Abstract
American trypanosomiasis is an emerging zoonosis in the Brazilian Amazon. Studies on benznidazole (BZ) chemotherapy with Trypanosoma cruzi from this region have great relevance, given the different discrete typing units (DTUs) that infect humans in the Amazon and other regions of Brazil. We performed a parasitological, histopathological, and molecular analysis of mice inoculated with strains of T. cruzi I, II, and IV that were BZ-treated during the acute phase of infection. Groups of Swiss mice were inoculated; 13 received oral BZ, whereas the other 13 comprised the untreated controls. Unlike parasitemia, the infectivity and mortality did not vary among the DTUs. Trypanosoma cruzi DNA was detected in all tissues analyzed and the proportion of organs parasitized varied with the parasite DTU. The BZ treatment reduced the most parasitological parameters, tissue parasitism and the inflammatory processes at all infection stages and for all DTUs. However, the number of significant reductions varied according to the DTU and infection phase.
Introduction
Currently, ∼12 million people are estimated to be infected with Trypanosoma cruzi, the etiologic agent of Chagas disease (CD) or American trypanosomiasis, in Latin America, and 75 million more are exposed to infection.1 In its chronic phase, CD causes significant disabilities and has high social and economic impacts. In Brazil, more than US$1.3 billion in wages and industrial productivity were lost because of CD complications.2 Some authors have emphasized the importance of preventive measures and have warned about the possibility that Amazonia might become an endemic region, as deforestation and orally acquired CD outbreaks often occur.3
Studies that have investigated CD in the Amazon region have highlighted clinical signs and symptoms observed mainly during the acute phase, including fever, headache, myalgia, dyspnea, facial edema, abdominal pain and exanthema, and myocarditis as the most frequent lesion.4 These signs are usually severe and differ from those in classical endemic areas. It is believed that the greater severity of the acute disease phase in the Brazilian Amazon is mostly caused by oral transmission, and some authors have reported the high morbidity of acute CD in this region.3,5 The chronic phase predominates in a latent form, with few cardiomyopathy records among serologically confirmed patients.6,7 However, in a study of CD characteristics in the State of Paraná, an older endemic area, it was observed that the cardiac form was present in most chronically hospitalized patients, followed by the digestive form.8
The unusual biological heterogeneity of T. cruzi isolates in terms of morphology, DNA content, virulence, pathogenicity, drug susceptibility, and other parameters is documented in the literature.9–12 According to a consensus reached in 2009, T. cruzi strains should be classified into discrete typing units (DTUs) as T. cruzi I (TcI) to T. cruzi VI (TcVI). To date, knowledge about these T. cruzi DTUs in the Amazon region is scarce, and pioneer studies from our group present a detailed understanding of the genetic, biological, and histopathological characteristics of parasites belonging to TcI and IV DTUs.13–17
The goal of etiologic treatment of CD is the parasite elimination from the infected individual, thereby inhibiting clinical evolution and breaking the disease transmission chain.18 Histologically, the acute phase of trypanosomiasis is a systemic infection, and intracellular forms of the parasite can be found in all organs.19 Therefore, an important parameter to evaluate is the drug effect at the tissue level for monitoring cure and recurrent disease progression even over a long term.
Given the lack of studies on the biological characteristics of Amazon T. cruzi strains, particularly TcIV, and the effects of etiologic treatment on the evolution of experimental infections in mice with different parasite DTUs, this study aims to evaluate and compare the impact of benznidazole (BZ) treatment when administered during the acute phase on the evolution of infections in mice inoculated with the T. cruzi strains TcI and TcIV from the State of Amazonas and TcII strains from Paraná, using parasitological, histopathological, and molecular (polymerase chain reaction) analyses.
Materials and Methods
Parasites.
A total of 18 T. cruzi strains were studied; 14 were isolated from patients during the acute phase of infection, vectors and wild reservoirs from the State of Amazonas and were classified as TcI (6) and TcIV (8),13and four strains were isolated from chronically infected patients residing in the State of Paraná and were genotyped as TcII20 (Table 1). Parasites were maintained in culture in liver infusion tryptose (LIT) medium by successive subculturing with alternating mouse-LIT-mouse passages and in blood passages in mice at the Chagas Disease Laboratory (Laboratório de Doença de Chagas), Maringá State University (Universidade Estadual de Maringá; UEM).
Table 1.
Discrete typing unit (DTU), source of isolation, geographical origin, infectivity rate (%INF), parasitemia clearance rate (%PC), and profile of in vivo susceptibility to benznidazole of Trypanosoma cruzi strains studied
| Strains of T. cruzi | DTU | Source of isolation | Geographical origin | %INF | % PC | Susceptibility to BZ |
|---|---|---|---|---|---|---|
| MHOM/BR/2008/AM49 | TcI | Acute phase patient | Coari/AM* | 100.0 (7/7) | 0.0 (0/6) | R |
| MDID/BR/2007/AM45 | TcI | Didelphis marsupialis | Manaus/AM | 71.4 (5/7) | 16.7 (1/6) | R |
| MDID/BR/2007/AM44 | TcI | Didelphis marsupialis | Manaus/AM | 71.4 (5/7) | 42.9 (3/7) | IS |
| MPHI/BR/2007/AM34 | TcI | Philander opossum | Manaus/AM | 100.0 (7/7) | 57.1 (4/7) | IS |
| TPIS/BR/2007/AM33 | TcI | Rhodnius pictipes | Manaus/AM | 100.0 (6/6) | 85.7 (6/7) | S |
| MDID/BR/2007/AM31 | TcI | Didelphis marsupialis | Manaus/AM | 57.1 (4/7) | 100.0 (7/7) | S |
| MHOM/BR/2007/PR1219 | TcII | Chronic phase patient | Maringá/PR† | 100.0 (13/13) | 0.0 (0/6) | R |
| MHOM/BR/2007/BS48 | TcII | Chronic phase patient | Colorado/PR | 100.0 (9/9) | 42.9 (3/7) | IS |
| MHOM/BR/2009/PR2259 | TcII | Chronic phase patient | Maringá/PR | 90.0 (9/10) | 100.0 (8/8) | S |
| MHOM/BR/2010/PR1226 | TcII | Chronic phase patient | Virgem da Lapa/MG‡ | 90.0 (9/10) | 100.0 (6/6) | S |
| MHOM/BR/2007/AM15 | TcIV | Acute phase patient | Coari/AM | 87.5 (7/8) | 0.0 (0/5) | R |
| MHOM/BR/2007/AM14 | TcIV | Acute phase patient | Coari/AM | 90.0 (9/10) | 14.3 (1/7) | R |
| TROB/BR/2009/AM57 | TcIV | Rhodnius robustus | Apuí/AM | 100.0 (9/9) | 28.6 (2/7) | R |
| MHOM/BR/2010/AM64 | TcIV | Acute phase patient | SIRN§/AM | 100.0 (9/9) | 28.6 (2/7) | R |
| MHOM/BR/2010/AM62 | TcIV | Acute phase patient | SIRN/AM | 100.0 (13/13) | 42.9 (3/7) | IS |
| MHOM/BR/2010/AM67 | TcIV | Acute phase patient | SIRN/AM | 100.0 (10/10) | 50.0 (4/8) | IS |
| MHOM/BR/2010/AM69 | TcIV | Acute phase patient | SIRN/AM | 77.8 (7/9) | 85.7 (6/7) | S |
| MHOM/BR/2010/AM68 | TcIV | Acute phase patient | SIRN/AM | 80.0 (8/10) | 100.0 (7/7) | S |
Brazilian states of
Amazonas;
Paraná;
Minas Gerais; and
Santa Isabel do Rio Negro.
R = resistent; IS = intermediate sensitivity; S = sensitive.
Inoculation.
For each T. cruzi strain, 26 male Swiss mice between 21 and 28 d of age were used; the mice were obtained from the Central Animal Facility at UEM. Mice were kept in a room with controlled humidity and temperature and light/dark cycles and were given water and food ad libitum. The animals were inoculated intraperitoneally (IP) with an inoculum of 1 × 104 blood trypomastigotes (BT)/animal (strains AM57, AM67, AM68, BS48, PR2259, and PR1226) or 2 × 106 culture metacyclic trypomastigotes (MT)/animal21 for the other strains (Table 1).
Treatment scheme.
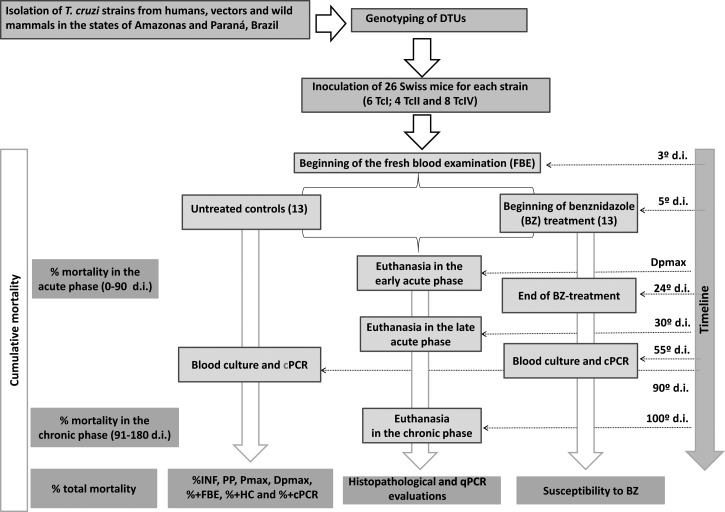
After inoculation, the mice were divided into two groups of 13 animals each (Figure 1); one group was treated with benznidazole (TBZ), whereas the other served as the untreated control (NT). The TBZ group animals received 100 mg/kg/d of BZ (Lafepe, Recife, Brazil) by gavage for 20 consecutive days from Day 5 of infection (d.i.).9
Figure 1.
