Abstract
We evaluated two novel, portable microscopes and locally acquired, single-ply, paper towels as filter paper for the diagnosis of Schistosoma haematobium infection. The mobile phone-mounted Foldscope and reversed-lens CellScope had sensitivities of 55.9% and 67.6%, and specificities of 93.3% and 100.0%, respectively, compared with conventional light microscopy for diagnosing S. haematobium infection. With conventional light microscopy, urine filtration using single-ply paper towels as filter paper showed a sensitivity of 67.6% and specificity of 80.0% compared with centrifugation for the diagnosis of S. haematobium infection. With future improvements to diagnostic sensitivity, newer generation handheld and mobile phone microscopes may be valuable tools for global health applications.
Introduction
Microscopy is an integral tool of clinical medicine and public health, however this basic technology is not routinely available in health centers in resource-constrained settings.1 Many individuals live in rural or underserviced locations with limited access to care, and where infections such as malaria, schistosomiasis, and soil-transmitted helminths are rife.2,3
Handheld microscopes,4–6 and more recently, mobile phone-based microscopes7,8 have been used in field settings for the diagnosis of malaria, schistosomiasis, and soil-transmitted helminthiasis. These devices have the benefit of being portable and relatively simple to use. Other recent innovations that support inexpensive diagnostic testing in underserviced locations include using locally procured paper products such as paper towels as low-cost filters for the diagnosis of Schistosoma haematobium infections.9 Such innovations hold promise for delivering low-cost laboratory-based technology to underserviced locations, and may have use in clinical and public health settings.
Here, we evaluate two novel handheld and mobile phone-based microscopes and inexpensive, locally acquired paper towels as filter paper for the diagnosis of S. haematobium infections in a rural Ghanaian community.
Methods
This study was integrated into an ongoing epidemiologic survey of schistosomiasis and soil-transmitted helminthiasis in the Central Region of Ghana between February and May 2014. The University of Cape Coast (Cape Coast, Ghana) granted ethical permission for this project. Headmasters at participating schools, pupils, and their parents consented for involvement in this study. For the purpose of this sub-study, we randomly selected 50 individuals enrolled in a participating school with 200 students 7–13 years of age in Sorodofo-Abaasa village, in the Abura Asebu Kwamankese district in the Central Region of Ghana, an area known to be endemic for S. haematobium. A urine sample from each participant was collected between 10:00 am and 14:00 pm,10 and processed on the same day of collection at the University of Cape Coast Hospital Laboratory. Urine samples were shaken and 20 mL was extracted by syringe, with 10 mL processed by centrifugation (as gold standard) and 10 mL by the experimental gravity filtration approach. The centrifuged urine was spun at 5,000 rpm for 5 minutes. The supernatant was discarded and sediment was transferred to a glass microscope slide with the addition of a coverslip. The other 10 mL of urine extracted was gravity filtered through single-ply paper towels that were purchased locally. The methods of this procedure are outlined elsewhere.9 Briefly, the paper towel was formed into a cone, and urine was poured into the center of the cone and allowed to filter through. The central portion of the paper towel where urine was poured through was cut out and placed directly onto a glass microscope slide. Conventional light microscopy (using 10× and 20× objective lenses) with a CX21LEDFS1 Olympus microscope (Olympus, Volketswil, Switzerland), and two novel handheld light microscope devices evaluated both centrifuged and filtered urine. Expert microscopists who were blinded to prior S. haematobium ova counts on each slide performed microscopy. The entire slide was evaluated for a minimum of 3 minutes. The presence or absence of ova was noted, and if present was quantified under conventional light microscopy. We only documented the presence or absence of ova with novel light microscopes. All data were directly entered into an Excel file (Microsoft Corp., Redmond, WA).
