Abstract
Directly observed therapy short-course (DOTS) requires direct observation of tuberculosis (TB) patients and manual recording of doses taken. Programmatically, manual tracking is both time-consuming and prone to human error. Our project in western Uganda assessed the impact on TB treatment outcomes of a comprehensive patient support program including eCompliance, a biometric medical record device, with the aim of increasing TB patient retention. Through an observational study of 142 patients, DOTS outcomes of patients in the intervention group were compared with two control groups. Descriptive statistical comparisons, case-cohort analysis, and difference in change over time were used to assess the impact. Intervention patients had a higher cure rate than all other patients (55.6% versus 28.3% [P < 0.01]) and the odds of having a “cured” outcome were 3.17 higher (P < 0.05). The intervention group had a statistically significantly lower odds of having a negative outcome (0% versus.17% [P < 0.01]) than patients from the control groups. Additionally, the intervention group had a lost to follow-up rate lower than all other groups (0% versus 7%) that was trending on significant. In resource-limited settings, implementing comprehensive DOTS including eCompliance may reduce the occurrence of negative DOTS outcomes for patients.
Background
Despite over 20 years of intensified global control efforts, tuberculosis (TB) still causes approximately 1.4 million deaths every year.1 One-third of the world's population is currently infected with the TB bacteria, leading to an estimated annual incidence of 8.8 million active cases of the disease worldwide. As with many other infectious diseases, the burden of TB falls disproportionately on the developing world,2 especially where the incidence of human immunodeficiency virus (HIV) co-infection is high.3,4 Tuberculosis is still mostly curable when the appropriate course of drug therapy is completed, but the full course of antibiotics lasts 6 months, and failure to adhere to complete treatment can lead not only to the return of symptoms, but also to drug resistance.5 Globally, the reported rate of TB patients lost to follow-up during TB treatment was > 12% in 2011.1 Consequently, the incidence of drug-resistant TB is on the rise: 310,000 cases of TB resistant to the two most potent antibiotics, termed multidrug-resistant TB (MDR-TB), were reported in 2011.6 As the incidence of MDR-TB grows, it is increasingly urgent to ensure case detection and adequate treatment completion. To compound this issue, incomplete treatment of TB leads to MDR-TB, but incomplete treatment of MDR-TB can lead to extensively drug resistant TB (XDR-TB), a form of TB that has a much higher mortality rate and is very costly to treat.7 Preventing drug-resistant TB through strict monitoring is therefore critical as mortality in drug-resistant cases is higher, and treatment is more difficult, lengthier, costlier, and associated with more severe side effects for the patients.
Directly observed therapy short-course (DOTS)—where a health worker watches a patient take the dose of TB antibiotics and then manually records the intake on an individual patient card—is the World Health Organization (WHO) standard approach to ensuring retention of patients in treatment programs.8 A DOTS treatment protocol is usually separated into two distinct phases: a 2-month intensive phase, with mandatory daily supervision of drug intake, usually at the clinic, followed by a 4-month continuation phase, with recommended daily supervision. The DOTS programs, as currently designed and implemented, place an important emphasis on monitoring therapy, but they cannot ensure that treatment adherence is fully maintained. Programmatically, manual tracking is time-consuming. It is nearly impossible for health workers to ensure that all patients have been seen and properly recorded, or to verify that records are correct, especially in high-burden, high-volume settings where hundreds of patients need daily supervision.9 Moreover, paper-based records delay the reporting of important patient and disease information, which limits potential implementation of interventions and policies that could greatly improve delivery of and adherence to TB care.
A suitable solution for both patients and healthcare providers is to implement a biometric system† known as “eCompliance” integrated into a comprehensive patient support package, to reduce the constraints of manual tracking.10 The eCompliance system simplifies the monitoring of all patients on treatment by scanning the patient's fingerprint at a netbook computer terminal during every visit, whether in the home or at a clinic. The eCompliance system streamlines the TB compliance recording process and ensures that health workers see all patients as scheduled by providing them with immediate access to accurate patient visit logs, removing any guesswork, human error, or time delay caused by the upkeep of manual DOTS recording. With this biometric system, each patient is registered by adding his or her fingerprints into the secure database. During all subsequent visits for DOTS, patient visits are logged by simply recording the patient's fingerprint again. Within seconds, the health workers can easily view the patients that have not been observed taking a scheduled dose and follow-up accordingly. The system allows health workers to adequately follow up on all patients. Those who discontinue treatment can easily be identified and actions can be taken by health workers to ensure TB treatment is completed.
In this way, the system has clear benefits for both health workers based in the field and in supervisory or monitoring positions. For those in the field, it can help them to ensure that they are seeing all of their patients, and verifying that they are seeing the correct patients. Additionally, it can help supervisors to ensure the efficacy of the work that their teams are doing, particularly as the biometric requirement ensures that all information reported by field workers is true, and that all patient visits are recorded at the actual time of visit—thereby limiting human error. It can also help both levels of health workers to review if there are any patterns that may be indicative of patients that require additional support.
The eCompliance system was developed in India by the technical team at Operation ASHA (http://www.opasha.org/) and has been tested in urban slums since 2010. Results in India show a significant decrease in the rate of patients lost to follow-up. The Indian Revised National TB Control Program (RNTCP) suggests average lost to follow-up rates of 7% in the country, and the Operation ASHA centers using the eCompliance system found that the lost to follow-up rate decreased to < 3%.10 The large gains by the Operation ASHA team have been determined to be largely a result of the system supporting concerns related to potential human error. In settings with large TB caseloads or where health workers are handling many different responsibilities besides TB management, it can be difficult for health workers to ensure that they are providing each TB patient with the support that they need to adhere to treatment. The eCompliance system supports health workers by providing them with active reminders for each patient and comprehensive, accurate records at the touch of button/scan of a fingerprint. In this way, it does not passively provide records, but instead plays an active role in giving health care providers and teams necessary support to ensure good outcomes.
The aim of our pilot project was to assess the transferability of the eCompliance system from an urban setting, where it had already been shown successful by Operation ASHA, to a rural setting in Uganda; we assessed whether eCompliance, when integrated into a comprehensive patient support package, can provide substantive improvements to TB DOTS programs. The ultimate goal of these improvements is to increase TB patient retention, which should reduce drug resistance and death caused by inadequate TB treatment.