Fluxogram depicting the experimental design.
Infectivity and susceptibility to BZ.
From the third d.i., animals in both groups were daily tested by fresh blood examinations (FBEs) to confirm the infections before starting treatment and to obtain parasitemia data. Some animals were treated without causing any patent parasitemia, especially those inoculated with TcI strains that have subpatent parasitemia. Both the TBZ and NT animals were submitted to FBE, hemoculture (HC),9 and conventional polymerase chain reaction (cPCR) on blood23 routinely used in our laboratory to determine the rate of infectivity (%INF) and susceptibility to BZ. Animals with at least one positive result were considered to be infected.22,23 However, TBZ group animals with negative results in all three tests (FBE, HC, and cPCR) after treatment could not be considered “cured” but rather as displaying a “sustained parasitemia clearance,” because T. cruzi DNA was found in the tissues of these animals. The rate of animals exhibiting parasitemia clearance (%PC) was obtained by calculating the ratio of the number of animals with parasitemia clearance and the total number of treated animals, multiplied by 100.22
Parasitological parameters.
The following were performed for animals in both the TBZ and NT groups: FBE was performed daily from the third d.i. until a negative result was obtained for at least 3 consecutive days for patent parasitemia strains and for 40 d for subpatent parasitemia strains. The percentage of animals with a positive FBE (%+FBE)24 was determined. Parasitemia curves were generated from the FBE data. The mean patent period (PP), the maximum peak of parasitemia (Pmax) and the day of maximum peak parasitemia (Dpmax)22 were determined. At 30 d after the end of treatment, the TBZ and NT animals underwent HC9 in LIT medium to obtain the percentage of mice with positive blood cultures (%+HC). Conventional PCR was performed with blood samples that were collected 30 d after treatment23,25,26 according to the protocol of Gomes,25 which was adapted to mice by Miyamoto and others.26 The percentage of cPCR-positive animals (%+cPCR) was obtained from the cPCR results. Finally, the cumulative mortality (%MOR) was recorded daily throughout the course of infection.
Histopathology.
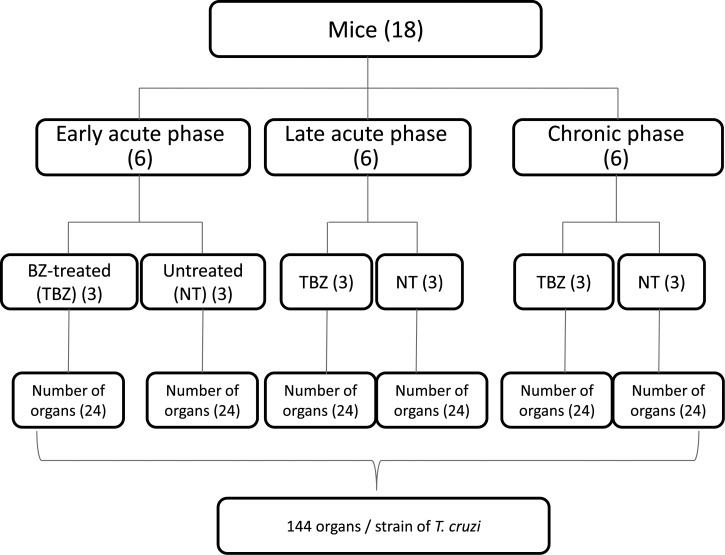
For the histopathological study, laparotomy was performed on animals that had been euthanized by deep anesthesia with an intraperitoneal administration of ketamine (50 mg/kg) and xylazine (10 mg/kg). For each T. cruzi strain, 18 animals were evaluated; nine were included in the TBZ group (to assess the impact of treatment on the tissue lesions), and nine were included in the NT group (to determine the strain pathogenicity). Of the nine animals in each group, three were euthanized at 1 d after Pmax to represent the early acute phase (eAP), three at 30 d.i. to study the late acute phase (lAP), and three at 100 d.i. to study the chronic phase (CP). Tissue sampling was made from the following organs/tissues: brain, heart, abdominal skeletal muscle, left hind paw, diaphragm, large intestine, liver, and spleen. Tissue fragments were first fixed in 10% formalin and, after 24 hr, preserved in 70% alcohol. The fragments were dehydrated, cleared, and embedded in paraffin. Five-μm-thick sections, separated by 25-μm intervals, were placed on microscopy slides and were subjected to a battery of hematoxylin/eosin (H/E) staining, permanently mounted, and examined under an optical microscope.
For each T. cruzi strain, a total of 144 organs were analyzed and read in quaduplicate; the end results were presented as averages. The number of strains studied differed between the DTUs (6 strains TcI, 4 strains TcII, and 8 TcIV) for total counts of 864 organs for TcI, 576 for TcII, and 1,152 organs for TcIV in the different infection stages and for both the TBZ and NT groups (Figure 2).
Figure 2.
Fluxogram showing the number of mice and organs assessed at each stage of infection and experimental groups for each strain of Trypanosoma cruzi studied.
Histopathological parameters.
The slides were analyzed, and the entire tissue lengths were analyzed in a double-blind study. The analyzed histopathological parameters included the presence and intensity of tissue parasitism (TP), the presence and intensity of inflammatory process (IP), and tissue tropism. For tissue parasitism, the presence of amastigote nests in different tissues was analyzed and classified as follows: absent (−), without nests; mild, 1–5 nests (+); moderate, 6–10 nests (+ +) and intense, > 10 nests (+ + +). Inflammatory process was considered as the presence of 10 or more inflammatory cells per microscopic field at 400× magnification. Two steps were followed during the IP analysis. First, inflammatory foci were classified as follows according to presence and intensity: absent (−), the minimum number of inflammatory cells was not found; mild, 10–25 cells (+); moderate, 26–50 cells (+ +) and intense, >50 cells (+ + +).27,28 Tissue changes in the spleen were classified as absent (−), mild hyperplasia (+, 1 focus/section), moderate (++, 2-5 foci/delimited/section), and intense (+++, more than 5 foci or throughout the length of the section). Second, the distribution of inflammatory infiltration per field was classified as follows: 1) focal, proximity between the infiltrating cells; 2) multifocal, more than one focus; and 3) diffuse, distance between the infiltrating cells.27 The following classifications were used for whole tissues: mild (+, single focus), moderate (+ +, 2–5 foci), intense (+++, 6–10 foci), and very intense (++++, > 10 foci).29
Real time polymerase chain reaction (qPCR).
Mice inoculated with six TcI strains (AM44, AM31, AM33, AM34, AM45, and AM49), two TcII strains (PR1219 and PR2259) and five TcIV strains (AM14, AM15, AM62, AM64, and AM69) were analyzed by qPCR.
Tissues analyzed in qPCR.
Fragments of 30 mg the same tissues collected for histological analysis were analyzed. One randomly selected animal was euthanized for each strain and for each stage of infection (eAP, lAP, and CP), from both TBZ and NT groups. A total of 48 organs were evaluated for each strain, 24 from TBZ animals and 24 from NT animals, eight in each stage of infection.
Extraction of genomic DNA.
Six hundred (600 μL) of a mixture of 0.5 M EDTA (pH 8.0) plus 50 mM NaOH and 18 μL of proteinase K 20 mg/mL (Invitrogen) were added on each tissue fragment. A water bath incubation was made for 2 hours at 55°C. Subsequently, 3 μL RNAse were added followed by incubation at 37°C for 15 minutes. To each sample, 200 μL of chloroform were added, and DNA was precipitated with 100% isopropanol, washed with 70% ethanol and resuspended in 20 μL of Milli-Q water.
qPCR.
For each sample analyzed in duplicate, the PCR reaction contained 2 μL genomic DNA, 0.20 mM of each primer, 5 μL SybrGreen PCR Mastermix, and Milli-Q water to a final reaction volume of 10 μL. The primers are oligonucleotides specific for a repeat of 195 base pairs (bp) of DNA from T. cruzi TCZ 5′–GCTCTTGCCCACAMGGGTGC-F–3′, where M = A or C (Invitrogen) and TCZ-R 5′–CCAAGCAGCGGATAGTTCAGG–3′, which amplify a product of 182 bp. The thermocycling program consisted of heating at 95°C for 10 minutes, 40 cycles of 94°C for 15 sec, 64.3°C for 1 minute, with fluorescence acquisition at 64.3°C. The amplification was immediately followed by melting with a program of initial denaturation at 95°C for 15 sec, cooling to 60°C for 1 minute, and gradual temperature increase of 0.3°C/sec to 95°C.30
Qualitative data analysis was performed considering the presence or absence of amplification. Tissue samples on which were detected the presence of T. cruzi DNA by qPCR were considered positive and organs of which the fragments were collected considered parasitized. The number of parasitized organs was obtained for each DTU, stage of infection (eAP, lAP, and CP) and experimental group (TBZ and NT). The parameter proportion of organs that showed positive results in qPCR (%+qPCR) was also obtained.
Statistics.
Data analysis was performed with the assistance of BioEstat software, version 5.3 (Belém, Pará, Brazil). The χ2 test was used to test for differences in proportions (%+FBE, %+HC, %+cPCR, %+qPCR, %INF, %MOR, and %PC), and Student's t test was used to test for differences between means (PP, Pmax, and Dpmax). Statistical comparisons were performed among the three DTUs (TcI versus TcII, TcI versus TcIV, and TcII versus TcIV), and among the three stages of infection within each DTU (eAP, lAP, and CP), and between groups (TBZ versus NT). The proportions of organs/tissues with pathological changes were also compared with the χ2 test. Statistical significance was considered if P < 0.05.
