasmin and yeast-form antigens and by immunodiffusion (ID) with histoplasmin. CF titers of ≥ 1:32 or the detection of M or both M and H bands with the ID assay were considered presumptive evidence of infection with H. capsulatum.4 A CF titer of 1:16 was considered borderline. A CF titer of ≤ 1:8 was considered negative.
Results
Proven or probable histoplasmosis was identified in 23 cases in our records, spanning a period from 2000 to 2011. The median age at diagnosis of our patients was 31 years (range: 19–66 years). The majority (74%) were males.
All but one had visited Central and/or South America. Only one of our cases acquired the infection in an endemic area of North America (Indianapolis, IN, in the Ohio River basin). All cases were immunocompetent.
Eight of our cases were four pairs of travel partners. We did not have any larger groups of cases.
Nine patients reported visiting a specific cave infested by bats in Lanquin, Guatemala. Three patients reported visiting other bat-infested caves. Two travel partners reported entering a hollow tree trunk inhabited by bats in the Amazon jungle in Bolivia 10–14 days prior to onset of symptoms. Thus, altogether 14/23 cases (61%) reported exposure to bat habitats. Notably, all four travel couples reported visiting bat habitats.
We divided our patients into two groups. The first group composed of 14 patients who were symptomatic (Table 1). Of these, 13 (93%) had fever; but only 10/14 (71%) complained of respiratory symptoms such as cough, dyspnea, and chest pain.
Table 1.
Symptomatic cases of proven or probable histoplasmosis
| Age (gender) | Probable location of infection | Visited bat habitat | Symptoms | Radiology | Travel partner with histoplasmosis† | Serology | Additional diagnostic tests | ||
|---|---|---|---|---|---|---|---|---|---|
| Fever | Respiratory* | ||||||||
| 1 | 29 (F) | Central America m/p Guatemala | Yes | Yes | Yes | 2 Nodules | Yes (#15) | Borderline | — |
| 2 | 28 (M) | Central America m/p Guatemala | Yes | Yes | Yes | Normal chest radiograph | No | Positive | — |
| 3 | 23 (M) | South America m/p Bolivia | Yes | Yes | Yes | 2 Nodules, 2 cavitated, LN | Yes (#4) | Positive | — |
| 4 | 22 (M) | South America m/p Bolivia | Yes | Yes | No | Several nodules, LN | Yes (#3) | Positive | — |
| 5 | 23 (M) | South America | No | Yes | Yes | Granuloma, LN | No | Positive | — |
| 6 | 54 (F) | Central America m/p Guatemala | Yes | Yes | Yes | Multiple nodules | Yes (#7) | Positive | — |
| 7 | 57 (M) | Central America m/p Guatemala | Yes | Yes | Yes | Multiple nodules | Yes (#6) | ND | — |
| 8 | 24 (M) | Guatemala | Yes | No | Yes | Nodules | No | Positive | — |
| 9 | 20 (F) | Central America m/p Guatemala | Yes | Yes | No | ND | No | Positive | — |
| 10 | 64 (M) | Bolivia or Brazil | No | Yes | No | 2 Nodules, LN | No | ND | Biopsy‡ |
| 11 | 57 (M) | Bolivia or Peru | No | Yes | No | RLL infiltrate | No | Positive | |
| 12 | 19 (F) | Peru | No | Yes | Yes | Multiple nodules | No | Negative | UrinaryAg§ |
| 13 | 46 (M) | Costa Rica | No | Yes | Yes | Multiple nodules | No | Positive | Biopsy∥ |
| 14 | 23 (F) | South America | No | Yes | Yes | 2 Nodules | No | Borderline | |
Ag = antigen; F = female; LN = lymphadenopathy (hilar or mediastinal); M = male; m/p = most probably; ND = no data; RLL = right lower lobe.
Cough, dyspnea, chest pain.
Proven or probable.
Serology uninterpretable because of anticomplementary activity, diagnosis based on biopsy of mediastinal lymph nodes showing necrotizing granulomata, with growth of Histoplasma capsulatum on culture of biopsy tissue.
Urinary Histoplasma antigen positive.
Transbronchial biopsies showed necrotizing granulomata and non-budding yeast forms.
