Abstract
Despite the use of accepted interventions to combat malaria, such as insecticide-treated bed nets and artemisinin-based combination therapy, malaria remains a leading cause of morbidity and mortality in Uganda. We investigated associations between household factors and malaria incidence in a cohort of children living in a highly endemic region of Uganda. Living in a modern house, defined as the use of non-earth floors, non-thatched roofs, and non-mud walls, was associated with approximately half malaria incidence compared with living in a traditional home (incidence rate ratio [IRR] = 0.54, P = 0.001). Other factors found to be associated with a lower incidence of malaria included living in town versus rural setting; sleeping in a room with openings to the outside (windows, eaves, and airbricks); and having an older and more educated primary caregiver. This study adds to the growing body of evidence that improved house construction may be associated with a lower risk of malaria.
Introduction
Malaria is a leading cause of morbidity and mortality in Uganda with an estimated 8–13 million cases and 103 deaths per 100,000 each year.1,2 Malaria is also a huge burden on the health-care system: The Uganda Ministry of Health reports that malaria accounts for 25–40% of outpatient visits to health facilities and is responsible for nearly half of inpatient pediatric deaths.3 Uganda's National Malaria Control Program relies heavily on donor funding of proven interventions including insecticide-treated bed nets (ITNs), indoor residual spraying (IRS) of insecticide, and effective case management with artemisinin-based combination therapy (ACT).3 However, despite scale-up of these interventions, the burden of malaria in Uganda remains high and may even be increasing in some areas. This underscores the necessity to expand and sustain interventions.2
One factor that may be contributing to the high malaria burden in Uganda and other resource-limited countries in sub-Saharan Africa is poor housing construction. Several studies have identified particular household characteristics as risk factors for an increased burden of malaria. An increase in the presence of household openings, such as windows and open eaves, has been associated with increases in mosquito entry into the home and parasite prevalence.4–7 Poor-quality household construction materials have also been associated with increased mosquito entry, malaria incidence, and parasite prevalence.4,5,8–11 Several studies classify housing structures into quality groupings according to the composition of the construction materials have found poor house quality to be associated with greater presence of mosquitoes in the home and higher malaria incidence.12,13
We recently completed a randomized control trial of malaria chemoprevention in a cohort of 600 children from different households followed from 6 months to 2 years of age in a rural area of Uganda where malaria is highly endemic. The primary outcome of the study was malaria incidence, defined as the number of episodes of symptomatic malaria per time at risk using passive surveillance. As part of a sub-study, researchers visited the homes of study participants and measured a variety of characteristics of the household members and the housing structure. Here, we present associations between the household characteristics and malaria incidence in children, with a focus on materials used in house construction.
Materials and Methods
Study site and population.
The study was conducted in Tororo District, a rural area in eastern Uganda characterized by subsistence farming, relatively high rates of poverty, intense year-round malaria transmission, and an entomological inoculation rate (EIR) estimated at 125 infectious bites per person-year in 2011–2012.14 This sub-study was conducted within the context of a larger randomized control trial evaluating three regimens for the prevention of malaria, the details of which have been described previously in other studies.15,16 In brief, between June 2010 and July 2011, 400 human immunodeficiency virus (HIV)–unexposed and 200 HIV-exposed children of age 4–6 months were recruited using convenience sampling at a dedicated study clinic located at Tororo District Hospital. Potential study subjects were recruited from an adjacent antenatal clinic and surrounding government health centers. The eligibility criteria for enrollment into the study included the following: 1) confirmed HIV status of the biological mother, 2) negative HIV DNA polymerase chain reaction test at the time of enrollment for infants born to HIV-infected mothers, 3) residency within a 30-km radius of the dedicated study clinic, 4) agreement to come to the study clinic for any febrile episode or other illness and avoid medications given outside the study protocol, 5) not living in the same household as a previously enrolled participant in the same study, 6) the absence of any active medical problem requiring inpatient evaluation or condition requiring frequent medical attention at the time of screening, and 7) the provision of informed consent by the parent/guardian.
Follow-up of study participants.
At enrollment, each household was given two long-lasting ITNs. Study participants were randomized to one of four chemoprevention arms: no chemoprevention; trimethoprim–sulfamethoxazole (Co-trimoxazole; Kampala Pharmaceutical Industries, Uganda) single dose once daily; sulfadoxine–pyrimethamine (Kamsidar, Kampala Pharmaceutical Industries, Uganda) single dose each month; and dihydroartemisinin–piperaquine (Duo-Cotexin; Holley-Cotec, Beijing, China) once daily for 3 consecutive days each month. HIV-unexposed children were randomized at 6 months of age, regardless of age of enrollment. HIV-exposed children were started on trimethoprim–sulfamethoxazole prophylaxis and then randomized after cessation of breast-feeding and confirmation of their HIV-negative status (median age 10 months). Chemoprevention drugs were administered unsupervised at home according to weight-based guidelines.