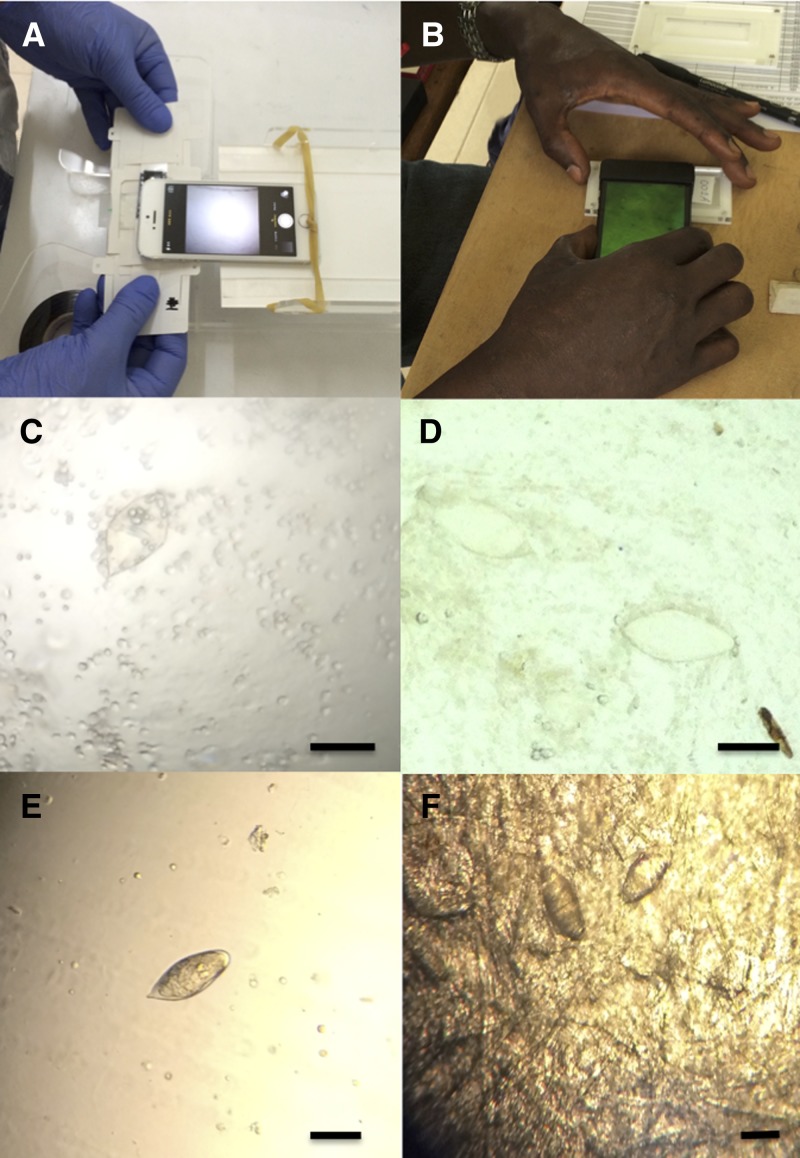
Experimental microscopes included the mobile phone-mounted Foldscope11 and a reversed-lens CellScope.12 The Foldscope is a small, handheld, paper-based microscope with a light-emitting diode (LED) light source. It can be held up to the eye for visualization of slides, however we secured it to the camera lens of an iPhone 5S (Apple, Cupertino, CA) by tape and magnets (Figure 1A). Microscope slides were manually manipulated under the phone-mounted Foldscope lens, and the presence or absence of ova was noted on the iPhone screen. The CellScope is a mobile phone microscope that integrates imaging and illumination optics directly with an unmodified mobile phone.13,14 The reversed-lens CellScope12 (Figure 1B) used in this study is a small three-dimensional-printed plastic attachment for the iPhone 5S with an embedded lens for microscopic imaging. It harnesses the mobile phone's light source to illuminate slides by reflecting light off of a white slide holder placed underneath the microscope slide. The lens embedded in the attachment aligns with the iPhone camera lens, and a magnified portion of the slide can be visualized on the screen of the mobile phone using the camera function. A microscopist held the device directly above a microscope slide and manually manipulated the device across the full slide, while examining the mobile phone screen for the presence or absence of S. haematobium ova.
Figure 1.
A mobile phone-mounted Foldscope (A), and reversed-lens CellScope (B). Schistosoma haematobium ova visualized by a mobile phone-mounted Foldscope after centrifugation (C), reversed-lens CellScope after centrifugation (D), conventional microscopy after centrifugation (E), and conventional microscopy after filtration with single-ply paper towel (F). Reference bars = ∼100 μm.
Using the same slides prepared by urine centrifugation, we measured the sensitivity and specificity of the two portable microscopes using conventional light microscopy as the gold standard, and compared dichotomous agreement using Cohen's Kappa. We similarly examined sensitivity, specificity, and agreement of urine filtration with centrifugation, using light microscopy. All analyses were performed using R (R Foundation for Statistical Computing, Vienna, Austria).