The second group of nine patients (Table 2) were asymptomatic and diagnosed during the evaluation of incidental radiological findings or because a travel partner had been suspected of Histoplasma infection.
Table 2.
Asymptomatic cases of proven or probable histoplasmosis (incidental radiological findings)
| Age (gender) | Probable location of infection | Visited bat habitat | Radiology | Travel partner with histoplasmosis* | Time lag (mo)† | Serology | Additional diagnostic tests | |
|---|---|---|---|---|---|---|---|---|
| 15 | 29 (M) | Central America m/p Guatemala | Yes | ND | Yes (#1) | 16 | Positive | — |
| 16 | 31 (M) | m/p Mexico | Yes | 3 Nodules of which 2 calcified | No | 108 | Negative‡ | — |
| 17 | 30 (F) | Guatemala | Yes | Multiple calcified nodules/masses | Yes (#18) | 96 | Negative§ | — |
| 18 | 32 (M) | Guatemala | Yes | 2 Nodules, 1 calcified | Yes (#17) | 96 | Negative§ | — |
| 19 | 37 (M) | Central America m/p Guatemala | Yes | 2 Partially calcified nodules | No | 36 | Positive | — |
| 20 | 66 (M) | South or Central America | No | RLL mass | No | 18 | Borderline∥ | Excisional biopsy |
| 21 | 33 (M) | Central America | No | Multiple calcified foci—lung and spleen | No | 120 | ND | — |
| 22 | 54 (M) | Indianapolis, IN | No | Multiple calcified nodules | No | 36 | ND | — |
| 23 | 52 (M) | Dominican Republic | Yes | Calcified nodule | No | 72 | ND | — |
F = female; M = male; m/p = most probably; ND = not done or uninterpretable; RLL = right lower lobe.
Proven or probable.
From return from endemic area until presentation.
Diagnosis (probable) based on typical radiological appearance.
Diagnosis based on radiological appearance and travel partner with proven or probable histoplasmosis.
Diagnosis based on histopathology of mass: necrotizing granulomata, yeasts with morphology of Histoplasma capsulatum in adjacent lymph node.
Although all of the symptomatic patients presented within 3 months of their return, the asymptomatic patients, in contrast, were diagnosed 16–120 months (median: 72 months) after their return from the endemic region.
Radiological findings also differed between the two groups. Symptomatic patients (Table 1) generally had two or more nodules that were not calcified. One had an infiltrate. Four had thoracic lymphadenopathy. In contrast, the majority of the asymptomatic, late presenters had calcified nodules without lymphadenopathy (Table 2).
In the symptomatic group, a proven diagnosis was obtained in 11 of the 14 patients (Table 1). In nine patients, serological testing for antibodies against Histoplasma was positive, in two patients, biopsy was positive (one was also seropositive), and in one case, urinary antigen was positive (despite negative serology).
In contrast, in the asymptomatic group with remote exposure, only 3 of 14 patients had a definitive diagnosis—two by positive serology and one by biopsy (Table 2). Thus, 75% of the recently exposed (≤ 3 months) symptomatic patients who had serological testing were positive, whereas only 22% of the asymptomatic cases, whose exposure was more remote, were positive.
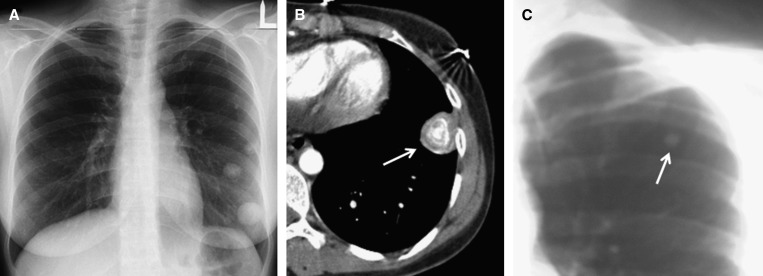
The circumspect way in which we supported some of our diagnoses of histoplasmosis is demonstrated by a woman who presented in the emergency room after a road accident (patient #17). A routine chest radiograph showed several nodules (Figure 1A). Computed tomography of the chest showed the nodules were calcified (Figure 1B). Thorough questioning about her travel history revealed that she had visited a bat-infested cave in Central America, and the diagnosis was confirmed by a chest radiograph of her travel partner who showed two calcified nodules (Figure 1C). Serological testing was negative in both the patient and her partner.