Ethical approval.
The use of T. cruzi strains obtained from humans in the states of Amazonas and Paraná was approved by the ethics committees of the Dr. Heitor Vieira Dourado Tropical Medicine Foundation (approval no. 360/07) and UEM (approval nos. 100/04 and 375/07). The maintenance and care of experimental animals complies with the National Council for the Control of Animal Experimentation (Conselho Nacional de Controle de Experimentação Animal – CONCEA) guidelines for the human use of laboratory animals, and use of mice was approved by the Ethics Committee for Animal Research of UEM (approval no. 113/09).
Results
Infectivity and parasitemia clearance rates.
The overall %INF in mice inoculated with 18 T. cruzi strains was 90.7% (146 of 161 animals). The %INF of TcI, TcII, and TcIV- inoculated animals did not significantly differ, with respective values of 82.9% (34 of 41), 95.2% (40 of 42), and 90.7% (146 of 161). Table 1 lists the %INF of each strain in the study. During the acute phase, BZ-treated infected mice had an overall parasitemia clearance rate (%PC) of 51.6% (63 of 122). The %PC for each strain are presented in Table 1. The TcI-, TcII-, and TcIV-inoculated animals had rates of 52.5% (21 of 40), 63% (17 of 27), and 45.5% (25 of 55), and the differences between the rates were not significant. However, Table 1 shows that the proportions of BZ-resistant (R), intermediately sensitive (IS), and sensitive (S) TcI strains were identical, whereas TcII strains included a higher proportion of S strains and TcIV included a higher proportion of R strains.
Impact of treatment on parasitological parameters.
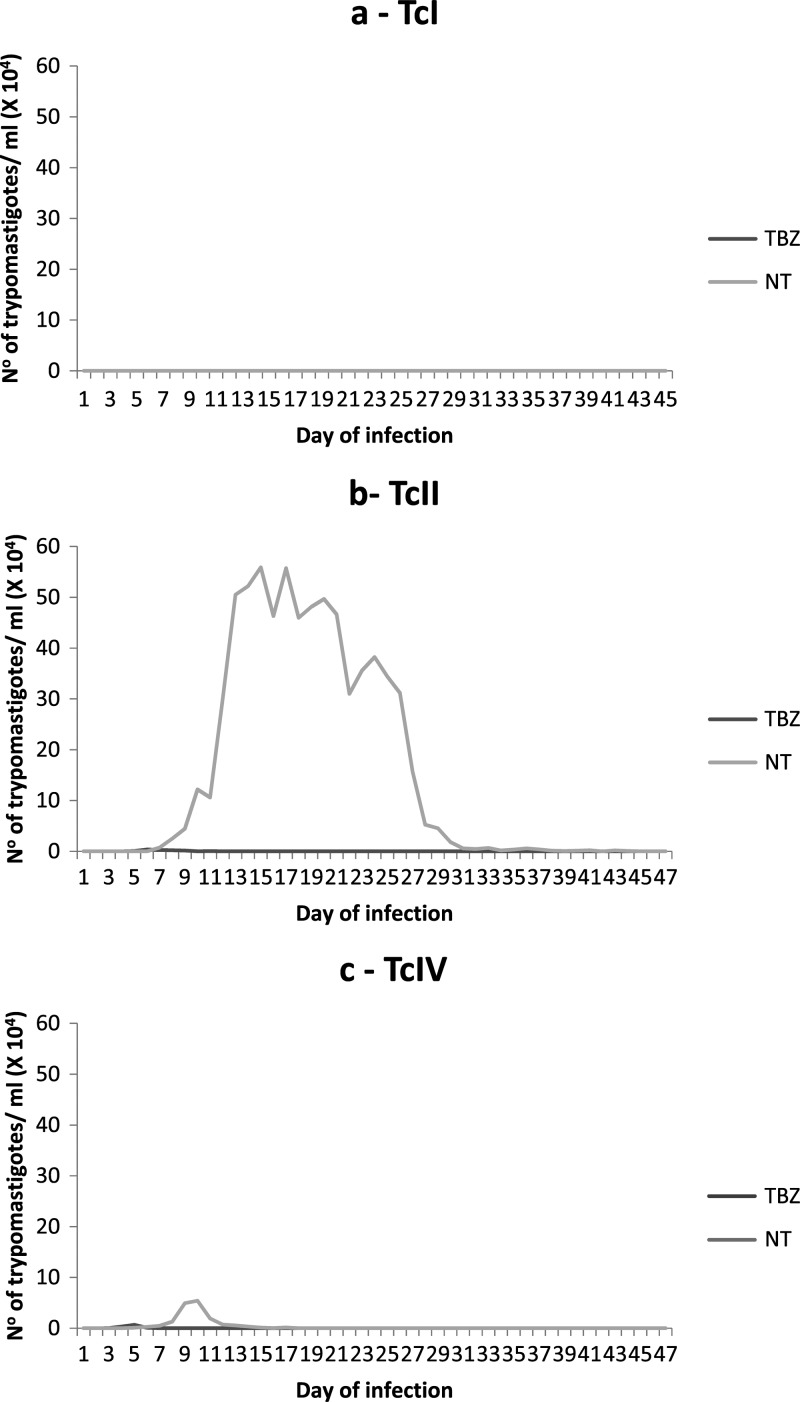
The mean parasitemia curves of the TBZ and NT mice inoculated with T. cruzi strains of each DTU are shown in Figure 3 (A-TcI, B-TcII, and C-TcIV). None of the six studied TcI strains determined patent parasitemia. Mice inoculated with the TcII and TcIV, which induce patent parasitemia, showed suppression of the same shortly after treatment initiation.
Figure 3.
Mean parasitemia curves in mice inoculated with strains of Trypanosoma cruzi I (A), II (B), and IV (C), treated with benznidazole (100 mg/kg/d- 20×) (TBZ) from Day 5 of infection and untreated controls (NT).
Regardless of the infecting DTU, BZ treatment during the acute phase significantly reduced five of the seven evaluated parasitological and molecular parameters (Table 2). There were no statistical differences between the groups TBZ and NT, for the parameters %+cPCR and %MOR.
Table 2.
Statistical comparisons of parasitological and molecular parameters of mice inoculated with strains of Trypanosoma cruzi I, II, and IV treated with benznidazole (TBZ), 100 mg/kg/day-20×, and untreated controls (NT)
| Parameter* | Total | TcI | TcII | TcIV | ||||
|---|---|---|---|---|---|---|---|---|
| NT × TBZ | P | NT × TBZ | P | NT × TBZ | P | NT × TBZ | P | |
| PP (in days) | 6.3 × 0.3 | < 0.001 | PA | − | 15.6 × 0.5 | 0.021 | 3.3 × 0.3 | < 0.001 |
| Pmax† (×103) | 21.7 × 0.35 | 0.009 | PA | − | 55.72 × 0.37 | 0.021 | 5.42 × 0.35 | 0.021 |
| DPmax‡ | 7.6 × 1.2 | 0.003 | PA | − | 15.3 × 5.5 | 0.021 | 7.5 × 2.0 | < 0.001 |
| %+FBE§ | 55.2 × 0.0 | < 0.001 | PA | − | 83.3 × 0.0 | 0.021 | 70.0 × 0.0 | < 0.001 |
| %+HC¶ | 68.9 × 36.4 | < 0.001 | 73.2 × 40.0 | 0.002 | 100.0 × 37.0 | < 0.001 | 50.9 × 33.3 | NS |
| %+cPCR∥ | 45.0 × 35.6 | NS | 14.0 × 12.0 | NS | 77.1 × 29.7 | < 0.001 | 17.0 × 23.0 | NS |
| %MOR** | 6.7 × 5.6 | NS | 6.0 × 2.0 | NS | 2.0 × 1.0 | NS | 7.0 × 6.0 | NS |
| No. of significant differences | 5/7 | 1/3 | 6/7 | 4/7 | ||||
The Student's t test was used in comparisons of PP, Pmax and Dpmax, and the χ2 for the other parameters.
Number of trypomastigotes/0.1 mL of blood at the peak of parasitemia.
Day of peak parasitemia.
Percentage of mice with positive fresh blood examination.
Percentage of mice with positive blood culture.
Percentage of mice with positive conventional polymerase chain reaction.
Cumulative mortality rate.
PP = mean patent period; PA = parasitemia absent; NS = not significant.
Statistical comparisons of these parameters between the TBZ and NT groups within the same DTU showed that the numbers of significant differences varied according to the parasite DTU. The animals inoculated with TcI showed no patent parasitemia, and it was therefore not possible to compare the parameters derived from the parasitemia curve. In mice inoculated with TcII, BZ treatment caused reductions in a greater number of parameters (6 of 7), and mice inoculated with TcIV showed a significant reduction only in the four parameters derived from the curve (Table 2).
Histopathology.
The histopathological analysis, which considered the TP and IP intensities, showed that the pathogenicity for mice varied significantly with the DTU and the phase of infection (Tables 3 and 4).
Table 3.