We implemented the eCompliance system at the Millennium Villages Project (MVP - www.millenniumvillages.org) cluster in Ruhiira, Uganda. The Ruhiira cluster lies in the southwestern region of Uganda in Isingiro district, about 40 kilometers from the nearest city, Mbarara. The cluster is made up of eight villages spread out over several hundred square kilometers. We selected Ruhiira to pilot eCompliance because of its previously reported high rates of TB patients who were lost to follow-up or died in 2010 (19% and 15.3%, respectively). The site also has a high TB caseload, with over 50 patients diagnosed a year in a population of about 50,000. Before the implementation of eCompliance at the site, health workers followed Uganda national TB guidelines implementing community-based DOTS (CB-DOTS): after diagnosis and treatment initiation at a clinic, a family member assigned to observe and record doses performed DOTS daily and manually at the household level. Family supervision was complemented with monthly supervision at home by a Community Health Worker (CHW). In addition to the high rate of patients lost to follow-up, many patients' adherence was not tracked using the official patient medical record card issued by the national TB control program. Instead, health workers often used paper notebooks to record TB patient information. This inconsistency in record keeping further complicated streamlining of services and ultimately, aggregation of data for reporting purposes.
Methods
Materials.
Three components were required to conduct the pilot project at the Ruhiira MVP site: a notebook computer (our choice: Asus Eee PC model, Taipei, Taiwan), the eCompliance software (developed by Operation ASHA in India, accessible at http://www.opasha.org/our-work/edots-innovation-and-health), and a fingerprint scanner (our choice: Digital Persona U.are.U 4500, Redwood City, CA). The individual cost of the materials was US $200, US $0, and US $70, respectively. Additional costs only included a one-time training/implementation cost ($500 for a 2-day training). There were no additional costs associated with airtime (the short message system [SMS] function was not active in our machines), hardware maintenance (we did not incur this cost for the duration of the pilot) and software support was provided free of charge by Operation ASHA.
eCompliance software.
The eCompliance software was found to be simple and straightforward for those that are not familiar with computers. At the Ruhiira cluster, it was operated by CHWs in the homes of each patient. Our description in this piece therefore assumes the CHWs as end-users. However, eCompliance can also be implemented at the clinic level for trained healthcare workers.
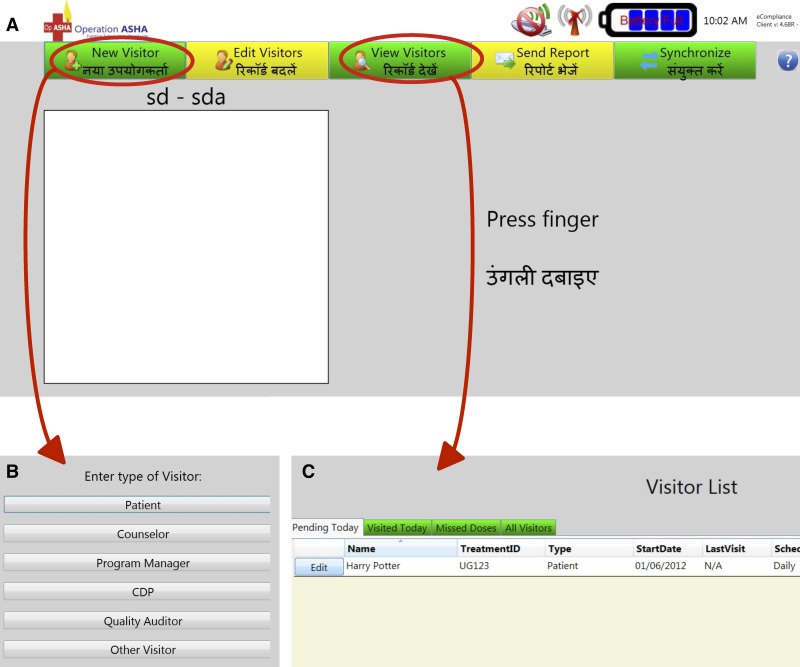
When the eCompliance system turns on, it automatically opens to the eCompliance Welcome Screen (Figure 1A). The software was developed to allow several types of visitors (Figure 1B). Visitors who are already registered can login by scanning their fingerprint. A patient login only allows registering a supervised dose. The CHW (or counselor) and program manager login allow full functionality of the software. The CHWs can select the “New Visitor” tab (Figure 1A) to register new visitors (including patients), edit an existing patient's status using the “Edit Visitors” tab (e.g., a CHW can change the status of a patient from “active” to “lost to follow-up”), view visitor logs and patients' missed doses (Figure 1C), send reports, and synchronize multiple eCompliance system records. However, the latter two functions were not used during the pilot project because of the small scale of the project and to simplify the support provided by eCompliance to the CHWs. Registering a new patient takes < 10 minutes, whereas registering a supervised dose takes less than a minute.
Figure 1.
Accessing eCompliance functions. (A) A registered visitor can instantly log a return visit on the Welcome Screen by simply scanning one finger twice, which takes less than a minute. Community health workers (CHWs) can access necessary functions of the eCompliance system by selecting one of the tabs at the top of the screen. (B) From the “New Visitor” page, the CHW can register different categories of visitors. Selecting the “Patient” option requires the most intensive registration process but will normally take < 10 minutes during the first visit. This process will establish a visit schedule for each patient that must be maintained (though can be edited) or the system will mark a missed dose in the log. The other registration processes require information according to the relevant task of the visitor. “Other visitor” is the most basic setting; it allows no access and affects no data recording—it is typically used for demonstrations. (C) From the “View Visitor” page, a health worker can access a list of patients to visit, or verify those already visited. Because the interface used in India was both in Hindi and English, the same interface was simply kept for Uganda where English is the main language.
Maintenance for both the hardware and the software was conducted by the MVP electronic Health (eHealth) team. Virus protection software was installed on machines prior to implementation, and CHWs were instructed to only use the netbooks for eCompliance work.
Ethical approval.