Figure 1.
(A) Chest radiograph of patient #17 showing several dense nodules in the left lower lung field. (B) Computed tomography of the chest showing a nodule (arrow) with “onion-skin” calcification. (C) Analog chest radiograph of her travel partner, which showed a calcified nodule (arrow) in the left upper lung field. An additional nodule was visible in the right mid-lung field. Clinical details are described in the text.
Discussion
Histoplasmosis is the most common systemic mycosis diagnosed among travelers returning from endemic areas.4,7 In recent years, a growing number of cases of histoplasmosis, both sporadic and in clusters, have been reported in travelers.4,6,8–11 This rise in apparent cases of histoplasmosis may be the result of increased international travel, and probably increased awareness among medical practitioners.
Although H. capsulatum was once isolated from soil sampled from a cave in Israel,12 autochthonous cases of histoplasmosis have not been reported in this country. We herein describe a series of 23 imported cases of proven/probable histoplasmosis in Israeli travelers returning from endemic areas. Our case series has several noteworthy features.
In agreement with the majority of reports of histoplasmosis in returning travelers, in all but one of our cases the infection was acquired in Central or South America. H. capsulatum is highly endemic in the Americas, but cases imported by travelers are almost all from Latin America (Central and South America) and are much more rarely reported from North America despite the fact that some regions of the United States are highly endemic. Histoplasmosis also exists in other regions of the world such as East Africa,11,13 the Indian subcontinent,14,15 and Australia,16 but there are hardly any cases reported by travelers to these regions.17 The reason for this pattern is unclear, but we suggest two possible explanations. First, foci of Histoplasma endemicity outside Latin America may not be popular travel destinations. Second, there may be an ascertainment bias related to a lack of awareness, among physicians serving nonendemic countries, that there are regions endemic for Histoplasma outside the well-described endemic parts of the Americas.
Most cases of imported histoplasmosis occur in clusters of individuals with a common source of infection. Often there is a group history of participation in activities that involve heavy exposure to bat or bird droppings, mostly spelunkers18,19 or volunteers with recent involvement in cleaning tasks or rehabilitation of old buildings,20 but one large outbreak in travelers returning from Mexico was reported in which the source of infection was a hotel where all the cases had resided during their trip.5 In our series, there were no groups, making the diagnosis more challenging, but 8 of the 23 cases formed 4 pairs of travel partners. This difference may simply reflect different travel habits, but may also reflect a higher index of suspicion for histoplasmosis in our setting—a specialist clinic for returning travelers and tropical disease whose staff are more likely to consider histoplasmosis even in isolated cases, as opposed to larger groups of returning travelers with a similar clinical syndrome, in whom the diagnosis may be more apparent.
The majority of our series (61%) reported visiting bat habitats in the region endemic for H. capsulatum. Nine cases (including four pairs of travel partners) were likely infected after visiting a single site—a bat-infested cave in Lanquin, Guatemala. Outbreaks of histoplasmosis reported in spelunkers are most probably related to bat infestation of the caves. Bird and bat droppings seem to encourage the growth of Histoplasma in the environment. Bats as well as numerous other mammalian species have been shown to be infected with histoplasmosis. Although birds may help spread of Histoplasma by carrying spores on their feet and wings, they are not infected, probably because the body temperature of birds is too high to support the growth of H. capsulatum.21
Our series divides naturally into two groups: acute, symptomatic cases (Table 1) presenting early (1–3 months) after the travelers' return from the endemic regions in Central and South America and asymptomatic cases diagnosed incidentally, as long as 10 years after the return from the endemic region (Table 2). Each group exemplifies challenges in the diagnosis of histoplasmosis in nonendemic countries.