Number and percentage of organs presenting tissue parasitism and inflammation in mice inoculated with strains of Trypanosoma cruzi I, II, and IV treated with benznidazole (TBZ) during acute phase and untreated controls (NT), at different stages of infection
| Parameter | Stage of infection | Total | TcI | TcII | TcIV | ||||
|---|---|---|---|---|---|---|---|---|---|
| NT (N = 432) | TBZ (N = 432) | NT (N = 144) | TBZ (N = 144) | NT (N = 96) | TBZ (N = 96) | NT (N = 192) | TBZ (N = 192) | ||
| Tissue parasitism | eAP* | 12 (2.7)§¶ | 2 (0.5) | 3 (2.1)§¶ | 0 (0.0) | 9 (9.4)§¶ | 0 (0.0) | 0 (0.0) | 2 (1.0) |
| lAP† | 9 (2.1)§∥ | 0 (0.0) | 3 (2.1)§∥ | 0 (0.0) | 6 (6.3)§∥ | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| CP‡ | 1 (0.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Inflammatory process | eAP | 183 (42.4)§** | 84 (19.5) | 60 (41.7)§** | 43 (29.9) | 40 (41.7)§ | 7 (7.3)¶ | 83 (43.2)§¶ | 34 (17.8) |
| lAP | 238 (55.1)§∥ | 67 (15.5)∥ | 85 (59.0)§∥ | 29 (20.1) | 52 (54.2)§ | 7 (7.3)∥ | 101 (52.6)§∥ | 31 (16.2) | |
| CP | 157 (36.4)§ | 103 (23.8) | 61 (42.4)§∥ | 42 (29.2) | 40 (41.7)§ | 26 (27.1) | 56 (29.2)§ | 35 (18.2) | |
n = total number of organs assessed by group and phase of infection.
Early acute phase;
Late acute phase;
Chronic phase.
The χ2 was used in comparing the parameters between experimental groups (NT × TBZ) and between phases of infection (eAP × lAP, eAP × CP, and lAP × CP) with a significant value of P < 0.05;
Significant differences between groups NT and TBZ,
eAP and CP,
lAP and CP and
eAP and lAP.
Table 4.
Statistical comparisons* (P values) among discrete typing units of the proportion of organs presenting pathological changes in mice inoculated with strains of Trypanosoma cruzi I, II, and IV, at different stages of infection
| TcI × TcII | TcI × TcIV | TcII × TcIV | ||
|---|---|---|---|---|
| Stage of infection | (n = 144) (n = 96) | (N = 144) (n = 192) | (n = 96) (n = 192) | |
| Tissue parasitism | eAP† | 2.1 × 9.4 (0.012) | 2.1 × 0.0 (0.024) | 9.4 × 0.0 < 0.001 |
| lAP‡ | 2.1 × 6.3 (NS) | 2.1 × 0.0 (0.024) | 6.3 × 0.0 < 0.001 | |
| CP§ | 0.0 × 1.0 (NS) | 0.0 × 0.0 (−) | 1.0 × 0.0 (NS) | |
| Inflammatory process | eAP | 41.7 × 41.7 (−) | 41.7 × 43.2 (NS) | 41.7 × 43.2 (NS) |
| lAP | 59.0 × 54.2 (NS) | 59.0 × 52.6 (NS) | 54.2 × 52.6 (NS) | |
| CP | 42.4 × 41.7 (NS) | 42.4 × 29.2 (0.012) | 41.7 × 29.2 (0.034) |
χ2 with significant value of P < 0.05.
Early acute phase;
Late acute phase;
Chronic phase.
NS = not significant; (−) = not applicable.
TP.
Regardless of the DTU and phase of infection, TP was observed in 5.3% (24 of 864) of the evaluated organs (22 NT animals and 2 TBZ animals; Table 3). Compared with the CP, significantly higher numbers of organs with TP were observed in eAP and lAP.
Mice infected with TcII, although fewer in number compared with those infected with the other two DTUs, had higher numbers of organs with TP, followed by TcI-infected mice, and this was the only DTU group in which amastigotes were observed during CP (Table 3).
Statistical comparisons of TP between the DTUs.
The statistical comparisons between the DTUs, which considered only the NT animals, showed significant differences in the TP during eAP and lAP (Table 4). During eAP, the number of organs with TP was significantly higher in TcII-infected animals compared with TcI (P = 0.012) and TcIV-infected animals (P < 0.001) and in TcI-infected animals, compared with TcIV-infected animals (P = 0.024). During lAP, significant differences were found between TcI- and TcIV-infected animals (P = 0.024) and between TcII- and TcIV-infected animals (P < 0.001), with TcIV-infected animals presenting a lower TP (Table 4).
Intensity of parasitism.
Regarding intensity, mild TP was most frequently observed during eAP for all three DTUs, in lAP for TcI and TcII, and during CP only for TcII (Table 5). Moderate intensity TP was observed only for TcII during eAP and lAP, and intense TP was not registered in any organ evaluated during this study.
Table 5.
Number and percentage of organs presenting tissue parasitism (TP) and inflammatory process (IP) of different intensities (+, ++, and +++) in mice inoculated with Trypanosoma cruzi I, II, and IV treated with benznidazole (TBZ) and untreated controls (NT), at different stages of infection
| Stage of infection | Intensity | TcI(n=144) | TcII(n=96) | TcIV(n=192) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TP | IP | TP | IP | TP | IP | ||||||||
| NT | TBZ | NT | TBZ | NT | TBZ | NT | TBZ | NT | TBZ | NT | TBZ | ||
| eAP | + | 3 (2.1) | 0 (0.0) | 43 (30)†‡ | 30 (21)†‡ | 6 (6.3)*‡ | 0 (0.0) | 21 (21.8)*‡ | 5 (5.2) | 0 (0.0) | 2 (1.0) | 56 (29)*†‡ | 23 (11.9)†‡ |
| ++ | 0 (0.0) | 0 (0.0) | 17 (11.8)§ | 13 (9.0)§ | 3 (3.1) | 0 (0.0) | 12 (12.5)* | 1 (1.0) | 0 (0.0) | 0 (0.0) | 21 (10.9)*§ | 10 (5.2)§ | |
| +++ | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 7 (7.2)* | 1 (1.0) | 0 (0.0) | 0 (0.0) | 6 (3.1) | 1 (0.5) | |
| lAP | + | 3 (2.1) | 0 (0.0) | 54 (37.5)*†‡ | 24 (16.6)†‡ | 4 (4.1)*‡ | 0 (0.0) | 39 (40.6)*†‡ | 4 (4.1)‡ | 0 (0.0) | 0 (0.0) | 69 (36)*†‡ | 22 (11.4)†‡ |
| ++ | 0 (0.0) | 0 (0.0) | 24 (16.6)*§ | 5 (3.5)§ | 2 (2.0) | 0 (0.0) | 9 (9.3) | 3 (3.1) | 0 (0.0) | 0 (0.0) | 27 (14)*§ | 9 (4.6)§ | |
| +++ | 0 (0.0) | 0 (0.0) | 7 (4.8)* | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (4.1)* | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (2.6)* | 0 (0.0) | |
| CP | + | 0 (0.0) | 0 (0.0) | 31 (21.5)‡ | 24 (16.6)† | 1 (1.0) | 0 (0.0) | 29 (30.2)†‡ | 19 (19.7)†‡ | 0 (0.0) | 0 (0.0) | 42 (21.8)*†‡ | 24 (12.5)†‡ |
| ++ | 0 (0.0) | 0 (0.0) | 27 (18.8)*§ | 15 (10.5)§ | 0 (0.0) | 0 (0.0) | 10 (10.5)§ | 6 (6.2) | 0 (0.0) | 0 (0.0) | 14 (7.2)§ | 10 (5.2)§ | |
| +++ | 0 (0.0) | 0 (0.0) | 3 (2.1) | 3 (2.1) | 0 (0.0) | 0 (0.0) | 1 (1.0) | 1 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.5) | |
n = number of organs evaluated by group and stage of infection: early (eAP) and late acute phases (lAP), and chronic phase (CP); + = mild; ++ = moderate; and +++ = intense;
significant differences between groups NT and TBZ,
+ and ++,
+ and +++, and
++ and +++;
χ2 with significance value of P < 0.05.
During eAP and lAP, the numbers of organs with mild TP were significantly higher than those with intense TP in TcII-infected animals (Table 5).
Inflammatory process (IP).
Regardless of the DTU, when only NT animals were considered, the proportions of organs with IP were 42.4% (183 of 432) during eAP, 55.1% (238 of 432) during lAP, and 36.3% (157 of 432) during CP (Table 3). During lAP, the number of organs with IP was significantly higher than those during eAP and CP.
Additionally, in NT animals, the proportions of organs with IP in mice infected with the three DTUs were similar during the acute phase, and no statistical differences were found between the DTUs during eAP or lAP (Table 4). During eAP, 41.7% of the organs in TcI (60 of 144) and TcII-infected animals (40 of 96) and 43.2% (83 of 192) in TcIV-infected animals presented with IP, whereas during lAP, 59.0% (85 of 144) of the organs in TcI, 54.2% (52 of 96) in TcII, and 52.6% (101 of 192) in TcIV-infected mice presented with IP (Table 3).
However, during CP, animals infected with TcI, TcII, and TcIV respectively presented with organ inflammation rates of 42.4% (61 of 144), 41.7% (40 of 96), and 29.2% (56 of 192) (Table 3); furthermore, TcIV-infected mice presented a number of organs with IP that was significantly lower than the numbers in TcI (P = 0.012) and TcII-infected mice (P = 0.034) (Table 4).
Inflammation severity during the three infection stages.
Regarding inflammation severity in NT animals during eAP, organs with mild to moderate IP intensity were observed in animals infected with the three DTUs, whereas intense IP was only observed in TcII and TcIV-infected animals (Table 5). During eAP, the number of organs with mild IP was significantly higher than the numbers of organs with moderate and intense IP in TcI and TcIV-infected animals and the number of organs with intense IP in TcII-infected animals. Additionally, the number of organs with moderate IP was significantly higher than the number with intense IP in TcI and TcIV-infected mice (Table 5).