This study was reviewed and approved by the Institutional Review Board at Columbia University (Protocol no.: IRB-AAAJ9911) and by Mbarara University of Science and Technology (Protocol no.: MUIRC 1/7). Approval was obtained for adult patients (> 18 years of age). Participants provided verbal informed consent to participate. Written consent was not obtained to prevent selection of participants on the basis of literacy. Participants' consent was recorded the following way: the consent form was read by a nurse or community health worker in the local language to each individual participant who was then asked to circle “yes” or “no” at the bottom of the form based on their intent to participate or not, and an index fingerprint was affixed next to their answer. The nurse or CHW obtaining consent subsequently signed and dated each form. Only patients circling “yes” were enrolled in the study. This consent procedure was approved by the ethics committee.
CHW selection and patient load.
Three CHWs from different clinics in the MVP cluster (Kabuyanda, Ntungu, and Ruhiira) were selected to oversee the eCompliance system. The CHWs were chosen based on their catchment area of service, to cover the three geographic areas of the project (lowlands, midlands, and highlands). All three had existing access to a motorbike and were literate.‡ None of them had used a computer before. Their patient load was determined by the number of TB cases at their respective clinics, and occasionally a patient was switched to a CHW other than the one at their primary clinic if logistically advantageous (e.g., caused by necessary time constraints, proximity to a different observation route, etc.).
Trainings.
The eCompliance system was introduced to the Ruhiira cluster through a series of classroom training sessions over 2 days and patient enrollments at the household level supervised by the research team. The training component included four sessions. During the first session, the research team introduced the project and the objectives to the CHWs, instructing them on individual tasks required by the project and the patient observation schedule: daily for patients in the intensive phase and weekly for those in the continuation phase. During the second and third sessions, the “Other Visitors” registration mode (Figure 1) was used for demonstration and individual practice. During the initial patient enrollment at the household level, direct support of the research team was immediately available to CHWs for questions related to patient registration, following patient consent. The CHWs were allowed to proceed independently once they had demonstrated confidence with and complete knowledge of enrolling and observing patients, as determined by trainer evaluation (enrolling two patients without support).
Subject selection and comparison cohorts.
All adult patients (> 18 years of age) enrolled into TB DOTS at clinics in the MVP cluster during the pilot period (July 2012–December 2012) were offered enrollment to be followed by CHWs using eCompliance until the completion of their TB treatment (6 months duration). All gave verbal consent. No patient refused to participate during or after enrollment. This cohort of patients followed daily by CHWs using eCompliance will be referred to as MVP 2012. Impact was then evaluated by comparing MVP 2012 against patients undergoing TB DOTS in the same rural district in Uganda (Isingiro). These non-intervention comparison patients fell into three groups of patients: MVP 2011, Isingiro 2011, and Isingiro 2012. MVP 2011 consisted of TB patients enrolled on TB DOTS during 2011 (July 2011–December 2011) at the same cluster of clinics as the eCompliance patients (i.e., MVP 2012). The latter two comparison cohorts consisted of patients who received care outside of the MVP cluster of clinics, but in the same district as the MVP cluster clinics. Patients in the Isingiro cohorts lived in similar rural conditions as the MVP cohorts, with comparable barriers to access as those in the Ruhiira cluster. Isingiro 2011 consisted of patients enrolled into TB treatment during the time period of July 2011–December 2011 and Isingiro 2012 consisted of patients enrolled between July 2012–December 2012. All three comparison groups followed Uganda national TB guidelines and were supervised daily by family members, and once monthly by a CHW. All patients were followed until a treatment outcome was available (Table 1) and were not blinded to enrollment on the eCompliance intervention.
Table 1.
Outcome classifications*
| Cured | A patient whose sputum smear or culture was positive at the beginning of the treatment but who was smear- or culture-negative in the last month of treatment and on at least one previous occasion. |
| Completed | A patient who has completed treatment but does not have a negative sputum smear or culture result in the last month of treatment and on at least one previous occasion. |
| Treatment success | The sum of cure and completed. |
| Transfer out | A patient who has been transferred to another recording and reporting unit and whose treatment outcome is unknown. |
| Failure | A patient who has a sputum smear or culture that is positive at 5 months or later during treatment. Also included in this definition are patients found to harbor a multidrug-resistant (MDR) strain at any point of time during the treatment, whether they are smear-negative or -positive. |
| Death | A patient who dies for any reason during the course of treatment. |
| Lost to follow-up | A patient whose treatment was interrupted for 2 consecutive months or more. |
| Negative outcome | The sum of failure, death, and default. |
Definitions adapted from the World Health Organization (WHO) Guidelines for tuberculosis treatment.
Five data points of interest were collected for each patient: age; gender; if the patient was undergoing retreatment; HIV status and therapy received and the outcome of treatment (Table 1), defined as cured, completed, transfer out, failure, death, or lost to follow-up.
Statistical methods and quantitative analysis.
Data for the group enrolled on eCompliance was exported directly from the system and data for comparison groups was collected from TB registers at the relevant clinics. All data was anonymized before being given to the research team for analysis.
The available summary statistics for the four cohorts were reviewed to determine if there was significant imbalance among groups that might bias the interpretation of the results. The continuous variable, age, was compared across cohorts by using 5-number summaries comparisons with T tests to assess differences of means. Categorical variables, gender, retreatment status, type of TB, HIV status, if HIV counseling/testing was received, if cotrimoxazole preventive therapy (CPT) was started, and if antiretroviral therapy (ART) for HIV co-infected patients was started, were assessed and compared by running crosstabs and Z tests for proportions.
Outcomes of observation were categorized into one or more possible results: cured, completed, treatment success, transferred out, failure, lost to follow-up, deceased, or negative outcome. Patients were then assigned a binomial result (yes or no) for each of these categories. The proportion of patients for cured, treatment success, lost to follow-up, deceased, and negative outcomes§ were then calculated for all four groups. A Z test for proportion and case-cohort odds ratio (OR) comparison was then carried out for each of those five outcomes in two comparisons: a test for statistical difference between MVP 2011 and MVP 2012, and for a composite control group (“non-intervention”) and MVP 2012.
Finally, a difference in differences comparison was done to assess the difference in change over time of outcome proportions in the MVP clusters as compared with the Isingiro clusters from the 2011–2012 cohorts. This was done by creating an interaction term between year of treatment and which clinic the patient was enrolled in (year*mvp), then running a logistical regression with OR.