Participants received all of their medical care at a designated Tororo District Hospital study clinic open every day. Parents/guardians were encouraged to bring their children to the clinic any time they were ill. For children who presented with a documented fever (tympanic temperature ≥ 38.0°C measured at the study clinic) or history of fever in the previous 24 hours, a blood sample was obtained by finger prick for a thick blood smear. If the smear was confirmed positive by the laboratory, the patient was diagnosed with malaria. Episodes of uncomplicated malaria were treated with artemether–lumefantrine (AL), the recommended first-line treatment in Uganda. AL was administered twice a day for 3 days, with the first daily dose given directly observed in the clinic and the second daily dose given to caregivers to administer at home. Episodes of complicated malaria (severe malaria or the presence of other danger signs) or treatment failures occurring within 14 days of prior therapy were treated with quinine. Study participants were followed until they reached 24 months of age or were prematurely withdrawn from the study for any of the following reasons: 1) movement out of the study area, 2) failure to be seen in the study clinic for over 60 consecutive days, 3) withdrawal of informed consent, or 4) inability to comply with the study schedule and procedures.
Household questionnaire.
A detailed survey collecting information on household demographics and possessions was administered on the day of enrollment as described previously.17 Specifically for this sub-study, a pretested, structured questionnaire to collect information on potential household risk factors was administered from June–August 2013 by trained interviewers in the local languages. The Uganda 2011 Demographic and Health Survey was used to develop the questionnaire. The questionnaire was tailored to only include household factors linked to malaria in previous studies and household factors with a biologic plausibility of being linked to malaria incidence.18 The questionnaire consisted of two parts, the primary caregiver questionnaire and the household questionnaire. The primary caregiver questionnaire contained questions related to primary caregiver demographic information and occupation, household food insecurity, and the study participant's daily routine. Staff at the Tororo District Hospital study clinic administered this questionnaire during appointments if caregivers visited the clinic or alternatively during household visits conducted specifically for the survey. The household questionnaire contained questions related to household size, house structure, sources of water, electricity, cooking fuel, proximity of animals to the house, and specific aspects of the room where the study participant slept: materials of floor, walls, and roof in the room; openings (doorways, windows, and eaves) to the room; place where the child sleeps; and number of household members who sleep with the child. The household questionnaire was only administered during home visits with all household information visually verified by study interviewers. During the home visits, the household geographic coordinates were recorded using a global positioning system (GPS) device. All questionnaires were administered using the Questionnaire Development System (Nova Research Company, Bethesda, MD) software on tablet computers.
Statistical analysis.
Data analysis was conducted using STATA version 12.1 (STATA Corp, College Station, TX) and ArcGIS version 10 (ESRI, Redlands, CA). A household wealth index was generated from the enrollment survey as previously described.17 To determine the location of residences, geographic coordinates were entered into ArcGIS geographic information software. Houses were classified as being in or outside of town based on administrative boundaries. Given the distribution of data and to simplify further analyses, the main materials used for floor, exterior walls, and roof were combined into a binary variable classifying homes as either “modern” (non-earth floors, non-thatched roofs, and non-mud walls) or “traditional” (all other homes). The number of rooms in the house, presence of entryways in the study participant's room, number of airbricks in the study participant's room, presence of eaves in the study participant's room, and the primary caregiver's level of education were recoded into binary variables for analysis. The primary caregiver's age was recoded as a categorical variable for analysis. The primary outcome was the incidence of malaria, defined as the number of incident episodes of malaria per time at risk. Any episode of malaria occurring within 14 days of a previous episode was not considered an incident event. Time at risk was defined as time between 6 and 24 months of age, minus 14 days after each incident episode. Data on study participants and their households were included if the study participant reached 6 months of age, were observed for at least 60 days, and were actively in the parent study at the time the household questionnaire was administered. Associations between covariates of interest and the incidence of malaria were estimated using both univariate and multivariate negative binomial regression models controlling for the assigned chemoprevention arm. The following covariates with a significance level of < 0.05 using univariate analysis were included in the multivariate model: house construction type, location of residence, number of rooms in the house, presence of an entryway to the outside in the study participant's room, presence of windows (defined as an opening in the wall > 1 ft.2 in size with or without screening or a covering) in the study participant's room, eaves, or airbricks in the study participant's room, location of sleeping space (i.e., sleeping directly on the ground or a bed on the ground versus sleeping on a bed raised off the ground), household wealth, and the primary caregivers' age and level of education. After running the multivariate binomial regression model, residual values were mapped and tested for spatial autocorrelation using Moran's I.19 Because there was evidence of spatial autocorrelation, a semivariogram was generated using the residual values to determine the distance over which spatial autocorrelation was present. This was then used to construct a hexagonal lattice, with cell diameter set to the semivariogram range value (4.1 km), to group households. The cell to which each household was located was then included as a first-level random effect in the final fully adjusted multivariate model.
Ethics statement.
Informed consent was obtained by all adult participants and from the parents or legal guardians of minors. Ethical approval was obtained from the Makerere University School of Medicine Research and Ethics Committee, the Uganda Council for Science and Technology, and the University of California, San Francisco Committee on Human Research.
Results
Study profile and characteristics of study participants.