During lAP, mild, moderate, and intense IP was recorded for the 3 DTUs, and the number of organs with mild IP was significantly greater than the number of organs with moderate and intense IP for all three DTUs (Table 5). Statistical differences were also found in the comparison between moderate and intense IP, as animals infected with TcI and TcIV had higher numbers of organs with moderate IP.
During CP, organs with mild and moderate IP were recorded with the three DTUs, and intense IP was only observed with TcI and TcII (Table 5). The number of organs with mild IP was significantly greater than the numbers of organs with moderate IP in TcII- and TcIV-infected animals and the number of organs with intense IP with all three DTUs. Significant differences were also recorded between the numbers of organs with moderate and intense IP among the three DTUs.
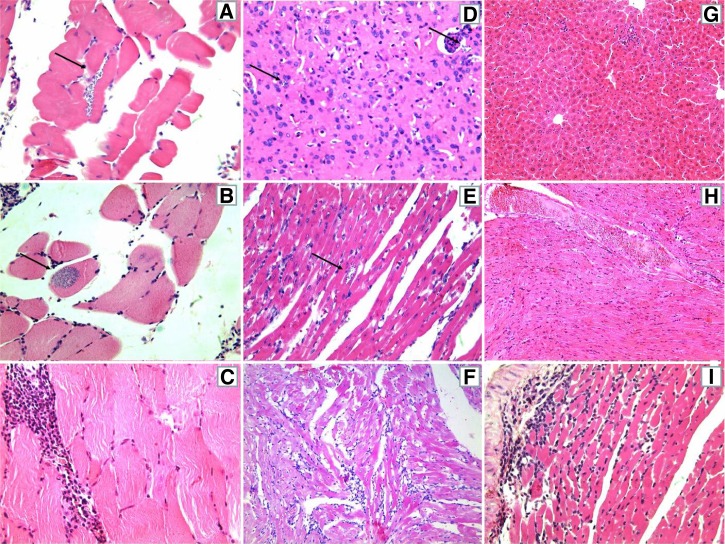
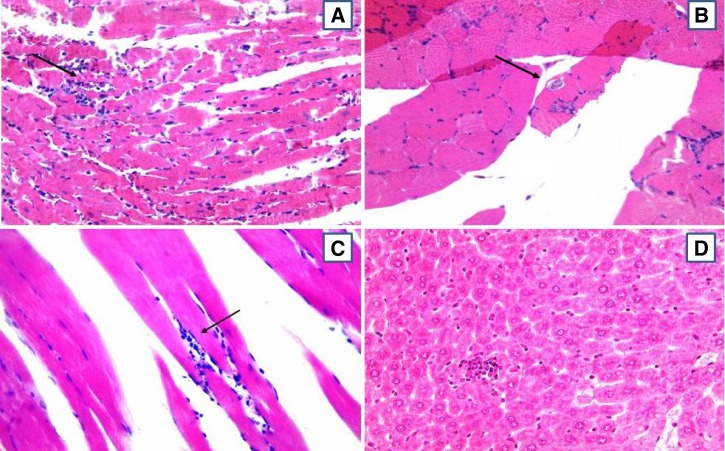
For TcI-infected animals from both groups (TBZ and NT), the inflammatory infiltrate was characterized as predominantly mild and focal at all stages of infection: eAP (Figure 4A), lAP (Figure 4B), and CP (Figure 4C). During the acute phase, TcII-infected animals also presented with mostly mild and focal inflammatory infiltration, although mild and diffuse inflammatory infiltrate predominated during CP (Figure 4F). The evolution of IP from mild/focal during eAP and lAP (Figure 4G and H, respectively) to mild/diffuse during CP (Figure 4I) was also observed for most of the TcIV-inoculated animals.
Figure 4.
Photomicrographs of histological sections of mice inoculated with strains of Trypanosoma cruzi TcI (A, B, and C), TcII (D, E, and F), and TcIV (G, H, and I), in the early (A, D, and G) and late (B, E, and H) acute phases, and chronic phase (C, F, and I) of infection. Striated skeletal muscle showing tissue parasitism (strains AM44 - A, and AM45 - B), and intense focal inflammatory infiltrate (strain AM33 - C). Brain with nests of amastigotes (strain PR1219) (D). Cardiac muscle presenting tissue parasitism and mild diffuse inflammatory infiltrate (strain PR1219 - E), and tissue injury and moderate diffuse inflammatory infiltrate (strain BS48 - F). Liver displaying mild and focal inflammatory infiltrate (strain AM64 - G). Heart muscle with diffuse infiltrate mild (strain AM64 - H) and moderate (AM14 - I). The arrows point to the nests of amastigotes. All photos were taken at 400× magnification, hematoxylin-eosin.
Impact of treatment on histopathological parameters.
Regardless of DTU, BZ treatment during the acute phase influenced the evolution of infection by promoting significant reductions in the numbers of organs with TP in both eAP and lAP and with IP in all three assessed phases (Table 3).
Statistical comparisons of TP between the TBZ and NT groups for each DTU showed that BZ treatment caused a significant reduction in the number of organs with TP during eAP and lAP in TcI- and TcII-infected animals (Table 3). There was no significant difference in TP between the TBZ and NT groups in TcIV-infected animals. However, amastigotes were observed early in treatment (during eAP) only in TBZ animals inoculated with the TcIV strains AM64 and AM68, which are considered R and S to BZ treatment, respectively (Table 1).
Statistical comparisons of IP between the TBZ and NT groups, independent of intensity, showed that although there remained organs with IP in the treated animals, the BZ caused significant reductions in their numbers during the three evaluated phases and for all three DTUs (Table 3).
Impact of treatment on lesion intensity.
Regarding histopathological lesion intensity, during the acute phase, TBZ animals infected with TcI showed no significant reductions in the number of organs with TP and IP during eAP (Table 5). However, BZ treatment caused a significant reduction in the numbers of organs with IP at different intensities during lAP and with moderate IP during CP.
For animals infected with TcII, BZ treatment significantly reduced the numbers of organs with mild TP during eAP and lAP, with different IP intensities during eAP, and with mild and intense IP during lAP (Table 5). Although the number of organs with pathological changes during CP was lower in the TBZ animals, this difference was not significant. The number of organs with IP in the TBZ group was significantly higher during CP (26) than during eAP (7) and lAP (7) (Table 3).
Animals infected with TcIV and BZ treated during the acute phase showed significant reductions in the numbers of organs with mild and moderate IP during eAP, with the three categories of IP during lAP and with mild IP during CP (Table 5).
In the NT group, tissues presented intense and diffuse IP during lAP and CP (Figure 5C), whereas in the TBZ group, this type of infiltration was observed only during CP for all three DTUs. Organs without pathological changes predominated during CP in the TBZ group.
Figure 5.
Photomicrographs of histological sections of mice inoculated with Trypanosoma cruzi in early (A and C) and late acute phases (B and D) of infection, treated with benznidazole (C and D) and untreated controls (A and B). (A) Cardiac muscle presenting tissue parasitism with mild and focal inflammatory infiltrate (TcII – strain PR1219). (B) Striated skeletal muscle presenting tissue parasitism (TcI - AM33). (C) Striated skeletal muscle presenting tissue parasitism with mild focal inflammatory infiltrate (TcIV - AM68). (D) Liver with mild and focal inflammatory infiltrate (TcIV - AM62).The arrows indicate the nests of amastigotes. All photos were taken at 400× magnification, hematoxylin-eosin.
Tissue tropism.
One to two amastigote nests were observed in the tissues from mice that were inoculated with 50% (3 of 6) of the TcI strains. The TP caused by the AM33 strain was observed in the liver (eAP), heart, and skeletal striated musculature (lAP) (Figure 5B), and TP caused by the AM44 and AM45 strains was observed in the heart and diaphragm (eAP), respectively (Figure 4A and B).
Tissue amastigotes were detected for all four investigated TcII strains in nine organs during eAP (Table 3). Two to 19 amastigote nests were present in the heart and left hind paw muscles (BS48 strain). Light parasitism in the heart and seven amastigote nests in the nervous tissues of the brain were observed during eAP for the PR1219 strain (Figure 4D), which was the only strain to present parasitism during CP. Mild TP in the abdominal and the left hind paw muscles (PR2259) and three amastigote nests in the heart (PR1226) were both observed during lAP.
In TBZ mice inoculated with 25% (2 of 8) of the TcIV strains, single amastigote nests could be observed in the abdominal wall (AM64) and thigh muscles (AM68) (Figure 5D) during eAP.
In this study, the skeletal muscle (10 cuts) was the only tissue in which amastigotes were observed for all three evaluated DTUs. The TP caused by TcI and TcII was also observed in the striated muscle of the heart (12 cuts). The TcII was the only DTU observed in the brain, whereas TcI was the only DTU observed in the liver. Amastigotes were not observed in the spleens and intestines of the evaluated animals.
Qualitative analyzes of tissues by qPCR.
The number and the proportion of organs positive in qPCR (%+qPCR), or presenting DNA of T. cruzi, for the three stages of the infection and DTUs studied are shown in Table 6. A high %+qPCR were observed, ranging from 37.2% to 68.8%. Considering the NT animals, the proportion of parasitized organs did not vary significantly between DTUs during eAP and CP but, during the lAP did, being higher (P = 0.031) for animals infected with TcI (68.8%) compared with other DTUs (Table 6). The %+qPCR did not vary significantly between the three stages within the DTU studied.
Table 6.