All analyses were conducted in Stata Statistics 12.0 (StataCorp, College Station, TX).
Results
Software adaptations.
The eCompliance software was originally developed by Operation ASHA for use in urban settings in India. The implementation of the biometric system in the new context resulted in changes to the coding of the software to follow Ugandan National TB Program guidelines. Four major updates to the software were made in the early stages of project implementation (before enrollment of patients mid-July 2012). These updates addressed errors found in the coding during CHW training and early project implementation and also provided updates to the system for the change in cultural context. The first update fixed an error that had caused patients to disappear after a status transition—e.g., from intensive phase to continuous. The second update fixed a glitch that caused the system to lose its ability to acknowledge fingerprint scans after successfully registering a patient until it was restarted. The final two updates during the pre-pilot phase fixed a coding error that caused the system to occasionally stop being responsive. During the final updates, two minor changes were also made to accommodate the specific local Ugandan context: allowing tracking for treatment on Sundays, which was not an option in the Indian version of the system; and changing the wording of patient registration to read “Scan patient's finger” rather than “Scan your finger” to avoid any confusion.
The system was fully functional upon enrollment of patients.
Demographics.
No patients declined enrollment for follow-up by a CHW using the eCompliance system. The total number of patients in all cohorts was 142. A 5-number summary showed that there were three patients who fell outside the 1.5 times interquartile range of ages. However, further analysis showed no significant relationship between age and any DOTS outcome so the patients were not removed (see Table 2). Additionally, it was determined by T test that there was not a statistically significant difference in age distribution between the enrolled patients and the non-intervention group (see Table 3). There was also no statistically significant difference following a Z test in the proportion of men to women. In an assessment of the number of patients receiving retreatment for TB, MVP 2012 had a significantly higher proportion of retreatment patients than the cumulative control group (22.2% as compared with 9.4%; P < 0.05); however, during later analysis, no statistically significant effect of being a retreatment patient on positive outcomes of TB DOTS treatment was observed, only a positive correlation with negative outcomes. Similarly, an assessment of the proportion of patients according to type of TB, HIV status, and enrollment on CPT and/or ART treatment showed no statistical difference between MVP 2012 and the control groups.
Table 2.
Baseline characteristics and outcomes of the cumulative group and by cohort (N = 142)
| Characteristics | n (%) | |||||
|---|---|---|---|---|---|---|
| All (N = 142) | Cumulative control (N = 106) | Isingiro 2011 (N = 40) | Isingiro 2012 (N = 32) | MVP 2011 (N = 34) | MVP 2012 (N = 36)* | |
| Sex | ||||||
| Male | 104 (73.2) | 79 (74.5) | 30 (75.0) | 23 (71.9) | 26 (76.5) | 25 (69.4) |
| Female | 36 (25.4) | 27 (25.5) | 10 (25.0) | 9 (28.1) | 8 (23.5) | 9 (25.0) |
| Unknown | 2 (1.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (5.6) |
| Age | ||||||
| 25% | 30 | 30 | 31 | 30 | 31 | 28 |
| Median | 40 | 40 | 39.5 | 38.5 | 41 | 40 |
| 75% | 49 | 48 | 49 | 45.5 | 46 | 52 |
| Patient category | ||||||
| New | 122 (85.9) | 95 (89.6) | 35 (87.5) | 28 (87.5) | 32 (94.1) | 27 (75) |
| Retreatment† | 18 (12.7) | 10 (9.4) | 4 (10) | 4 (12.5) | 2 (5.9) | 8 (22.2) |
| Unknown | 2 (1.4) | 1 (0.9) | 1 (2.5) | 0 (0) | 0 (0) | 1 (2.8) |
| Type of tuberculosis | ||||||
| Pulmonary, sputum positive | 113 (79.6) | 87 (82.1) | 36 (90.0) | 27 (84.4) | 24 (70.6) | 26 (82.2) |
| Pulmonary, sputum negative | 19 (13.4) | 13 (12.3) | 2 (5.0) | 1 (3.1) | 10 (29.4) | 6 (16.7) |
| Extra-pulmonary | 8 (4.9) | 6 (5.7) | 2 (5.0) | 4 (12.5) | 0 (0) | 2 (5.6) |
| Unknown | 2 (2.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (5.6) |
| HIV status | ||||||
| HIV-positive | 55 (38.7) | 43 (40.6) | 15 (37.5) | 12 (37.5) | 16 (47.0) | 12 (33.3) |
| HIV-negative | 80 (56.3) | 60 (56.6) | 24 (60.0) | 20 (62.5) | 16 (47.0) | 22 (61.1) |
| Unknown | 8 (4.9) | 3 (2.8) | 1 (2.5) | 0 (0) | 2 (5.9) | 2 (5.6) |
| Patient knows HIV status and received counseling | ||||||
| Yes | 132 (93.0) | 100 (94.3) | 36 (90.0) | 32 (100) | 32 (94.1) | 34 (94.4) |
| No | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Unknown | 10 (7.0) | 6 (5.7) | 4 (10.0) | 0 (0) | 2 (5.9) | 2 (5.6) |
| Enrolled on CPT, where applicable (% total/percentage of applicable) | ||||||
| Yes | 52 (36.6/94.5) | 40 (37.7/93.0) | 15 (37.5/100) | 11 (34.4/91.7) | 14 (41.2/87.5) | 12 (33.3/100) |
| No | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Unknown | 3 (2.1/5.5) | 3 (2.8/7.0) | 0 (0) | 1 (3.1/8.3) | 2 (5.9/12.5) | 0 (0) |
| Enrolled on ART, where applicable (% total/percentage of applicable) | ||||||
| Yes | 21(14.8/38.2) | 17 (16.0/39.5) | 8 (20.0/53.3) | 5 (15.6/41.7) | 4 (11.8/25.0) | 4 (33.3/11.1) |
| No | 2 (1.4/3.6) | 1 (0.1/2.3) | 0 (0) | 0 (0) | 1 (2.9/6.3) | 1 (2.8/8.3) |
| Unknown | 32 (22.5/40.0) | 25 (23.5/58.1) | 7 (17.5/46.7) | 7 (21.9/58.3) | 11 (32.4/68.8) | 7 (19.4/5.8) |
| Outcome | ||||||
| Cured | 48 (33.8) | 30 (28.3) | 13 (32.5) | 6 (18.8) | 11 (32.4) | 20 (55.6) |
| Success | 108 (76.1) | 79 (74.5) | 28 (70.0) | 26 (81.3) | 25 (73.5) | 29 (80.6) |
| Transfer | 16 (11.3) | 9 (8.5) | 5 (12.5) | 3 (9.4) | 1 (2.9) | 7 (19.4) |
| Failure | 1 (0.01) | 1 (0.9) | 0 (0) | 1 (3.1) | 0 (0) | 0 (0) |
| Lost to follow-up | 8 (5.6) | 8 (7.6) | 5 (12.5) | 0 (0) | 3 (8.8) | 0 (0) |
| Died | 9 (6.3) | 9 (8.5) | 2 (5) | 2 (6.3) | 5 (14.7) | 0 (0) |
| Negative outcome | 18 (12.7) | 18 (17) | 7 (17.5) | 3 (9.4) | 8 (23.5) | 0 (0) |
Enrolled on eCompliance.