Of a total 600 infants enrolled in the parent study (one infant per household), 7 were withdrawn before reaching 6 months of age, 70 were withdrawn before the household questionnaire was administered, and 8 were observed for < 60 days; this left 515 infants in this analysis. Characteristics of the study participants are presented in Table 1. Approximately 75% of the study participants were not exposed to HIV whereas 25% were HIV-exposed, equally distributed across the four chemoprevention arms. All but three study participants (99.4%) were followed to 24 months of age and compliance with ITNs was high—over 98% of caregivers reported that the child slept under an ITN the prior evening at the time of monthly routine assessments. A total of 3,024 incident episodes of malaria were diagnosed over 633 person-years at risk, resulting in an overall incidence of malaria of 4.78 episodes per person-years at risk. Stratified by chemoprevention, the incidence of malaria per person-years at risk was 6.34 in the no chemoprevention arm, 5.94 in the monthly sulfadoxine–pyrimethamine arm, 4.28 in the daily trimethoprim–sulfamethoxazole arm, and 2.80 in the monthly dihydroartemisinin–piperaquine arm.
Table 1.
Characteristics of study participants and their households (N = 515)
| Categories | Characteristics | Frequency (%) |
|---|---|---|
| Study participants | ||
| Female gender | 255 (49.5) | |
| HIV exposed | 131 (25.4) | |
| Assigned chemoprevention arm | No chemoprevention | 131 (25.4) |
| Daily TS | 132 (25.6) | |
| Monthly SP | 126 (24.5) | |
| Monthly DP | 126 (24.5) | |
| Reported sleeping under an ITN the prior evening* | 98.5% | |
| Followed to 24 months of age | 512 (99.4) | |
| Material used for household construction | ||
| Materials used for floor | Earth | 440 (85.4) |
| Cement | 72 (14.0) | |
| Stones | 2 (0.4) | |
| Bricks | 1 (0.2) | |
| Materials used for walls | Mud and sticks or mud bricks | 425 (82.5) |
| Cement bricks or plaster | 90 (17.5) | |
| Materials used for roof | Thatched | 293 (56.9) |
| Iron sheets | 221 (42.9) | |
| Cement | 1 (0.2) | |
HIV = human immunodeficiency virus; ITN = insecticide-treated bed nets; TS = trimethoprim-sulfamethoxazole; SP = sulfadoxine-pyrimethamine; DP = dihydroartemisinin-piperaquine.
At the time of monthly routine assessments.
Materials used in household construction.
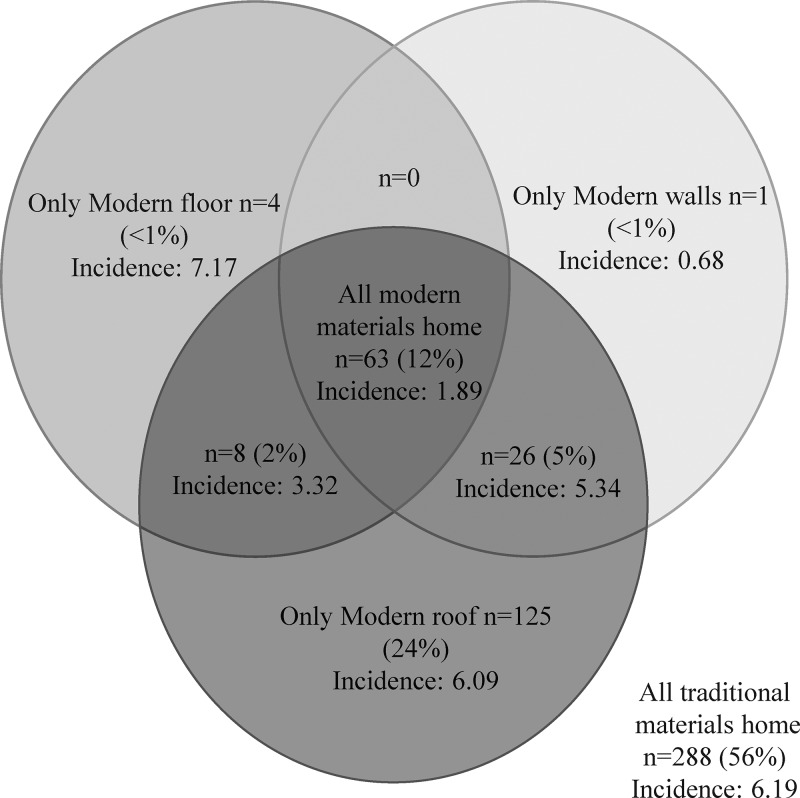
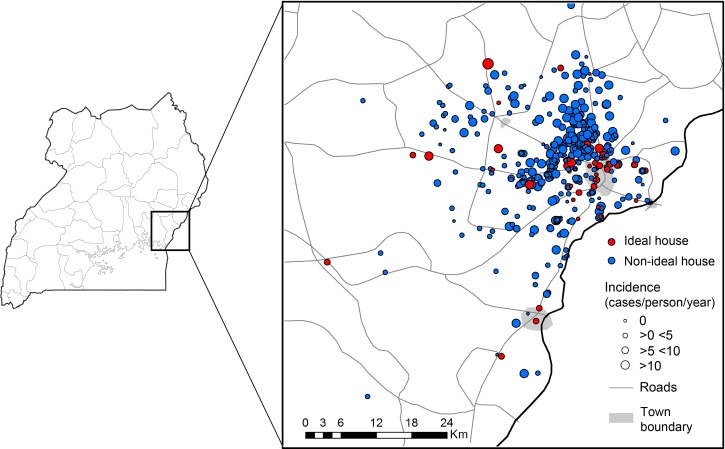
The frequencies of various materials used in household construction are presented in Table 1. The majority of homes used traditional materials defined as earth floors (85.4%), mud and sticks or mud brick walls (82.5%), and thatched roofs (56.9%). The frequencies of all observed combinations of materials used for household construction with the observed incidence of malaria for each combination are presented as a Venn diagram in Figure 1 . The most frequent combinations observed were traditional materials for the floor, walls, and roof (55.9%); traditional materials for the floor and walls with a modern roof (24.3%); modern materials for the floor, walls, and roof (12.2%); with all other combinations represented by 5% households or less. Given the strong degree of correlation between materials used for house construction, it was not possible to evaluate the independent associations between house construction