Number and percentage of parasitized organs (in which DNA was detected by qPCR) in mice inoculated with Trypanosoma cruzi I, II, and IV, treated with TBZ during acute phase and untreated controls, at different stages of infection
| Stage of infection | TcI (N = 288) | TcII (N = 96) | TcIV (N = 240) | |||
|---|---|---|---|---|---|---|
| NT (N = 144, 48/stage) | TBZ (N = 144) | NT (N = 48, 16/stage) | TBZ (N = 48) | NT (N = 120, 40/stage) | TBZ (N = 120) | |
| eAP* | 24 (50.0) | 23 (47.9) | 6 (37.5) | 1 (6.3)† | 21 (52.5) | 12 (30.0)† |
| lAP‡ | 33 (68.8)§ | 22 (45.8)† | 10 (62.5) | 2 (12.5)† | 17 (42.5) | 13 (32.5) |
| CP¶ | 25 (52.1) | 25 (52.1) | 6 (37.5) | 6 (37.5) | 19 (47.5) | 14 (35.0) |
Early acute phase (one day after the peak of parasitemia);
Significant difference between untreated controls (NT) and benznidazole treated mice (TBZ), and
among DTUs.
Late acute phase (30 days after inoculation).
Chronic phase (100 days after inoculation).
qPCR = real time-polymerase chain reaction.
Impact of treatment with BZ on the results of qPCR.
The proportion of parasitized organs was significantly reduced by treatment with BZ performed during the acute phase. Mice infected with TcI and BZ-treated showed %+qPCR significantly lower (P = 0.039) compared with NT animals, only in the lAP (Table 6). Those infected with TcII displayed reductions in eAP (P = 0.032) as in the lAP (P = 0.003), and with TcIV only in the eAP (P = 0.041). In the CP, the %+qPCR did not vary significantly between TBZ and NT animals, for any of the DTUs studied.
Discussion
The results obtained by evaluating experimental T. cruzi infections in laboratory animals cannot be directly extrapolated to naturally infected wild animals or to infected humans, especially if we consider the complex biological and genetic species diversity. However, even with this caveat, the study of the biological behaviors of strains from different parasite DTUs in experimentally infected BZ-treated mice can provide important insights and promote a better comprehension of the pathological and epidemiological aspects of CD.
In this study, it was found that the Amazonas TcI and TcIV strains (%INF of 83% and 91%, respectively), and the Paraná TcII strains (95%) had high infectivity in mice, with no significant differences among them, in agreement with a previous study.16 The susceptibilities of these strains to BZ varied from 0% to 100%, and there was no correlation between this parameter and the DTU strain classifications, as reported previously.17 However, TcII showed a higher proportion of BZ-sensitive strains, i.e., whose inoculated animals exhibited a sustained parasitemia clearance after treatment with BZ and TcIV resistant ones. Small differences between parasitemia clearance rates of each strain or DTU of the current study and the cure rates obtained in the previous study17 might be caused by the developmental stages used for inoculation and the techniques used to monitor cure, and the selection (as a result of laboratory manipulation) of different genotypes that might be present within the same strain.31
The beneficial effect of BZ treatment was shown when the parasitological, molecular, and histopathological parameters were assessed. Regardless of the DTU strains, etiological treatment caused a significant reduction in 5 of the 7 parasitological parameters (PP, Pmax, Dpmax, %+FBE, and %+HC). When the DTUs were considered separately, the numbers of significant reductions promoted by each treatment varied, with a greater number of reductions in TcII, both in the parasitological parameters (6 of 7) and in the percentage of parasitized organs (%+qPCR) in the eAP and lAP. Mice inoculated with TcII strains showed parasitemia levels up to 10-fold higher than mice infected with the other strains,16 and some authors associate TcII with a greater sensitivity to drugs.22,32 Mice infected with TcI and TcIV strains and BZ-treated showed no significant reduction in the parameter %+cPCR in relation to NT animals, because the percentages presented by the latter were already low, 14% and 17%, respectively. Strains belonging to six different T. cruzi DTUs have dissimilar DNA content.33 The detection capability of PCR depends on, among other factors, the molecular targets analyzed. In this study the cPCR targeted the k-DNA minicircle of T. cruzi. Therefore, we can speculate that the lower analytical sensitivity of the our cPCR test to detect DNA from TcI and TcIV DTUs respect to the other ones, could be caused by a lower minicircle copy number.
In this study, histopathological changes were mainly observed during the acute phase of infection, with a greater TP intensity at 1 d after peak parasitemia (eAP) and greater IP at 30 d.i. (lAP). During CP, these changes appeared in fewer organs. Mice inoculated with the three DTUs generally presented with focal and low-intensity inflammatory infiltration in tissues, which predominantly comprised lymphocytes. Mild TP H/E-detectable was observed in mice inoculated with 2 of the 8 TcIV strains, 3 of the 6 TcI strains, and with all TcII strains, during the acute phase, but only with the PR1219 strain (TcII) during CP. Animals infected with PR1219 were also the only to present with moderate acute-phase TP, suggesting the greater pathogenicity of TcII for mice, which is consistent with other studies (Meza and others, unpublished data).34
In this study, as shown by the H/E technique, there was no direct association between tissue tropism and parasite DTU. The TcI strains showed tropism for skeletal muscle, heart and liver, whereas TcII strains showed tropism for the heart (++), skeletal muscle and central nervous tissue, and TcIV strains for skeletal muscle. Data from a previous study in mice inoculated with TcII strains from Paraná and TcIV strains from Amazonas suggested the preferential tropism of TcII for the heart and TcIV for the central nervous system, because these DTUs produced a parasitism of greater intensity in those tissues (Meza and others, unpublished data). Although most of the same strains were used in both studies, the observed differences in tissue tropism might be caused by the different evolutionary forms used to inoculate the mice.35
Qualitative qPCR analysis showed the presence of T. cruzi DNA in all eight different organs analyzed and its positivity varied with the parasite DTU. Caldas and others36 detected T. cruzi DNA by qPCR in 100% of cardiac tissue obtained from mice infected with a TcII strain. The use of this technique in tissue confirmed and extended the parasitological and histopathological findings and a quite different biological behavior for strains of the three DTUs was observed with the parameters used. The TcI strains from the Amazonas determining subpatent parasitemia in mice prevents the morphometric study of the blood trypomastigotes. Parasite populations that determining earlier or subpatent parasitemias are predominantly constituted by slender trypomastigotes, which are more sensitive to circulating antibodies, more adapted to penetrate tissue cells, and more infectious to mice.37 Several aspects of behavior of the strains TcI studied are consistent with populations of the parasite that have a predominance of slender blood forms, as shown by the results of the H/E and qPCR analysis of tissue: tropism by mononuclear phagocyte system organs (of the three DTUs studied, TcI was the only to display TP in the liver) and a proportion of parasitized organs (68.8%) significantly higher than the other two DTUs, indicating greater tissue invasiveness.
Among the three DTUs studied, TcI was intermediate with regard to pathogenicity in mice, with fewer and more organs with pathological changes, compared with TcII and TcIV, respectively, at different stages of infection. The TP caused by TcI was only observed during eAP and lAP, and the inoculated animals had 1–2 amastigote nests by tissue analyzed, suggesting less intracellular replicative capacity. These data agree with the results of a study of Amazonas TcI and TcIV strains, in which TP only occurred with TcI and the same inflammatory infiltration characteristics were observed.14
The TcI-infected mice and treated with BZ presented with significantly lower IP at all stages of infection and a lower proportion of parasitized organs (%+qPCR) at lAP after treatment, and no amastigote nests were observed in any organs from these animals. A study of a TcI strain from Nicaragua showed the benefits of early BZ treatment, which led to significant reductions in parasitemia, mortality, and the number of parasites in the skeletal muscle during CP, but no significant differences were observed in the frequencies of lesions in the heart and smooth muscles of these mice.38
The pathological changes observed with TcII confirm the results of previous studies that used TcII strains of different origins8,39–41 and from chronic patients in Paraná (Meza SKL and others, unpublished data).34 The TcII parasites invade heart cell cultures in lower numbers than TcI parasites; however, intracellular multiplication of TcII parasites was more efficient than that of TcI parasites, strongly suggesting that intracellular development is important to determine parasite tissue tropism.42 These parasites showed tropism for the heart muscles, a greater amount of tissue amastigote nests, and higher pathogenicity for mice. Regarding infection progression in TcII-infected animals BZ-treated during the acute phase, no amastigote nests were observed in the tissues of these animals at any infection stage. In other words, the specific treatment promoted the removal of H/E-detectable tissue parasites and significant reductions in the inflammatory process at all infection stages. However, there was a 3.7-fold increase in the number of organs with inflammatory infiltration during CP relative to the initial infection stages, although this number remained lower than that of the NT animals. These data corroborate the findings of several studies of TcII strains,32,43 with an exception where the reduction in the parasite load could not be associated with the intensity of the cardiac chronic lesions.43,44
Tissue parasitism H/E-detectable was scarce in TcIV-infected mice. During the acute phase, the number of organs with TP in these mice was significantly lower than the numbers for TcI and TcII, as was the IP intensity during CP. The TcIV was the only DTU in which there was observed the presence of intracellular parasites in the tissues of TBZ animals, although this was only true during the early phase of infection (eAP). Another study of Amazonas TcIV strains also reported low pathogenicity, and TP was observed only during eAP for 1 of the 8 strains studied (AM62) (Meza SKL and others, unpublished data).