Retreatment, a patient who is returning after default, or receiving retreatment after initial treatment failure, or who has relapsed; for outcome definitions see Table 1.
Isingiro 2011 represents those patients enrolled on manually recorded DOTS at clinics outside of the MVP cluster in 2011; Isingiro 2012 represents those patients enrolled on manually recorded DOTS at clinics outside of the MVP cluster in 2012; MVP 2011 represents those patients enrolled on manually recorded DOTS at clinics inside the MVP cluster in 2011; MVP 2012 represents those patients enrolled on DOTS at clinics in the MVP cluster in 2012 where eCompliance was used as the method of recording observations; and cumulative control represents the composite of Isingiro 2011, Isingiro 2012, and MVP 2011.
Table 3.
Results of demographic and outcome comparisons
| MVP 2012* vs. cumulative control | MVP 2012* vs. MVP 2011 | |
|---|---|---|
| P value | P value | |
| Demographics | ||
| Sex† | 0.552 | 0.509 |
| Age | 0.375 | 0.779 |
| Retreatment‡ | 0.046§ | 0.051 |
| Type of tuberculosis | ||
| Pulmonary, sputum positive | 0.205 | 0.880 |
| Pulmonary, sputum negative | 0.503 | 0.204 |
| Extra-pulmonary | 0.981 | 0.163 |
| Unknown | 0.015§ | 0.163 |
| HIV status | ||
| HIV-positive | 0.442 | 0.241 |
| HIV-negative | 0.636 | 0.238 |
| Unknown | 0.443 | 0.953 |
| Patient knows HIV status and received counseling | ||
| Yes | 0.981 | 0.435 |
| No | − | − |
| Unknown | 0.981 | 0.953 |
| Enrolled on CPT, where applicable (of all patients/of those applicable) | ||
| Yes | 0.636/0.347 | 0.497/0.204 |
| No | − | − |
| Unknown | 0.308/0.347 | 0.140/0.204 |
| Enrolled on ART, where applicable (of all patients/of those applicable) | ||
| Yes | 0.472/0.696 | 0.932/0.630 |
| No | 0.420/0.326 | 0.967/0.832 |
| Unknown | 0.607/0.990 | 0.217/0.570 |
| Outcomes | ||
| Cured | 0.003¶ | 0.050§ |
| Success | 0.464 | 0.484 |
| Lost to follow-up | 0.090 | 0.068 |
| Dead | 0.071 | 0.017§ |
| Lost or dead | 0.010¶ | 0.002¶ |
| Negative outcomes | 0.008¶ | 0.002¶ |
Enrolled on eCompliance.
Given records with unknown gender the values report reflect the most statistically significant Z and P values.
Retreatment, a patient who is returning after default, or receiving retreatment after initial treatment failure, or who has relapsed.
Statistically significant at P < 0.05.
Statistically significant at P < 0.01.
A comparison of proportions for the demographic characteristics and outcome indicators for the MVP 2012 cohort, who received the treatment, against the proportions for the control group in the MVP cluster in 2011 and for all of the patients in a control group, respectively.
Of the 142 patients in the study, 36 (25.4%) were in MVP 2012, and therefore enrolled on the follow-up by a CHW using the eCompliance biometric system. Of all patients, 108 (76.1%) had a successful treatment outcome (of which 50 patients, or 35.2%, were considered cured of TB), and 16 (11.3%) transferred to different clinics for treatment. Of the remaining patients, 18 (12.7%) had negative outcomes of treatment as follows: 9 (6.3%) died, 1 was deemed a treatment failure, and 8 (5.6%) were lost to follow-up (for outcome according to cohort see Table 2).
Cured and treatment success outcomes.
Positive outcomes appeared in two forms in this population, as defined by the WHO (Table 1): “treatment completed” and “cured,” and the aggregate of these two outcomes, defined as treatment success. Evaluation was done only for cure outcomes and treatment success.
When MVP 2012 patients were evaluated against non-intervention patients, the difference in proportions of patients cured was found to be statistically significantly greater at 55.6% compared with 28.3%, respectively (P < 0.05) (see Table 3). A case-cohort OR shows that MVP 2012 patients had 3.17 higher odds (P < 0.05) of having DOTS resulting in a cured outcome than all other patients (see Table 4). Similarly, MVP 2012 had a statistically significant higher proportion of patients with a cured outcome than MVP 2011: 55.7% and 32.4%, respectively (P < 0.05) (see Table 3). A case-cohort OR shows that MVP 2012 patients had 2.61 higher odds (P < 0.05) of having DOTS resulting in a cured outcome than MVP 2011 patients (see Table 4). Correspondingly, the logistic regression shows positive correlation between enrollment on eCompliance and odds of having a cure outcome on the cohorts over time, with log odds of 1.13 (P < 0.05) and an OR of 3.09 (P < 0.05) greater likelihood of receiving a DOTS “cure” outcome when in the intervention group (see Table 5).
Table 4.