materials and the incidence of malaria. The one exception was roof type, where a sufficient number of houses had only a modern roof, but this was not associated with a significant difference in the incidence of malaria compared with houses with only traditional materials (6.09 versus 6.19 episodes of malaria per person-years at risk, P = 0.64). Once combined into a binary variable, 12.2% homes were modern homes (non-traditional materials used for floor, walls, and roof) and 87.8% homes were traditional homes (any traditional materials used). The spatial distribution of modern and traditional homes categorized by the incidence of malaria is presented in Figure 2 . Houses located in town were significantly more likely to be modern compared with those in rural areas (45.0% versus 9.5%, P < 0.001).
Figure 1.
Relationship between materials used for household construction. Incidence is defined as cases/person/person-year at risk. Traditional floors are defined as earth floors, whereas modern floors are defined as cement, stone, or brick floors. Traditional walls are defined as mud and sticks or mud brick walls, whereas modern walls are defined as cement bricks or plaster. Traditional roofs are defined as thatched roofs, whereas modern roofs are defined as iron sheets or cement.
Figure 2.
Spatial distribution of household construction type variable. Incidence is defined as cases/person/person-year at risk. Modern homes defined as structure where nontraditional materials are used for floor, walls, and roof. Traditional homes defined as structure where any traditional materials are used.
Factors associated with the incidence of malaria.
After controlling for the assigned chemoprevention arm, the following covariates were significantly positively associated with the incidence of malaria in univariate analyses: house construction type, location of residence, number of rooms in the house, presence of an entryway to the outside in the study participant's room, presence of windows (defined as an opening in the wall > 1 ft.2 in size with or without screening or a covering) in the study participant's room, eaves or airbricks in the study participant's room, location of the study participant's sleeping space (i.e., sleeping directly on the ground or a bed on the ground versus sleeping on a bed raised off the ground), household wealth, and the primary caregivers' age and level of education (Table 2). In multivariate analysis, after additional adjustment for spatial autocorrelation, living in a modern home was associated with approximately half the incidence of malaria compared with living in a traditional home (incidence rate ratio [IRR] = 0.54, P = 0.001). Other factors independently associated with a lower incidence of malaria in multivariate analysis included living in town versus a rural area (IRR = 0.37, P < 0.001), the presence of one or more windows in the study participant's room (IRR = 0.71, P < 0.001), presence of eaves in the study participant's room (IRR = 0.81, P = 0.01), four or more airbricks present in the study participant's room (IRR = 0.77, P = 0.04), having a primary caregiver above 21 years of age (22–35 years: IRR = 0.75, P = 0.003; 36–68 years: IRR = 0.68, P = 0.003), and having a primary caregiver with more than a primary school level of education (IRR = 0.78, P = 0.04) (Table 2).
Table 2.