Returning travelers with symptomatic histoplasmosis are often treated by physicians unacquainted with the epidemiology, clinical features, and diagnostic tests of histoplasmosis. Indeed several of our patients who were seen first in other facilities were treated initially for tuberculosis without considering histoplasmosis. Historically, misdiagnosis as tuberculosis was common before the discovery of histoplasmosis in the mid-twentieth century.22 A thorough travel history, including not only the travel itinerary but also specific questioning regarding exposure to bat habitats, is essential for considering the diagnosis. Histoplasmosis is rarely considered in the differential diagnosis of fever of unknown origin after tropical exposure. In fact, although all of our symptomatic cases had primary lung infections, in 28% (4/14 patients) the presentation was of febrile illness without respiratory symptoms.
Even when the level of awareness is high, diagnostic tests recommended in the literature such as serology and urinary antigen assay23 are not available in many nonendemic countries. Moreover, although the sensitivity of serology is reported to be 90%,23 only 75% of our acute, symptomatic cases were seropositive. Urine antigen testing was not performed in our cohort because of lack of availability, except in one case tested in another hospital.
The asymptomatic group, with remote exposure to Histoplasma (1.5–10 years after exposure) offers a different challenge. Those patients presented for a variety of different reasons and were found to have pulmonary nodules (often calcified). The differential diagnosis of these nodules, especially in countries nonendemic for histoplasmosis, may trigger invasive diagnostic procedures. Those cases with remote exposure highlight the importance of taking a travel history in the evaluation of patients with asymptomatic pulmonary nodules.
Theoretically, serological testing for histoplasmosis may confirm the diagnosis in late presenters. However, anti-Histoplasma antibody titers wane over the years,23 as can be seen in our case series from the fact that only 22% of late presenters had a positive serological test, and these cases had a relatively shorter interval between exposure and diagnosis (≤ 3 years).
Histoplasmin skin testing, once widely used, has been abandoned, largely because of high-background skin test positivity in endemic areas (50–80%). Indeed the histoplasmin reagent is no longer commercially available. However, in the traveler returning to the nonendemic native country, the skin test might be useful in revealing exposure, since background histoplasmin sensitivity in nonendemic regions (including Israel) is negligible.24,25 Therefore, reviving the histoplasmin skin test in nonendemic countries for use in returning travelers may reveal exposure to Histoplasma in “late presenters,” as well as those with a subacute presentation in whom symptoms may continue for several weeks. Further research is warranted to determine the utility of the skin test in this context.
In conclusion, several important lessons may be learned from this case series.
First, histoplasmosis should be considered in travelers within weeks of return from Central or South America who present with acute/subacute onset of cough, dyspnea, or chest pain, or febrile illness even without respiratory symptoms, particularly if exposed to bat habitats. Immunocompromised travelers should be warned of the danger of Histoplasma infection when traveling to Latin America, and should at least avoid bat habitats in these areas. Since histoplasmosis does exist in other continents as well, vigilance to the possibility of import of the disease from other regions is important.
Second, a travel history should be elicited in the evaluation of pulmonary nodules.
Third, questioning and diagnostic testing of travel partners may be a useful adjunct in the returning traveler suspected of having current or healed histoplasmosis.
Finally, serology and/or urinary antigen are useful for the diagnosis of “early” presenters but wane over time and thus are of limited utility in late presenters. The use of the histoplasmin skin test as a tool for histoplasmosis diagnosis in nonendemic countries should be further evaluated.
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Authors' addresses: Michael J. Segel, Institute of Pulmonology, Chaim Sheba Medical Center, Tel HaShomer, Ramat Gan, Israel, and Sackler Medical School, Tel Aviv University, Tel Aviv, Israel, E-mail: Michael.Segel@gmail.com. Judith Rozenman, Department of Imaging, Chaim Sheba Medical Center, Tel HaShomer, Ramat Gan, Israel, and Sackler Medical School, Tel Aviv University, Tel Aviv, Israel, E-mail: Yehudit.Rozenman@Sheba.health.gov.il. Mark D. Lindsley, Mycotic Diseases Branch, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: mil6@cdc.gov. Tamar Lachish, Infectious Diseases Unit, Shaare-Zedek Medical Center, Jerusalem, Israel, E-mail: LachishT@yahoo.com. Neville Berkman, Institute of Pulmonology, Hadassah-Hebrew University Medical Center, Jerusalem, Israel, E-mail: Neville@Hadassah.org.il. Ami Neuberger, Rappaport Faculty of Medicine, Technion—Israel Institute of Technology, Haifa, Israel, and Unit of Infectious Diseases, Internal Medicine B, Rambam Medical Center, Haifa, Israel, E-mail: A_Neuberger@Rambam.health.gov.il. Eli Schwartz, Internal Medicine “C” and Center for Geographic Medicine, Chaim Sheba Medical Center, Tel HaShomer, Ramat Gan, Israel, and Sackler Medical School, Tel Aviv University, Tel Aviv, Israel, E-mail: elischwa@post.tau.ac.il.