Estimated magnitude of intervention effect
| Outcome | MVP 2012* vs. cumulative control | MVP 2012* vs. MVP 2011 | ||||
|---|---|---|---|---|---|---|
| Odds ratio | P value | OR 95% CI | Odds ratio | P value | OR 95% CI | |
| Cured | 3.17 | 0.003† | 1.35, 7.46 | 2.61 | 0.050‡ | 0.891, 7.49 |
| Success | 1.49 | 0.484 | 0.42, 5.34 | 1.46 | 0.461 | 0.52, 4.27 |
Enrolled on eCompliance.
Statistically significant at P < 0.01.
Statistically significant at P < 0.05.
An estimation of the magnitude of effect of the intervention using a case-cohort comparison. OR = odds ratio; CI = confidence interval.
Table 5.
Logistic regression outcome using year*mvp
| Outcome | year*mvp | |||
|---|---|---|---|---|
| Coefficient | Odds Ratio | OR P value | OR 95% CI | |
| Cured | 1.13 | 3.09 | 0.006† | 1.38, 6.9 |
| Treatment success | 0.56 | 1.76 | 0.293 | 0.61, 5.01 |
For outcome definitions see Table 1. The interaction term year*mvp compares the difference in change in proportion of a TB DOTS outcome from 2011 to 2012 between patients enrolled on eCompliance, and those not enrolled on the system. OR = odds ratio; CI = confidence interval.
Statistically significant P < 0.05.
Statistically significant at P < 0.01.
The MVP 2012 had a higher proportion of patients classified as “treatment success” (80.6%) compared with MVP 2011 (73.5%) and all non-intervention patients (74.5%). However, neither difference between MVP 2012 and the non-intervention groups was statistically significant (see Table 3). Similarly, neither the logistic regression nor a case-cohort analysis shows a statistically significant correlation between being enrolled on eCompliance and odds of having a DOTS “success” outcome (see Tables 4 and 5).
Negative treatment outcomes.
Negative outcomes appeared in three forms in this population, as defined by the WHO (Table 1): lost to follow-up, deceased, and treatment failure. Only one patient in the group had an outcome of treatment failure, thus only the first two outcomes were compared on an individual level. Additionally, because of perfect prediction errors, no case-cohort or difference in difference analysis could be calculated for the negative outcomes.
When MVP 2012 patients were evaluated against non-intervention patients for the proportion lost to follow-up, the 0% lost to follow-up rate in MVP 2012 was trending on significantly lower than the 7.6% lost to follow-up rate of all other cohorts (see Table 3). Similarly, MVP 2012 had a lost to follow-up rate of 0%, which was trending on statistically different when compared with the 8.8% lost to follow-up rate in MVP 2011 (see Table 3).
When MVP 2012 patients were evaluated against the non-intervention patients for proportion of death, MVP 2012 had a lower proportion of death at 0% as compared with a 8.5% death rate for all other cohorts, trending on significant (see Table 3). More strongly, MVP 2012s proportion of death was lower than MVP 2011s and statistically significant with 0% of patients dying and 14.7% in MVP 2011 dying (see Table 3).
Overall, the reduction in negative outcomes was statistically significant on both comparisons as well. None (0%) of MVP 2012 patients logged a negative treatment outcome, whereas non-intervention patients had a significantly higher negative outcome proportion of 17% (P < 0.05; see Table 3). Similarly, MVP 2011 patients had a significantly higher negative outcome proportion of 23.5%, much higher than the 0% proportion of MVP 2012 (P < 0.05; see Table 3).
Discussion
Health care workers monitoring DOTS, especially in clinics in resource-constrained settings, are rarely focused solely on TB activities. Their responsibility to follow-up on the appropriate outcome of treatment is combined with a multitude of other tasks and obligations in other fields, such as malaria, HIV/AIDS and antenatal care, that are as important or time-consuming. Although the “directly-observed” component of DOTS seems logical to prevent non-adherence to treatment, which may lead to increased risks of deaths and/or drug resistance for TB patients, it also places a substantial burden on already overworked staff. As a result, patient TB cards summarizing individual monitoring are inadequately completed, if at all, and there is no simple system in place to help healthcare workers effectively monitor TB patients under their supervision and prevent patients from being lost to follow-up.
We have piloted a home-based DOTS system employing CHWs using a biometric system, originally designed for urban slums in India, and introduced it in a rural village in Uganda. This system, entitled eCompliance, facilitates the monitoring of TB patients adherence to treatment by using fingerprints at a netbook terminal during every visit, whether in the home or at a clinic. The system was easily adapted to Ugandan National TB Program requirements of daily supervision of DOTS through the full course of TB drugs, and was fully functional within a couple weeks, before enrollment of patients.
Compared with non-intervention patients, the patients monitored by CHWs using eCompliance (MVP 2012) had improved treatment outcomes at multiple levels. The difference in the rates of negative outcomes for MVP 2012 (0%) was significantly lower than that for the non-intervention cohorts (17%) and when compared just to those patients treated at clinics in the MVP cluster (23.5%). Similarly, the rate of patients cured of TB following DOTS was significantly higher for MVP 2012 (55.6%) when compared with non-intervention cohorts (28.3%), including when compared with only patients treated at the same cluster of clinics (32.4%) the previous year. It is also important to note that MVP 2012 had a proportion of retreatment patients significantly higher than other groups; these patients are typically more difficult to treat and usually have worse outcomes than new cases; yet that cohort still had significantly better outcomes.
The improvement in both the proportions of patients cured and deceased were consistently the most statistically significant. Compared with all groups, MVP 2012 improved on both of these indicators with statistical significance (P < 0.05), with the exception of the death rate comparison with the non-intervention group, which is only trending on significance. “Died” and “Cured” indicators have the most exactly defined measurements, and are the most difficult to improperly report. These results suggest a great potential for correlation between implementing a comprehensive DOTS program using eCompliance and improving positive outcomes and decreasing mortality. Our study was limited in size and these outcomes could not be shown unequivocally. There is therefore the necessity to conduct further larger studies using eCompliance to confirm these trends.