Associations between household factors and the incidence of malaria
| Category | Group (number of houses) | Incidence of malaria PPYR* | Univariate† | Multivariate‡ | ||
|---|---|---|---|---|---|---|
| IRR§ (95% CI) | P value | IRR§ (95% CI) | P value | |||
| House construction∥ | Traditional home (N = 452) | 5.26 | Reference | − | Reference | − |
| Modern home (N = 63) | 1.66 | 0.30 (0.23–0.40) | < 0.001 | 0.54 (0.39–0.78) | 0.001 | |
| Location of residence | Lives in rural area (N = 475) | 5.11 | Reference | − | Reference | − |
| Lives in town (N = 40) | 1.22 | 0.29 (0.20–0.42) | < 0.001 | 0.37 (0.22–0.61) | < 0.001 | |
| Number of rooms in the house | One room (N = 260) | 5.46 | Reference | − | Reference | − |
| More than one room (N = 255) | 4.13 | 0.75 (0.63–0.88) | 0.001 | 0.94 (0.75–1.18) | 0.58 | |
| Whether participant's room opens to outside | No entryway open to outside (N = 281) | 5.27 | Reference | − | Reference | − |
| At least one entryway open to outside (N = 234) | 4.21 | 0.79 (0.66–0.93) | 0.005 | 0.99 (0.79–1.24) | 0.97 | |
| Whether participant's room has windows | No windows (N = 283) | 5.76 | Reference | − | Reference | − |
| One or more windows (N = 232) | 3.67 | 0.60 (0.51–0.71) | < 0.001 | 0.71 (0.61–0.84) | <0.001 | |
| Whether participant's room has eaves | No eaves (N = 191) | 3.82 | Reference | − | Reference | − |
| Eaves present (N = 324) | 5.36 | 1.36 (1.15–1.62) | < 0.001 | 0.81 (0.69–0.96) | 0.01 | |
| Number of airbricks in participant's room | 0–3 airbricks (N = 410) | 5.31 | Reference | − | Reference | − |
| Four or more airbricks (N = 105) | 2.88 | 0.53 (0.43–0.66) | < 0.001 | 0.77 (0.60–0.98) | 0.04 | |
| Location of participant's sleeping space | Sleeps on ground or bed on the ground (N = 212) | 5.40 | Reference | − | Reference | − |
| Sleeps on bed raised off the ground (N = 303) | 4.39 | 0.81 (0.69–0.96) | 0.02 | 0.92 (0.76–1.12) | 0.41 | |
| Household wealth index | Lowest tertile (N = 166) | 5.40 | Reference | − | Reference | − |
| Middle tertile (N = 173) | 5.23 | 0.90 (0.73–1.10) | 0.30 | 0.91 (0.76–1.1) | 0.33 | |
| Highest tertile (N = 176) | 3.80 | 0.67 (0.55–0.82) | < 0.001 | 0.86 (0.72–1.03) | 0.11 | |
| Primary caregiver's age | 16–21 years (N = 79) | 7.42 | Reference | − | Reference | − |
| 22–35 years (N = 302) | 4.75 | 0.71 (0.56–0.89) | 0.004 | 0.75 (0.63–0.91) | 0.003 | |
| 36–68 years (N = 134) | 3.46 | 0.57 (0.44–0.75) | < 0.001 | 0.68 (0.52–0.88) | 0.003 | |
| Primary caregiver's level of education | None or primary school (N = 439) | 5.05 | Reference | − | Reference | − |
| More than primary school (N = 76) | 3.29 | 0.61 (0.48–0.77) | < 0.001 | 0.78 (0.62–0.99) | 0.04 | |
CI = confidence interval; IRR = incidence rate ratio; PPYR = per person-years at risk.
Episodes of malaria PPYR.
Only adjusted for chemoprevention arm.
Adjusted for chemoprevention arm, clustering of episodes of malaria for participants living in the same geographical area, and all variables listed.
Incidence rate ratio.
Modern homes defined as structure where non-traditional materials are used for floor, walls, and roof. Traditional homes defined as structure where any traditional materials are used.
Discussion
We investigated associations between household factors and the incidence of malaria in a cohort of children living in a highly endemic area, with a focus on materials used in house construction. The incidence of malaria was remarkably high in this setting despite the use of ITNs and a range of chemopreventive regimens. Living in a modern house, defined as having non-earth floors, non-thatched roofs, and non-mud walls, was associated with an almost 50% lower incidence of malaria compared with living in a traditional home. Other factors found to be associated with a lower incidence of malaria included living in town, sleeping in a room with openings to the outside (windows, eaves, and airbricks), and having a primary caregiver of older ages and a higher level of education.
Malaria has long been recognized as a disease of poverty.20 Although recent progress has been made in reducing the burden of malaria, largely due to the scale-up of proven control interventions including ITNs, IRS, and ACTs, additional interventions aimed at supporting socioeconomic development could prove to be highly effective and sustainable.20 One such approach is improving the quality of housing in resource-limited settings characteristic of much of sub-Saharan Africa based on the growing body of evidence that well-built, modern housing is protective against malaria. In studies from the Gambia, South Africa, Tanzania, and Bangladesh, homes with mud walls were associated with a higher vector density and increased risk of clinical malaria compared with homes with walls made of other materials including cement or concrete.4,5,8,9 Homes with roofs made of earthen materials, such as mud, grass, or “thatch,” were associated with an increased malaria prevalence and incidence of malaria compared with homes with roofs made of iron sheets in studies from Burkina Faso and Ethiopia.7,10 Several studies classified housing structures into quality groupings according to the composition of the construction materials and found poorer house quality to be associated with greater presence of mosquitoes in the home or a higher incidence of malaria. A study from Sri Lanka identified poor housing construction—defined as lacking brick walls and a roof of either tiles, corrugated iron, or asbestos—as being associated with a higher vector density.12 A similar study from Sri Lanka concluded that living in the worst type of house construction (mud or thatched walls and thatched roofs) was associated with a higher incidence of malaria compared with those living in houses with completed brick and plaster walls and tiled roofs.13 In a larger study of intermittent preventive treatment of malaria conducted in Tanzania, high-quality housing based on an index of dwelling characteristics was associated with a lower incidence of malaria and vector density.21
Quality housing is thought to provide protection from malaria by blocking entry and reducing the density of the mosquito vector. Poor-quality material used for walls, such as mud, may provide more entry points through cracks in their surface.5 Anopheles gambiae, one of the primary vectors in Africa, typically approaches a home via scent, flies up the external wall, and enters the house through open eaves, attracted by the microclimate and odors of humans coming from the house.22,23 Similarly, traditional thatched roofs may be associated with holes and eaves with large gaps that enable mosquito vectors common in Africa to enter.22 Traditional floors made of earth may also provide an odorous and moist environment, attracting mosquitoes to the dwelling.