The overall parasitemia clearance rate of 53.6% obtained in this study with FBE, HC, and cPCR on blood was not very different from the cure rates obtained in a previous study (60.5%)17 that used these same techniques, classically used in cure control of CD in the past, in addition to the enzyme-linked immunosorbent assay (Elisa) test to evaluate most of these strains and suggested that a considerable proportion of animals are not parasitologically cured. Negative blood test results only indicate that the parasitemia levels were below the limit of detection of the methods used and provide no information on the parasite burden in the extravascular organs. Even showing a reduction in the proportion of parasitized organs by qPCR, mice inoculated with the three DTUs and BZ-treated during the acute phase still showed T. cruzi DNA on tissues. These findings corroborate those of other authors who have shown that cPCR tests performed on tissues from animals considered cured after BZ treatment still detected T. cruzi DNA, most probably indicating residual infection.45 However, the animals benefitted from BZ treatment even if they were not cured; according to the parasitological, histopathological, and molecular evaluation results, BZ treatment during the acute phase resulted in significant reductions in the levels of parasitemia, numbers of organs with TP, IP, and displaying T. cruzi DNA, for all three DTUs.
Longitudinal studies to compare the evolution of chronic chagasic untreated patients with that of BZ-treated patients showed controversial results and the data gathered from clinical practice are not convincing to support prescription of etiological treatment as routine for indeterminate and cardiac chronic CD patients.46 However, our preclinical study and others who used BZ also indicates that etiological treatment is beneficial in reducing cardiac lesions in animals infected with T. cruzi, preventing, stopping, or reversing myocarditis.
This study used TcII strains from chronic patients who lived in the State of Paraná, which is considered an old endemic area, and TcI and TcIV strains from humans, insects, and wild animals in the State of Amazonas that were obtained during two orally acquired CD outbreaks; the latter state is considered an emerging disease area. We conclude that in mice that have been experimentally infected with these strains, BZ treatment during the acute phase provides benefits in terms of significant reductions in blood and tissue parasitism and the frequency and severity of inflammation throughout the course of infection, contributing to ensure the potential for applicability in different disease-endemic regions. Our results suggest that the beneficial effects of BZ might vary according to the parasite DTU and the experimental infection phase, thus justifying its use to treat patients in the Amazon region, and an urgent search for new and more effective chemotherapeutic agents for CD treatment.
ACKNOWLEDGMENTS
We thank the National Council for Scientific and Technological Development (CNPq) for providing a productivity grant to MJOT (304593/2011-7) and a Scientific Initiation scholarship to MM (3681/2012).
Footnotes
Financial support: The Araucária Foundation for Scientific and Technological Development (13055/14/2008) funded the research.
Authors' addresses: Ana Paula Gruendling, Miyoko Massago, Ana Paula M. Teston, Edilson N. Kaneshima, Silvana M. Araújo, Mônica L. Gomes, and Max Jean O. Toledo, Departamento de Ciências Básicas da Saúde, Universidade Estadual de Maringá, Maringá, PR, Brazil, E-mails: anap.gru@gmail.com, mi_massago@hotmail.com, anapeteston@hotmail.com, enkaneshima@uem.br, smaraujo@uem.br, mlgomes@uem.br, and mjotoledo@uem.br. Wuelton M. Monteiro and Maria das Graças V. Barbosa, Fundação de Medicina Tropical Dr. Heitor Vieira Dourado, Manaus, Amazonas, Brazil, E-mails: wueltonmm@ibest.com.br, and barbosamgvale@gmail.com.
References
- 1.Zingales B. Trypanosoma cruzi: one, two or various Chagas disease parasites? RevBiol. 2011;6:44–48. [Google Scholar]
- 2. DNDi 2013. Neglected Diseases–Chagas Disease. Drugs for Neglected Diseases Initiative–DNDi Available athttp://www.dndi.org.br/pt/doencas-negligenciadas/doenca-de-chagas.html Accessed January 7, 2013
- 3.Coura JR, Junqueira ACV. Risks of endemicity, morbidity and perspectives regarding the control of Chagas disease in the Amazon Region. Mem Inst Oswaldo Cruz. 2012;107:145–154. doi: 10.1590/s0074-02762012000200001. [DOI] [PubMed] [Google Scholar]
- 4.Pinto AY, Valente SA, Valente VC, Ferreira-Junior AG, Coura JR. Acute phase of Chagas disease in the Brazilian Amazon region. A study of 233 cases from Pará, Amapá and Maranhão observed between 1988 and 2005. Ver Soc Bras Med Trop. 2008;41:602–614. doi: 10.1590/s0037-86822008000600011. [DOI] [PubMed] [Google Scholar]
- 5.Valente SA, Valente VC, Frahia-Neto H. Considerations on the epidemiology and transmission of Chagas disease in the Brazilian Amazon. Mem Inst Oswaldo Cruz. 1999;94:395–398. doi: 10.1590/s0074-02761999000700077. [DOI] [PubMed] [Google Scholar]
- 6.Albajar PV, Laredo SV, Terrazas MB, Coura JR. Dilated cardiomyopathy in patients with chronic chagasic infection: report of two fatal autochthonous cases from Rio Negro, State of Amazonas, Brazil. Rev Soc Bras Med Trop. 2003;36:401–407. [PubMed] [Google Scholar]
- 7.Xavier SS, Sousa AS, Viñas PA, Junqueira AC, Bóia MN, Coura JR. Chronic chagasic cardiopathy in Rio Negro, State of Amazonas. Report of three new autochthonous cases confirmed by serology, clinical examination, chest x-ray, and electro and echocardiography. Rev Soc Bras Med Trop. 2006;39:211–216. doi: 10.1590/s0037-86822006000200015. [DOI] [PubMed] [Google Scholar]
- 8.Andrade SG. Morphological and behavioral characterization of Trypanosoma cruzi strains. Rev Soc Bras Med Trop. 1985;18:39–46. [Google Scholar]
- 9.Filardi LS, Brener Z. Susceptibility and natural resistance of Trypanosoma cruzi strains to drugs used clinically in Chagas disease. Trans R Soc Trop Med Hyg. 1987;81:755–759. doi: 10.1016/0035-9203(87)90020-4. [DOI] [PubMed] [Google Scholar]
- 10.Machado CA, Ayala FJ. Nucleotide sequences provide evidence of genetic exchange among distantly related lineages of Trypanosoma cruzi. Proc Natl Acad Sci USA. 2001;98:7396–7401. doi: 10.1073/pnas.121187198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murta SM, Gazinelli RT, Brener Z, Romanha AJ. Molecular characterization of susceptible and naturally resistant strains of Trypanosoma cruzi to benznidazole and nifurtimox. Mol Biochem Parasitol. 1998;93:203–214. doi: 10.1016/s0166-6851(98)00037-1. [DOI] [PubMed] [Google Scholar]
- 12.Bozelli CE, Araújo SM, Guilherme AL, Gomes ML. Clinical and epidemiological profiles of patients with Chagas disease at the University Hospital in Maringá, Paraná, Brazil. Cad Saude Publica. 2006;22:1027–1034. doi: 10.1590/s0102-311x2006000500015. [DOI] [PubMed] [Google Scholar]
- 13.Monteiro WM, Magalhães LK, de Sá AR, Gomes ML, Toledo MJ, Borges L, Pires I, Guerra JA, Silveira H, Barbosa MG. Trypanosoma cruzi IV causing outbreaks of acute Chagas disease and infections by different haplotypes in the Western Brazilian Amazonia. PLoS ONE. 2012;7:e41284. doi: 10.1371/journal.pone.0041284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monteiro WM, Magalhães LK, Oliveira JC, Guerra JA, Silveira H, Ferreira LC, Toledo MJ, Barbosa MG. Biological behavior of Trypanosoma cruzi stocks obtained from the State of Amazonas, Western Brazilian Amazon, in mice. Rev Soc Bras Med Trop. 2012;45:209–214. doi: 10.1590/s0037-86822012000200014. [DOI] [PubMed] [Google Scholar]
- 15.Monteiro WM, Teston AP, Gruendling AP, Reis D, Gomes ML, Araújo SM, Bahia MT, Magalhães LK, Guerra JA, Silveira H, Toledo MJ, Barbosa MG. Trypanosoma cruzi I and IV Stocks from Brazilian Amazon are divergent in terms of biological and medical properties in mice. PLoS Negl Trop Dis. 2013;7:e2069. doi: 10.1371/journal.pntd.0002069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reis D, Monteiro WM, Bossoloni GD, Teston AP, Gomes ML, Araújo SM, Barbosa MG, Toledo MJ. Biological behavior in mice of Trypanosoma cruzi isolates from Amazonas and Paraná states, Brazil. Exp Parasitol. 2012;30:321–329. doi: 10.1016/j.exppara.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 17.Teston AP, Monteiro WM, Reis D, Bossoloni GD, Gomes ML, Araújo SM, Bahia MT, Barbosa MG, Toledo MJ. In vivo susceptibility to benznidazole of Trypanosoma cruzi strains from the western Brazilian Amazon. Trop Med Int Health. 2012;18:85–95. doi: 10.1111/tmi.12014. [DOI] [PubMed] [Google Scholar]