In contrast, the increase in proportion of patients with a treatment success outcome was not statistically significant. Because “treatment success” combines patients who are cured and who completed treatment, we believe this can be explained largely to a significantly higher proportion of completed cases in non-intervention compared with MVP 2012. Those patients adhered to the full course of drugs, but did not get their final sputum checked for bacteriological confirmation of cure. Although the WHO target for treatment monitors treatment success, the preferred outcome remains a demonstrated cure over a completed treatment, which was statistically significantly higher in MVP 2012. It is also interesting to note that, based on WHO recommendations, the calculation of overall outcome of treatment still takes into account patients who have transferred out, even though these patients are no longer under the care of the health system that diagnosed them. If the calculation of treatment success were based only on patients who completed treatment where it was initiated, the treatment success for MVP 2012 would be 100% (Table 2).
These results strongly suggest that there are improvements on actual patient outcomes. Some patient outcome improvements are expected because patients who were adequately monitored on a daily basis, with the support of CHWs and eCompliance, did not skip pills, did not default, and therefore had a higher chance of completing treatment. Larger follow-up studies would be very valuable to confirm these indications, particularly the correlation between the use of the eCompliance system and the decrease in patient mortality.
Additionally, eCompliance provides the opportunity for the CHWs and any monitoring team to collect more detailed and verified dose observation records for each of the patients enrolled on eCompliance: total number of missed doses; the number of individuals with missed doses; the number of individuals with repeated missed doses; and the number of individuals that had missed 2 or more consecutive weeks of doses (and therefore were potentially believed to be at risk of discontinuing TB treatment). This information provides more insight into how regularly patients are missing doses and how often consecutive missed doses lead to being at risk for non-adherence. This information is particularly useful to health workers as it allows them to easily assess which patients require extra support and education during care. We believe that the large gains made on patient outcomes during the pilot are largely a result of the support that the eCompliance system gives to health care providers to ensure they are reaching every single patient for TB care and support. In this case, we believe that better, more accurate documentation that is also easily and quickly accessible by health teams can ultimately lead to improved patient support and, therefore, better outcomes. Although available, we did not present these details for our cohort in this study because they were difficult or impossible to retrieve for the patients in non-intervention cohorts. This suggests the importance of a system such as eCompliance that seamlessly tracks all these additional indicators for each patient. The system could also be updated to collect further information for patient records, such as HIV status and, if applicable, enrollment on ART and CPT. This would allow CHWs to have a more complete view of each patient, and quickly assess if patients are at higher risk of being lost to follow-up. Additionally, it could allow for TB and HIV programs to be further linked to support co-infected patients.
It may be interesting in further studies using eCompliance to add a qualitative assessment regarding why patients miss doses, particularly when they miss consecutive observations. This information would provide further insight into potential health system improvements, especially if there is a pattern regarding when patients would require additional education and/or support during their time on DOTS.
Though there was no formal qualitative survey of the eCompliance pilot project, feedback from the CHWs and patients was largely positive. Both the CHWs and the patients commented that the system was helping in fully following up with patients, and therefore were more likely to have positive treatment outcomes of TB DOTS. The CHWs felt that eCompliance helped them to be more efficient and to increase community and patient confidence in the health program. Patients reported that the system had reassured them that they were being properly cared for and that their TB treatment would be correctly monitored in its entirety. Additionally, no patients or CHWs expressed concern about added stigma related to use of eCompliance, even when prompted. Conversely, in the clinics outside of the MVP cluster, where eCompliance was not implemented, the CHWs were frustrated with the current inefficacy of the DOTS program in adequately monitoring patients—and even felt they may have been doing the patients a disservice. Future reviews of eCompliance may wish to formally review CHW and patient perceptions of the system, and changes in satisfaction of the associated healthcare system.
The effectiveness of eCompliance in this pilot project focused solely on the use of patient outcomes as indicators. However, it may also be interesting to evaluate eCompliance based on its potential increase in health worker efficiency. Because health workers are able to easily access patient schedules, view a verified record of observations, and quickly log accurate patient observations, eCompliance may reduce the amount of time spent on paperwork, facilitating the timely follow-up of patients. In addition, eCompliance can also send daily text message to CHWs informing which patients require follow-up, though that feature was not used during this pilot. It also allows for the option that multiple systems can synchronize, therefore a patient could potentially be enrolled on multiple machines and transfer between clinics or CHWs while maintaining complete and accurate DOTS records. This feature would be particularly interesting for patients visiting more than one local clinic for care as their profile would be available wherever they go, or if an absent CHW needs to be replaced by a colleague using a different machine. We expect that these options would further support efficiency of TB DOTS programs, particularly as they allow for adaptation of eCompliance within various health systems, and being viable options in rural areas because they communicate through an SMS-based platform, and do not required internet access.
Similarly, this pilot project limited the use of eCompliance and the biometric medical record system to TB patients. However, given the success, we expect that the eCompliance technology, using modified software, could provide support to other challenges in health programs in resource-limited settings. These functions could be as straightforward as monitoring the follow-up of other health programs, such as antenatal care, antiretroviral treatment, and primary care. Additionally, the instantaneous and accurate information regarding patient loads and TB burdens can be used to predict reagent, commodity, and drug needs and direct resources to where they are needed most. Streamlining information about supply chain needs ensures that all patients are provided medicines improving treatment completion. Additionally, the system provides additional security for patient information because it requires biometric login of health care workers to access patient information and history. Once stored in the system, the biometric information is securely encrypted, and cannot be exported out of the eCompliance system, preventing fraudulent use.
Limitations.
The sample size used for this study was relatively small. Although still considered large enough to show statistical significance, a larger sample size would have provided more insight into the effect of enrolling patients on eCompliance.
Our intervention revolved around daily supervision by a CHW using eCompliance, whereas the control groups relied on daily supervision by a family member, with a monthly visit by an appointed CHW. We chose this protocol for the intervention cohort because daily supervision represents the most stringent of conditions for implementation of DOTS, and in several countries, daily supervision is the recommended treatment protocol. We showed that this frequency is achievable and does not constitute a burden for the health workers. However, the improved outcomes of the intervention group could be attributed to the increased frequency of a visit by a CHW. Because it was already shown that direct observation for DOTS can be successfully undertaken by either a family member or a CHW,11,12 a larger follow-up study to our pilot should segregate daily supervision by a CHW from daily supervision by a CHW using eCompliance to determine the added impact of the biometric monitoring tool.