Results
One urine sample was lost during laboratory processing, leaving 49 urine samples for evaluation. Based on gold standard conventional light microscopic examination by centrifugation, 34 of the 49 urine samples were positive for S. haematobium ova (mean: 8.7 eggs/10 mL), and of those infected, all but one had a light intensity infection (≤ 50 eggs/10 mL).15 When evaluating centrifuged urine samples prepared on a microscope slide, the mobile phone-mounted Foldscope and reversed-lens CellScope showed a sensitivity of 55.9% (95% confidence interval [CI]: 38.1–72.4%) and 67.6% (95% CI: 49.4–82.0%), respectively, and a specificity of 93.3% (66.0–99.7%) and 100.0% (74.7–100.0%), respectively (Table 1). Images of S. haematobium ova captured by each device can be viewed in Figure 1C and D. Agreement between conventional microscopy and the phone-mounted Foldscope (κ = 0.39, 95% CI: 0.18–0.60) was fair, and agreement between conventional microscopy and reversed-lens CellScope (κ = 0.56, 95% CI: [0.36–0.77]) was moderate. Neither device could reliably detect S. haematobium ova on slides prepared by gravity filtration through single-ply paper towels, and hence were not quantified.
Table 1.
Sensitivity, specificity, and Kappa comparing conventional light microscopy with single-ply paper towels to urine centrifugation, and conventional light microscopy to the mobile phone-mounted Foldscope and reversed-lens mobile phone microscope for the diagnosis of Schistosoma haematobium infection
| Single-ply filter paper | Phone-mounted Foldscope | Reversed-lens CellScope | |||||
|---|---|---|---|---|---|---|---|
| Negative | Positive | Negative | Positive | Negative | Positive | ||
| Urine centrifugation and conventional light microscopy | Negative | 12 | 3 | 14 | 1 | 15 | 0 |
| Positive | 11 | 23 | 15 | 19 | 11 | 23 | |
| Kappa | 0.41, 95% CI (0.17–0.66) | 0.39, 95% CI (0.18–0.60) | 0.56, 95% CI (0.36–0.77) | ||||
| Sensitivity | 0.68 | 0.56 | 0.68 | ||||
| Specificity | 0.80 | 0.93 | 1.000 | ||||
CI = confidence interval.
Gravity filtration of urine samples through single-ply paper towels and examined with conventional microscopy showed sensitivity of 67.6% (95% CI: 49.4–82.0%), specificity of 80.0% (51.4–94.7%), and fair to moderate correlation (κ = 0.41, 95% CI: 0.17–0.66) compared with slides prepared with centrifuged urine (Table 1). Schistosoma haematobium ova centrifuged or filtered through single-ply paper towel and examined with conventional microscopy can be viewed in Figure 1E and F, respectively.
Discussion
Robust, simple, and inexpensive diagnostic tests are greatly needed in resource-constrained settings. Handheld and mobile phone-based microscopes may have an important role in providing such diagnostic support.13,16 In this work we studied two novel, handheld, mobile-phone compatible microscopes and single-ply paper towels to filter urine for the diagnosis of S. haematobium in individuals with low-intensity infections in an endemic setting.
The mobile phone-mounted Foldscope11 had limited sensitivity, but excellent specificity for the diagnosis of S. haematobium infection. This device, which uses a 2.38 mm ball lens for magnification and is illuminated by a small battery powered LED, was effective at focusing on ova when in the field of view. The Foldscope weighs < 8 g, costs < 1 United States Dollar (USD). The sensitivity of this device may be low because of a combination of some challenges with manually manipulating microscope slides underneath the lens, and the low infection intensities in the study setting. The Foldscope used in this project was able to magnify images by 140×, however other greater power magnifications are available as well. Based on preliminary trials, we felt the 140× magnification was easiest to use, and when coupled with the digital zoom of the mobile phone camera would provide adequate magnification for S. haematobium ova.