References
- 1.Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev. 2007;20:115–132. doi: 10.1128/CMR.00027-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wheat LJ. Histoplasmosis: a review for clinicians from non-endemic areas. Mycoses. 2006;49:274–282. doi: 10.1111/j.1439-0507.2006.01253.x. [DOI] [PubMed] [Google Scholar]
- 3.Wheat J. Histoplasmosis. Experience during outbreaks in Indianapolis and review of the literature. Medicine (Baltimore) 1997;76:339–354. doi: 10.1097/00005792-199709000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Panackal AA, Hajjeh RA, Cetron MS, Warnock DW. Fungal infections among returning travelers. Clin Infect Dis. 2002;35:1088–1095. doi: 10.1086/344061. [DOI] [PubMed] [Google Scholar]
- 5.Morgan J, Cano MV, Feikin DR, Phelan M, Monroy OV, Morales PK, Carpenter J, Weltman A, Spitzer PG, Liu HH, Mirza SA, Bronstein DE, Morgan DJ, Kirkman LA, Brandt ME, Iqbal N, Lindsley MD, Warnock DW, Hajjeh RA. A large outbreak of histoplasmosis among American travelers associated with a hotel in Acapulco, Mexico, Spring 2001. Am J Trop Med Hyg. 2003;69:663–669. [PubMed] [Google Scholar]
- 6.Gascon J, Torres JM, Jimenez M, Mejias T, Trivino L, Gobbi F, Quinto L, Puig J, Corachan M. Histoplasmosis infection in Spanish travelers to Latin America. Eur J Clin Microbiol Infect Dis. 2005;24:839–841. doi: 10.1007/s10096-005-0050-6. [DOI] [PubMed] [Google Scholar]
- 7.Leder K, Torresi J, Libman MD, Cramer JP, Castelli F, Schlagenhauf P, Wilder-Smith A, Wilson ME, Keystone JS, Schwartz E, Barnett ED, von Sonnenburg F, Brownstein JS, Cheng AC, Sotir MJ, Esposito DH, Freedman DO. GeoSentinel surveillance of illness in returned travelers, 2007–2011. Ann Intern Med. 2013;158:456–468. doi: 10.7326/0003-4819-158-6-201303190-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Norman FF, Martin-Davila P, Fortun J, Dronda F, Quereda C, Sanchez-Sousa A, Lopez-Velez R. Imported histoplasmosis: two distinct profiles in travelers and immigrants. J Travel Med. 2009;16:258–262. doi: 10.1111/j.1708-8305.2009.00311.x. [DOI] [PubMed] [Google Scholar]
- 9.Miyaji M, Kamei K. Imported mycoses: an update. J Infect Chemother. 2003;9:107–113. doi: 10.1007/s10156-003-0236-8. [DOI] [PubMed] [Google Scholar]
- 10.Farina C, Rizzi M, Ricci L, Gabbi E, Caligaris S, Goglio A. Imported and autochthonous histoplasmosis in Italy: new cases and old problems. Rev Iberoam Micol. 2005;22:169–171. doi: 10.1016/s1130-1406(05)70034-6. [DOI] [PubMed] [Google Scholar]
- 11.Cottle LE, Gkrania-Klotsas E, Williams HJ, Brindle HE, Carmichael AJ, Fry G, Beeching NJ. A multinational outbreak of histoplasmosis following a biology field trip in the Ugandan rainforest. J Travel Med. 2013;20:83–87. doi: 10.1111/jtm.12012. [DOI] [PubMed] [Google Scholar]
- 12.Ajello L, Kuttin ES, Beemer AM, Kaplan W, Padhye A. Occurrence of Histoplasma capsulatum Darling, 1906 in Israel, with a review of the current status of histoplasmosis in the Middle East. Am J Trop Med Hyg. 1977;26:140–147. doi: 10.4269/ajtmh.1977.26.140. [DOI] [PubMed] [Google Scholar]