In this study, we found that compared with living in a rural environment, living in town was associated with a lower incidence of malaria. Although there was a strong association between living in town and having a modern home, the associations between both house construction and location were independently associated with malaria incidence in the multivariate analysis. These findings suggest that a higher concentration of good-quality housing may provide benefits for the surrounding community, in addition to protecting their occupants. Alternatively, living in a more urban setting may be associated with factors other than house quality that are protective against malaria, such as negative impacts of urbanization on the larval ecology of mosquitoes.24
An interesting finding in this study was the association between an increase in the number of openings in the room where the study participant slept (presence of windows or eaves) and a lower incidence of malaria, which is contradictory to the findings from several other studies.4–6,22,23,25 Indeed, conventional wisdom would suggest that more openings would lead to a higher mosquito density and an increased risk of malaria. In a randomized controlled trial from the Gambia, full or ceiling screening was associated with a reduction in vector density and childhood anemia, but had no effect on the prevalence of malaria infection.25 A possible explanation for these findings seen in our study is the effect of openings on the temperature and airflow in the house and compliance with ITNs. The presence of windows, eaves, and airbricks may cool the home at night and deter indoor-biting mosquitoes. This hypothesis is supported by a study from Ethiopia, where homes with windows were found to have a lower density of indoor biting mosquitoes compared with homes without windows.23 Increased openings may also increase airflow and reduce thermal discomfort, therefore improving compliance with sleeping under an ITN throughout the night.26
In addition to reducing the risk of malaria infection, studies have shown that improved household construction is also associated with a reduction of other infectious diseases. Non-dirt floors and cinder block walls have been associated with lower prevalence of vector-borne Chagas disease.27 People living in rural households with dirt floors are at higher risk of acquiring soil-transmitted helminths Ascaris lumbricoides and Trichuris trichiura infections.28 Improved natural ventilation in a household because of an increase in windows and entryways is associated with lower transmission of air-borne pathogens such as tuberculosis.29 The high cost of household improvements could therefore be justified by the multifaceted protection they may offer against these common diseases in resource-limited settings.
There were several limitations to this study. The observational study design limits one's ability to make causal inference about factors associated with a lower incidence of malaria. We did not collect any entomological data, precluding our ability to make linkages between risk factors of interest, vector density, and clinical outcomes. Other studies reporting similar associations between poor house construction and an increased incidence of malaria have successfully included this absent information to give a clearer picture.21 Another limitation is self-reported ITN use. Over 98% study participants reported sleeping under an ITN the previous night at the time of routine assessments, however, actual compliance with ITNs was not confirmed through visual inspection at the home. Finally, our household questionnaire was conducted at a single point in time and therefore we were unable to account for possible changes in household characteristics over time.
In summary, the burden of malaria may remain high in some settings despite the use of widely accepted control interventions, suggesting the need for additional novel approaches. This study adds to the growing body of evidence that improved house construction may be associated with a lower risk of malaria. Future, well-designed studies should evaluate interventions aimed at improving house quality as a sustainable and cost-effective means of reducing the burden of malaria.
ACKNOWLEDGMENTS
We are grateful to all the parents and guardians for kindly giving their consent and to the study participants for their cooperation. We also thank all the members of the study team for their tireless effort and excellent work.
Footnotes
Financial support: This study was funded by the National Institutes of Health (HD059454) and the University of California, Berkeley Center for Global Public Health.
Authors' addresses: Katherine Snyman, Tamara D. Clark, Bryan Greenhouse, and Grant Dorsey, Department of Medicine, San Francisco General Hospital, University of California, San Francisco, CA, E-mails: katherine.snyman@ucsf.edu, tclark@medsfgh.ucsf.edu, bgreenhouse@medsfgh.ucsf.edu, and gdorsey@medsfgh.ucsf.edu. Florence Mwangwa, Infectious Diseases Research Collaboration, Kampala, Uganda, E-mail: fmwangwa@idrc-uganda.org. Victor Bigira, Clinton Health Access Initiative, Kampala, Uganda, E-mail: vbigira@gmail.com. James Kapisi, Department of Medicine, Makerere University, Kampala, Uganda, E-mail: kapisij@gmail.com. Beth Osterbauer, Children's Hospital Los Angeles, Los Angeles, CA, E-mail: b.osterbauer@gmail.com. Hugh Sturrock, Roly Gosling, and Jenny Liu, Department of Global Health Sciences, University of California, San Francisco, CA, E-mails: hugh.sturrock@ucsf.edu, goslingr@globalhealth.ucsf.edu, and jenny.liu2@ucsf.edu.