The reversed-lens CellScope12 showed modest sensitivity but excellent specificity for S. haematobium diagnosis in this study. As with the Foldscope, the modest sensitivity may be caused by our manual manipulation of the device over slides with very light intensity infections. The device was easy to handle and did not have issues with sample contamination from the microscope slide. The reversed-lens CellScope weighs 5 g, uses a wide-field lens that costs < 6 USD, and provides ≤ 5 μm resolution over an ∼10 mm2 field of view.12 When coupled with the optical zoom function of the mobile phone camera, the device can provide adequate screen magnification for S. haematobium ova identification.
These two novel devices show early promise and may be a stepping stone for future portable diagnostic devices. They could be used in clinical and epidemiologic settings in the near future with technical adjustments to increase diagnostic sensitivity, such as increasing the field of view and enabling magnification of different powers, in addition to validating these devices on other pathogens.
Locally procured single-ply paper towel did not have adequate sensitivity for S. haematobium diagnosis when evaluated by conventional light microscopy. Furthermore, we could not detect S. haematobium ova on filter paper with the mobile phone-mounted Foldscope or reversed-lens CellScope in the configurations used in this study, possibly because of how light scattered off of the corrugated surface of the paper when illuminated from above. Early results using inexpensive, locally procured filter paper evaluated by conventional light microscopy were encouraging,9 however it appears that this type of paper is not suitable for routine laboratory use. Schistosoma haematobium ova may have passed through larger pores in the paper or may have been aligned in vertical or diagonal positions caused by the corrugated surface of the paper. Microscopists may not have counted S. haematobium ova in these unusual positions. Single-ply filter paper is strong and easy to manipulate when wet and it does not tear easily when transferred to a microscope slide. Inexpensive filter paper used in future studies should have these positive attributes but will need to have a smoother surface to reduce light refraction, while ensuring pores are small enough such that ova cannot readily pass through.
Other recent innovations may aid in the field diagnosis of S. haematobium infection as well, including a low-cost Millipore filtration device,17 or on-chip imaging of ova,18 both of which showed promise in early proof-of-concept studies. However, all will require vigorous field testing in clinical and public health settings before implementation.
Our study was limited by a few factors. We only detected the presence or absence of S. haematobium ova with experimental microscopes and did not quantify infection intensity. Future studies should quantify ova counts, and also evaluate a cohort where there are both high and low intensity infections. In addition, laboratory technicians rather than expert microscopists should be included in evaluating slides to more closely proxy real-world settings.
Both the mobile phone-mounted Foldscope and reversed-lens CellScope have excellent specificity for diagnosing S. haematobium in those with light intensity infections in an endemic setting. With future modifications to improve sensitivity, such devices may be useful diagnostic tools in resource-constrained clinical and public health settings.
Footnotes
Financial support: Isaac Bogoch is supported by Grand Challenges Canada.
Authors' addresses: Richard K. D. Ephraim and Evans Duah, Division of Medical Laboratory Technology, University of Cape Coast, Cape Coast, Ghana, E-mails: kdephraim@yahoo.com and ivanprecy@gmail.com. James S. Cybulski and Manu Prakash, Department of Mechanical Engineering, Stanford University, Stanford, CA, and Department of Bioengineering, Stanford University, Stanford, CA, E-mails: cybulski@stanford.edu and manup@stanford.edu. Michael V. D'Ambrosio and Daniel A. Fletcher, Department of Bioengineering, University of California, Berkeley, CA, E-mails: mdambrosio@berkeley.edu and fletch@berkeley.edu. Jennifer Keiser, Department of Medical Parasitology and Infection Biology, Swiss Tropical and Public Health Institute, Basel, Switzerland, and University of Basel, Basel, Switzerland, E-mail: jennifer.keiser@unibas.ch. Jason R. Andrews, Division of Infectious Diseases and Geographic Medicine, Stanford University School of Medicine, Stanford, CA, E-mail: jandr@stanford.edu. Isaac I. Bogoch, Divisions of Internal Medicine and Infectious Diseases, Toronto General Hospital, Toronto, ON, Canada, E-mail: isaac.bogoch@uhn.ca.