- 13.Lofgren SM, Kirsch EJ, Maro VP, Morrissey AB, Msuya LJ, Kinabo GD, Saganda W, Diefenthal HC, Ramadhani HO, Wheat LJ, Crump JA. Histoplasmosis among hospitalized febrile patients in northern Tanzania. Trans R Soc Trop Med Hyg. 2012;106:504–507. doi: 10.1016/j.trstmh.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gopalakrishnan R, Nambi PS, Ramasubramanian V, Abdul Ghafur K, Parameswaran A. Histoplasmosis in India: truly uncommon or uncommonly recognised? J Assoc Physicians India. 2012;60:25–28. [PubMed] [Google Scholar]
- 15.Subramanian S, Abraham OC, Rupali P, Zachariah A, Mathews MS, Mathai D. Disseminated histoplasmosis. J Assoc Physicians India. 2005;53:185–189. [PubMed] [Google Scholar]
- 16.McLeod DS, Mortimer RH, Perry-Keene DA, Allworth A, Woods ML, Perry-Keene J, McBride WJ, Coulter C, Robson JM. Histoplasmosis in Australia: report of 16 cases and literature review. Medicine (Baltimore) 2011;90:61–68. doi: 10.1097/MD.0b013e318206e499. [DOI] [PubMed] [Google Scholar]
- 17.Mahvi A, Nachega J, Piron A, Blomme C, Deneys V, Provoost N, Boland B. Chronic disseminated histoplasmosis in an apparently immuno-competent Belgian patient. Acta Clin Belg. 2004;59:102–105. doi: 10.1179/acb.2004.015. [DOI] [PubMed] [Google Scholar]
- 18.Nasta P, Donisi A, Cattane A, Chiodera A, Casari S. Acute histoplasmosis in spelunkers returning from Mato Grosso, Peru. J Travel Med. 1997;4:176–178. doi: 10.1111/j.1708-8305.1997.tb00815.x. [DOI] [PubMed] [Google Scholar]
- 19.Lyon GM, Bravo AV, Espino A, Lindsley MD, Gutierrez RE, Rodriguez I, Corella A, Carrillo F, McNeil MM, Warnock DW, Hajjeh RA. Histoplasmosis associated with exploring a bat-inhabited cave in Costa Rica, 1998–1999. Am J Trop Med Hyg. 2004;70:438–442. [PubMed] [Google Scholar]
- 20.CDC Outbreak of histoplasmosis among travelers returning from El Salvador–Pennsylvania and Virginia, 2008. MMWR. 2008;57:1349–1353. [PubMed] [Google Scholar]
- 21.Segel MJ, Schwartz E. Histoplasmosis and other endemic fungal infections. In: Schwartz E, editor. Tropical Diseases in Travelers. Oxford, United Kingdom: Wiley-Blackwell; 2010. pp. 282–293. [Google Scholar]
- 22.Furcolow ML, Schubert J, Tosh FE, Doto IL, Lynch HJ., Jr Serologic evidence of histoplasmosis in sanatoriums in the U.S. JAMA. 1962;180:109–114. doi: 10.1001/jama.1962.03050150015003. [DOI] [PubMed] [Google Scholar]
- 23.Wheat LJ. Current diagnosis of histoplasmosis. Trends Microbiol. 2003;11:488–494. doi: 10.1016/j.tim.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Baum GL, Racz I, Hofshi E. Skin sensitivity to histoplasmin and coccidiodin in Israel. Am J Trop Med Hyg. 1965;14:643–646. [Google Scholar]
- 25.Daoud KA, Schwabe CW. A skin test survey for histoplasmosis and coccidioidomycosis in the Middle East. Am J Trop Med Hyg. 1958;7:643. doi: 10.4269/ajtmh.1958.7.643. [DOI] [PubMed] [Google Scholar]