Reprint requests: Grant Dorsey, San Francisco General Hospital, 1001 Portrero Avenue, Building 30, Room 3420, San Francisco, CA 94110, E-mail: gdorsey@medsfgh.ucsf.edu.
References
- 1.World Health Organization . World Health Statistics 2012. 2012. http://www.who.int/gho/publications/world_health_statistics/2012/en/ Available at. Accessed February 6, 2014. [Google Scholar]
- 2.Yeka A, Gasasira A, Mpimbaza A, Achan J, Nankabirwa J, Nsobya S, Staedke SG, Donnelly MJ, Wabwire-Mangen F, Talisuna A, Dorsey G, Kamya MR, Rosenthal PJ. Malaria in Uganda: challenges to control on the long road to elimination: I. Epidemiology and current control efforts. Acta Trop. 2012;121:184–195. doi: 10.1016/j.actatropica.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.President's Malaria Initiative . Uganda Country Profile. 2013. http://www.pmi.gov/docs/default-source/default-document-library/country-profiles/uganda_profile.pdf?sfvrsn=12 Available at. Accessed October 28, 2014. [Google Scholar]
- 4.Lwetoijera DW, Kiware SS, Mageni ZD, Dongus S, Harris C, Devine GJ, Majambere S. A need for better housing to further reduce indoor malaria transmission in areas with high bed net coverage. Parasit Vectors. 2013;6:57–63. doi: 10.1186/1756-3305-6-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirby MJ, Green C, Milligan PM, Sismanidis C, Jasseh M, Conway DJ, Lindsay SW. Risk factors for house-entry by malaria vectors in a rural town and satellite villages in the Gambia. Malar J. 2008;7:2. doi: 10.1186/1475-2875-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradley J, Rehman AM, Schwabe C, Vargas D, Monti F, Ela C, Riloha M, Kleinschmidt I. Reduced prevalence of malaria infection in children living in houses with window screening or closed eaves on Bioko Island, equatorial Guinea. PLoS ONE. 2013;8:e80626. doi: 10.1371/journal.pone.0080626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghebreyesus TA, Haile M, Witten KH, Getachew A, Yohannes M, Lindsay SW, Byass P. Household risk factors for malaria among children in the Ethiopian highlands. Trans R Soc Trop Med Hyg. 2000;94:17–21. doi: 10.1016/s0035-9203(00)90424-3. [DOI] [PubMed] [Google Scholar]
- 8.Haque U, Glass GE, Bomblies A, Hashizume M, Mitra D, Noman N, Haque W, Kabir MM, Yamamoto T, Overgaard HJ. Risk factors associated with clinical malaria episodes in Bangladesh: a longitudinal study. Am J Trop Med Hyg. 2013;88:727–732. doi: 10.4269/ajtmh.12-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coleman M, Coleman M, Mabaso MLH, Mabuza AM, Kok G, Coetzee M, Durrheim DN. Household and microeconomic factors associated with malaria in Mpumalanga, south Africa. Trans R Soc Trop Med Hyg. 2010;104:143–147. doi: 10.1016/j.trstmh.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Yé Y, Hoshen M, Louis V, Séraphin S, Traoré I, Sauerborn R. Housing conditions and Plasmodium falciparum infection: protective effect of iron-sheet roofed houses. Malar J. 2006;5:8. doi: 10.1186/1475-2875-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Temu EA, Coleman M, Abilio AP, Kleinschmidt I. High prevalence of malaria in Zambezia, Mozambique: the protective effect of IRS versus increased risks due to pig-keeping and house construction. PLoS ONE. 2012;7:e31409. doi: 10.1371/journal.pone.0031409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konradsen F, Amerasinghe P, Hoek WVD, Amerasinghe F, Perera D, Piyaratne M. Strong association between house characteristics and malaria vectors in Sri Lanka. Am J Trop Med Hyg. 2003;68:177–181. [PubMed] [Google Scholar]
- 13.Gamage-Mendis AC, Carter R, Mendis C, De Zoysa AP, Herath PR, Mendis KN. Clustering of malaria infections within an endemic population: risk of malaria associated with the type of housing construction. Am J Trop Med Hyg. 1991;45:77–85. doi: 10.4269/ajtmh.1991.45.77. [DOI] [PubMed] [Google Scholar]