References
- 1.Petti CA, Polage CR, Quinn TC, Ronald AR, Sande MA. Laboratory medicine in Africa: a barrier to effective health care. Clin Infect Dis. 2006;42:377–382. doi: 10.1086/499363. [DOI] [PubMed] [Google Scholar]
- 2.Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, Hotez PJ. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–1532. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 3.Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J. Helminth infections: the great neglected tropical diseases. J Clin Invest. 2008;118:1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stothard JR, Kabatereine NB, Tukahebwa EM, Kazibwe F, Mathieson W, Webster JP, Fenwick A. Field evaluation of the Meade Readiview handheld microscope for diagnosis of intestinal schistosomiasis in Ugandan school children. Am J Trop Med Hyg. 2005;73:949–955. [PubMed] [Google Scholar]
- 5.Stothard JR, Nabatte B, Sousa-Figueiredo JC, Kabatereine NB. Towards malaria microscopy at the point-of-contact: an assessment of the diagnostic performance of the Newton Nm1 microscope in Uganda. Parasitology. 2014;141:1–7. doi: 10.1017/S0031182014000833. [DOI] [PubMed] [Google Scholar]
- 6.Bogoch II, Andrews JR, Speich B, Ame SM, Ali SM, Stothard JR, Utzinger J, Keiser J. Quantitative evaluation of a handheld light microscope for field diagnosis of soil-transmitted helminth infection. Am J Trop Med Hyg. 2014;91:1138–1141. doi: 10.4269/ajtmh.14-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bogoch II, Andrews JR, Speich B, Utzinger J, Ame SM, Ali SM, Keiser J. Mobile phone microscopy for the diagnosis of soil-transmitted helminth infections: a proof-of-concept study. Am J Trop Med Hyg. 2013;88:626–629. doi: 10.4269/ajtmh.2013.12-0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bogoch II, Coulibaly JT, Andrews JR, Speich B, Keiser J, Stothard JR, N'Goran EK, Utzinger J. Evaluation of portable microscopic devices for the diagnosis of Schistosoma and soil-transmitted helminth infection. Parasitology. 2014;141:1–8. doi: 10.1017/S0031182014000432. [DOI] [PubMed] [Google Scholar]
- 9.Ephraim RK, Duah E, Andrews JR, Bogoch II. Ultra-low-cost urine filtration for Schistosoma haematobium diagnosis: a proof-of-concept study. Am J Trop Med Hyg. 2014;91:544–546. doi: 10.4269/ajtmh.14-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weber MD, Blair DM, Clark VV. The pattern of schistosome egg distribution in a micturition flow. Cent Afr J Med. 1967;13:75–88. [PubMed] [Google Scholar]
- 11.Cybulski JS, Clements J, Prakash M. Foldscope: origami-based paper microscope. PLoS ONE. 2014;9:e98781. doi: 10.1371/journal.pone.0098781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Switz NA, D'Ambrosio MV, Fletcher DA. Low-cost mobile phone microscopy with a reversed mobile phone camera lens. PLoS ONE. 2014;9:e95330. doi: 10.1371/journal.pone.0095330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breslauer DN, Maamari RN, Switz NA, Lam WA, Fletcher DA. Mobile phone based clinical microscopy for global health applications. PLoS ONE. 2009;4:e6320. doi: 10.1371/journal.pone.0006320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skandarajah A, Reber CD, Switz NA, Fletcher DA. Quantitative imaging with a mobile phone microscope. PLoS ONE. 2014;9:e96906. doi: 10.1371/journal.pone.0096906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO Expert Committee Prevention and control of schistosomiasis and soil-transmitted helminthiasis. World Health Organ Tech Rev Ser. 2002;912(i–vi):1–57. [PubMed] [Google Scholar]
- 16.Smith ZJ, Chu K, Espenson AR, Rahimzadeh M, Gryshuk A, Molinaro M, Dwyre DM, Lane S, Matthews D, Wachsmann-Hogiu S. Cell-phone-based platform for biomedical device development and education applications. PLoS ONE. 2011;6:e17150. doi: 10.1371/journal.pone.0017150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gyorkos TW, Ramsan M, Foum A, Khamis IS. Efficacy of new low-cost filtration device for recovering Schistosoma haematobium eggs from urine. J Clin Microbiol. 2001;39:2681–2682. doi: 10.1128/JCM.39.7.2681-2682.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linder E, Grote A, Varjo S, Linder N, Lebbad M, Lundin M, Diwan V, Hannuksela J, Lundin J. On-chip imaging of Schistosoma haematobium eggs in urine for diagnosis by computer vision. PLoS Negl Trop Dis. 2013;7:e2547. doi: 10.1371/journal.pntd.0002547. [DOI] [PMC free article] [PubMed] [Google Scholar]