- 18.Sosa-Estani S, Segura EL. Etiological treatment in patients infected by Trypanosoma cruzi: experiences in Argentina. Curr Opin Infect Dis. 2006;19:583–587. doi: 10.1097/01.qco.0000247592.21295.a5. [DOI] [PubMed] [Google Scholar]
- 19.Lenzi H, Oliveira D, Lima M, Gattass CR. Trypanosoma cruzi: pan infectivity of CL strain during murine acute infection. Exp Parasitol. 1996;84:16–27. doi: 10.1006/expr.1996.0086. [DOI] [PubMed] [Google Scholar]
- 20.Abolis NG, Araújo SM, Toledo MJ, Fernandez MA, Gomes ML. Trypanosoma cruzi I-III in southern Brazil causing individual and mixed infections in humans, sylvatic reservoirs and triatomines. Acta Trop. 2011;120:167–172. doi: 10.1016/j.actatropica.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Araújo SM, Falavigna-Guilherme AL, Toledo MJ, Oliveira PJ, Silva JC, Gomes ML. Bioloy of Trypanosoma cruzi isolated from chagasic patients from different geographic origins residing in northwestern region of the state of Paraná, Brasil. Acta Scientiarum. 1999;21:229–235. [Google Scholar]
- 22.Toledo MJ, Bahia MT, Carneiro CM, Martins-Filho OA, Tibayrenc M, Barnabé C, Tafuri WL, Lana M. Chemotherapy with benznidazole and itraconazole for mice infected with different Trypanosoma cruzi clonal genotypes. Antimicrob Agents Chemother. 2003;47:223–230. doi: 10.1128/AAC.47.1.223-230.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyamoto CT, Gomes ML, Marangon AV, Araújo SM, Bahia MT, Martins-Filho AO, Lana M, Toledo MJ. Usefulness of the polymerase chain reaction for monitoring cure of mice infected with different Trypanosoma cruzi clonal genotypes following treatment with benznidazole. Exp Parasitol. 2008;1:45–49. doi: 10.1016/j.exppara.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 24.Brener Z. Therapeutic activity and criterion of cure on mice experimentally infected with Trypanosoma cruzi. Rev Inst Med Trop Sao Paulo. 1962;4:389–396. [PubMed] [Google Scholar]
- 25.Gomes ML, Macedo AM, Vago AR, Pena SD, Galvão LM, Chiari E. Trypanosoma cruzi: optimization of polymerase chain reaction for detection in human blood. Exp Parasitol. 1998;88:28–33. doi: 10.1006/expr.1998.4191. [DOI] [PubMed] [Google Scholar]
- 26.Miyamoto CT, Gomes ML, Marangon AV, Araújo SM, Bahia MT, Lana M, Toledo MJO. Trypanosoma cruzi: sensitivity of the polymerase chain reaction for detecting the parasite in the blood of mice infected with different clonal genotypes. Exp Parasitol. 2006;112:198–201. doi: 10.1016/j.exppara.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Michailowsky V, Silva NM, Rocha CD, Vieira LQ, Lannes-Vieira J, Gazzinelli RT. Pivotal role of interleukin-12 and interferon-axis in controlling tissue parasitism and inflammation in the heart and central nervous system during Trypanosoma cruzi infection. Am J Pathol. 2001;159:1723–1733. doi: 10.1016/s0002-9440(10)63019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oliveira KM, Souza NB, Jr, Mata FR, Sabóia-Morais SMT, Aversi-Ferreira TA, Mata JR. Acute alterations induced in Wistar rats by Trypanosoma cruzi. Revista Eletrônica de Farmácia. 2007;4:86–94. [Google Scholar]
- 29.Devera R, Illarramendi X, Montoya-Araújo R, Pirmez C, Fernandes O, Coura JR. Biodemes of Trypanosoma cruzi strains isolated from humans from three endemic areas in Minas Gerais State. Ver Soc Bras Med Trop. 2002;35:323–330. doi: 10.1590/s0037-86822002000400008. [DOI] [PubMed] [Google Scholar]
- 30.Cummings KL, Tarleton RL. Rapid quantitation of Trypanosoma cruzi in host tissue by real-time PCR. Mol Biochem Parasitol. 2003;1:53–59. doi: 10.1016/s0166-6851(03)00093-8. [DOI] [PubMed] [Google Scholar]
- 31.Caldas S, Santos FM, Lana M, Diniz LF, Machado-Coelho GL, Veloso VM, Bahia MT. Trypanosoma cruzi: acute and long-term infection in the vertebrate host can modify the response to benznidazole. Exp Parasitol. 2008;118:315–323. doi: 10.1016/j.exppara.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 32.Andrade SG, Magalhães JB. Biodemes and zymodemes of Trypanosoma cruzi strains: correlations with clinical data and experimental pathology. Rev Soc Bras Med Trop. 1997;30:27–35. doi: 10.1590/s0037-86821997000100006. [DOI] [PubMed] [Google Scholar]
- 33.Schijman AG, Bisio M, Orellana L, Sued M, Duffy T, Jaramillo AM, Cura C, Auter F, Veron V, Qvarnstrom Y, Deborggraeve S, Hijar G, Zulantay I, Lucero RH, Velazquez E, Tellez T, Leon SZ, Galvão L, Nolder D, Rumi MM, Levi JE, Ramirez JD, Zorrilla P, Flores M, Jercic MI, Crisante G, Anez N, Castro AM, Gonzalez CI, Viana KA, Yachelini P, Torrico F, Robello C, Diosque P, Chavez OT, Aznar C, Russomando G, Buscher F, Assal A, Guhl F, Estani SS, Silva A, Britto C, Luquetti A, Ladzins J. International study to evaluate PCR methods for detection of Trypanosoma cruzi DNA in blood samples from Chagas disease patients. PLoS Negl Trop Dis. 2011;5:e931. doi: 10.1371/journal.pntd.0000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toledo MJ, Falavigna-Guilherme AL, Silva JC, Gasperi MV, Mendes AP, Gomes ML, Araújo SM. Trypanosoma cruzi: chemotherapy with benznidazole in mice inoculated with strains from Paraná state and from different endemic areas of Brazil. Rev Inst Med Trop Sao Paulo. 1997;39:283–290. doi: 10.1590/s0036-46651997000500007. [DOI] [PubMed] [Google Scholar]
- 35.Carneiro CM, Martins-Filho OA, Reis AB, Veloso VM, Aráujo FM, Bahia MT, de Lana M, Machado-Coelho GL, Gazzinelli G, Correa-Oliveira R, Tafuri WL. Differential impact of metacyclic and blood trypomastigotes on parasitological, serological and phenotypic features triggered during acute Trypanosoma cruzi infection in dogs. Acta Trop. 2007;101:120–129. doi: 10.1016/j.actatropica.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 36.Caldas S, Caldas IS, Diniz LD, Lima WG, Oliveira RD, Cecílio AB, Ribeiro I, Talvani A, Bahia MT. Real-time PCR strategy for parasite quantification in blood and tissue samples of experimental Trypanosoma cruzi infection. Acta Trop. 2012;123:170–177. doi: 10.1016/j.actatropica.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Brener Z. Intraspecific variation in Trypanosoma cruzi: two types of parasite population presenting distinct characteristic. PAHO Sci Publ. 1977;347:11–21. [Google Scholar]
- 38.Grosso NL, Bua J, Perrone AE, Gonzalez MN, Bustos PL, Postan M, Fichera FE. Trypanosoma cruzi: biological characterization of an isolate from an endemic area and its susceptibility to conventional drugs. Exp Parasitol. 2010;126:239–244. doi: 10.1016/j.exppara.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andrade SG. Characterization of Trypanosoma cruzi strains isolated from Recôncavo Baiano. Rev Pat Trop. 1974;3:165–221. [Google Scholar]
- 40.Andrade SG. Influence of Trypanosoma cruzi strain on the pathogenesis of chronic myocardiopathy in mice. Mem Inst Oswaldo Cruz. 1990;85:17–25. doi: 10.1590/s0074-02761990000100003. [DOI] [PubMed] [Google Scholar]
- 41.Andrade LO, Machado CR, Chiari E, Pena SD, Macedo AM. Differential tissue distribution of diverse clones of Trypanosoma cruzi in infected mice. Mol Biochem Parasitol. 1999;100:163–172. doi: 10.1016/s0166-6851(99)90035-x. [DOI] [PubMed] [Google Scholar]
- 42.Andrade LO, Galvão LM, Meirelles MN, Chiari E, Pena SD, Macedo AM. Differential tissue tropism of Trypanosoma cruzi strains: an in vitro study. Mem Inst Oswaldo Cruz. 2010;105:834–837. doi: 10.1590/s0074-02762010000600018. [DOI] [PubMed] [Google Scholar]
- 43.Toledo MJ, Bahia MT, Veloso VM, Carneiro CM, Machado-Coelho GL, Alves CF, Martins HR, Cruz RE, Tafuri WL, Lana M. Effects of specific treatment on parasitological and histopatological parameters in mice infected with different Trypanosoma cruzi clonal genotypes. J Antimicrob Chemother. 2004;53:1045–1053. doi: 10.1093/jac/dkh224. [DOI] [PubMed] [Google Scholar]
- 44.Caldas IS, Talvani A, Caldas S, Carneiro CM, de Lana M, da Matta Guedes PM, Bahia MT. Benznidazole therapy during acute phase of Chagas disease reduces parasite load but does not prevent chronic cardiac lesions. Parasitol Res. 2008;103:413–421. doi: 10.1007/s00436-008-0992-6. [DOI] [PubMed] [Google Scholar]
- 45.Martins HR, Figueiredo LM, Valamiel-Silva JC, Carneiro CM, Machado-Coelho GL, Vitelli-Avelar DM, Bahia MT, Martins-Filho OA, Macedo AM, Lana M. Persistence of PCR-positive tissue in benznidazole-treated mice with negative blood parasitological and serological tests in dual infections with Trypanosoma cruzi stocks from different genotypes. J Antimicrob Chemother. 2008;61:1319–1327. doi: 10.1093/jac/dkn092. [DOI] [PubMed] [Google Scholar]
- 46.Guedes PM, Gutierrez FR, Nascimento MS, Matta MA, Silva JS. Antiparasitical chemotherapy in Chagas' disease cardiomyopathy: current evidence. Trop Med Int Health. 2012;17:1057–1065. doi: 10.1111/j.1365-3156.2012.03025.x. [DOI] [PubMed] [Google Scholar]