- 14.Kilama M, Smith DL, Hutchinson R, Kigozi R, Yeka A, Lavoy G, Kamya MR, Staedke SG, Donnelly MJ, Drakeley C, Greenhouse B, Dorsey G, Lindsay SW. Estimating the annual entomological inoculation rate for Plasmodium falciparum transmitted by Anopheles gambiae s.l. using three sampling methods in three sites in Uganda. Malar J. 2014;13:111. doi: 10.1186/1475-2875-13-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamya MR, Kapisi J, Bigira V, Clark TD, Kinara S, Mwangwa F, Muhindo MK, Kakuru A, Osterbauer B, Aweeka FT, Huang L, Jagannathan P, Achan J, Havlir DV, Rosenthal PJ, Dorsey G. Efficacy and safety of three regimens for the prevention of malaria in young HIV-exposed Ugandan children: a randomized controlled trial. AIDS. 2014;28:2701–2709. doi: 10.1097/QAD.0000000000000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bigira V, Kapisi J, Clark TD, Kinara S, Mwangwa F, Muhindo MK, Osterbauer B, Aweeka FT, Huang L, Achan J, Havlir DV, Rosenthal PJ, Kamya MR, Dorsey G. Protective efficacy and safety of three antimalarial regimens for the prevention of malaria in young Ugandan children: a randomized controlled trial. PLoS Med. 2014;11:e1001689. doi: 10.1371/journal.pmed.1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osterbauer B, Kapisi J, Bigira V, Mwangwa F, Kinara S, Kamya MR, Dorsey G. Factors associated with malaria parasitaemia, malnutrition, and anaemia among HIV-exposed and unexposed Ugandan infants: a cross-sectional survey. Malar J. 2012;11:432. doi: 10.1186/1475-2875-11-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uganda Bureau of Statistics . Demographic and Health Survey 2011. 2012. http://dhsprogram.com/pubs/pdf/FR264/FR264.pdf Available at. Accessed February 11, 2015. [Google Scholar]
- 19.Moran PAP. Notes on continuous stochastic phenomena. Biometrika. 1950;37:17. [PubMed] [Google Scholar]
- 20.Tusting LS, Willey B, Lucas H, Thompson J, Kafy HT, Smith R, Lindsay SW. Socioeconomic development as an intervention against malaria: a systematic review and meta-analysis. Lancet. 2013;382:963–972. doi: 10.1016/S0140-6736(13)60851-X. [DOI] [PubMed] [Google Scholar]
- 21.Liu JX, Bousema T, Zelman B, Gesase S, Hashim R, Maxwell C, Chandramohan D, Gosling R. Is housing quality associated with malaria incidence among young children and mosquito vector numbers? Evidence from Korogwe, Tanzania. PLoS ONE. 2014;9:e87358. doi: 10.1371/journal.pone.0087358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Njie M, Dilger E, Lindsay SW, Kirby MJ. Importance of eaves to house entry by anopheline, but not culicine, mosquitoes. J Med Entomol. 2009;46:505–510. doi: 10.1603/033.046.0314. [DOI] [PubMed] [Google Scholar]
- 23.Animut A, Balkew M, Lindtjørn B. Impact of housing condition on indoor-biting and indoor-resting Anopheles arabiensis density in a highland area, central Ethiopia. Malar J. 2013;12:393. doi: 10.1186/1475-2875-12-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robert V, Macintyre K, Keating J, Trape J-F, Duchemin J-B, Warren M, Beier JC. Malaria transmission in urban sub-Saharan Africa. Am J Trop Med Hyg. 2003;68:169–176. [PubMed] [Google Scholar]
- 25.Kirby MJ, Ameh D, Bottomley C, Green C, Jawara M, Milligan PJ, Snell PC, Conway DJ, Lindsay SW. Effect of two different house screening interventions on exposure to malaria vectors and on anaemia in children in the Gambia: a randomised controlled trial. Lancet. 2009;374:998–1009. doi: 10.1016/S0140-6736(09)60871-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Seidlein L, Ikonomidis K, Bruun R, Jawara M, Pinder M, Knols BG, Knudsen JB. Airflow attenuation and bed net utilization: observations from Africa and Asia. Malar J. 2012;11:200. doi: 10.1186/1475-2875-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bustamante DM, De Urioste-Stone SM, Juárez JG, Pennington PM. Ecological, social and biological risk factors for continued Trypanosoma cruzi transmission by Triatoma dimidiata in Guatemala. PLoS ONE. 2014;9:e10. doi: 10.1371/journal.pone.0104599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quintero K, Durán C, Duri D, Medina F, Garcia J, Hidalgo G, Nakal S, Echeverria-Ortega M, Albano C, Incani RN, Cortez J, Jiménez S, Díaz M, Maldonado C, Matute F, Rodriguez-Morales AJ. Household social determinants of ascariasis and trichuriasis in north central Venezuela. In Health. 2012;4:103–110. doi: 10.1016/j.inhe.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Escombe AR, Oeser CC, Gilman RH, Navincopa M, Ticona E, Pan W, Martínez C, Chacaltana J, Rodríguez R, Moore DAJ, Friedland JS, Evans CA. Natural ventilation for the prevention of airborne contagion. PLoS Med. 2007;4:e68. doi: 10.1371/journal.pmed.0040068. [DOI] [PMC free article] [PubMed] [Google Scholar]