Additionally, the quality and quantity of control group data points was fairly limited for analysis. Although eCompliance collects and verifies each dose and outcome through its biometric technology, the control group data is subject to human error. The data was also only outcome based and did not look at the progression of treatment or the individual patient level data because of this limitation. Similarly, patient level data such as missed doses was available for those enrolled on eCompliance, and yet could not be used as a comparison because information was limited from the control groups. The nature of the population, in terms of which variables could be controlled for, was limited as well: for instance, we did not have access to factors such as education level, nutritional intake, household income, etc., which may have influenced outcomes at the patient level. However, by comparing the eCompliance data against outcomes for patients taken from the same population within the Ruhiira cluster, and from neighboring communities in Isingiro district, we believe that these factors should be fairly controlled. Additionally, when considering the success of the eCompliance project, the quality of CHWs must be considered. Across the Ruhiira cluster cohorts, this should be standardized as they all receive the same training and are held to the same accountability. Finally, this study did not take into account the clustering effect on CHWs, which should be considered in a larger follow-up study.
Also of note, the high rate of patients that transfer to new clinics provides a challenge for all DOTS programs, and is a limitation to understanding the actual outcomes of patients observed in this study. However, given the ability to transfer patient information and synchronize systems using the biometric technology, this would not be a challenge should eCompliance be used at a national level as clinics can easily connect to verify patient transfer and adherence. eCompliance was designed to identify patients locally on the machine, which makes it a distributed system. A distributed system consists of a collection of autonomous computers, connected through a network, which enables computers to coordinate their activities and to share the resources of the system, so that users perceive the system as a single, integrated computing facility. Additionally, each machine supports up to 10,000 fingerprints with identification speed of 3,000 templates per seconds at more than 99.5% accuracy. eCompliance can therefore be scaled up to a national level.
Conclusions
The TB eCompliance pilot project at the Ruhiira site shows that there is transferability of the system to rural and resource-limited settings for use by community health workers, with adaptations of the software to local context. Indeed, the various DOTS projects across continents will require that the eCompliance system be properly adjusted to fit within the individual country setting. However, this can be achieved with basic software updates as was accomplished for the pilot project in Uganda, ensuring that the specifications of the system properly match the local and national TB programs. It also shows that the system can be used successfully in a non-clinic based model, and when managed by CHWs. Unique challenges, such as finding available charging stations and transport to patients' homes, exist. However, where functioning health programs exist, the eCompliance system can overcome these challenges.
Ultimately, the eCompliance pilot project showed that this system can help improve follow-up of TB patients in a resource-limited rural setting, thereby increasing positive TB DOTS outcomes and decreasing negative outcomes. By implementing eCompliance in resource-limited settings, the occurrence of patients lost to follow-up may be halted, which would support the goal of reducing drug resistance and minimizing TB mortality.
ACKNOWLEDGMENTS
We are grateful to Robert Mugabe, John Asiimwe Museveni, and Sam Twinomujuni for their help with the implementation of the eCompliance system. We also thank Anne Liu, Saira Qureshi, and Jilian Sacks for critical reading of the manuscript.
Disclaimer: All authors declare that they have no financial or non-financial competing interest.
Footnotes
Financial support: This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through Grant no. UL1 TR000040. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Authors' addresses: Sarah Jane Snidal, LifeNet International, Global Health Corps, Bujumbura, Burundi, E-mail: sarahjane.snidal@gmail.com. Genevieve Barnard, The Schlesinger Fund for Global Healthcare Entrepreneurship, Babson College, Wellesley, E-mail: gvbarnard@gmail.com. Emmanuel Atuhairwe, Millennium Villages Project, Mbarara, Uganda, E-mail: atuhairwe.emmanuel@gmail.com. Yanis Ben Amor, The Earth Institute, Columbia University, NY, E-mail: yba2101@columbia.edu.
A means of identifying humans through unique traits such as fingerprints.
All CHWs in Uganda are literate.
The “cured” and “treatment success” outcomes were used as the primary proportions for evaluation, “completed” is the difference of the two and provides less information about project improvement, therefore it was not separately assessed. The proportions for patients transferred out were not analyzed because of the inability to know their official treatment outcome. Finally, failure was not evaluated individually as only one patient had this outcome in the cohort.
References
- 1.WHO . Global Tuberculosis Report. Geneva, Switzerland: WHO; 2012. http://www.who.int/tb/publications/global_report/en/ Available at. Accessed June 25, 2014. [Google Scholar]
- 2.Zumla A, Raviglione M, Hafner R, von Reyn CF. Tuberculosis. N Engl J Med. 2013;368:745–755. doi: 10.1056/NEJMra1200894. [DOI] [PubMed] [Google Scholar]
- 3.Gray JM, Cohn DL. Tuberculosis and HIV coinfection. Semin Respir Crit Care Med. 2013;34:32–43. doi: 10.1055/s-0032-1333469. [DOI] [PubMed] [Google Scholar]
- 4.Lonnroth K, Jaramillo E, Williams BG, Dye C, Raviglione M. Drivers of tuberculosis epidemics: the role of risk factors and social determinants. Soc Sci Med. 2009;68:2240–2246. doi: 10.1016/j.socscimed.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 5.Zumla A, Nahid P, Cole ST. Advances in the development of new tuberculosis drugs and treatment regimens. Nat Rev Drug Discov. 2013;12:388–404. doi: 10.1038/nrd4001. [DOI] [PubMed] [Google Scholar]
- 6.Glaziou P, Falzon D, Floyd K, Raviglione M. Global epidemiology of tuberculosis. Semin Respir Crit Care Med. 2013;34:3–16. doi: 10.1055/s-0032-1333467. [DOI] [PubMed] [Google Scholar]
- 7.Ben Amor Y, Day MS, Schluger NW. Preventing the next generation of extensively drug-resistant tuberculosis. Int J Tuberc Lung Dis. 2010;14:525–527. [PubMed] [Google Scholar]
- 8.Lienhardt C, Glaziou P, Uplekar M, Lonnroth K, Getahun H, Raviglione M. Global tuberculosis control: lessons learnt and future prospects. Nat Rev Microbiol. 2012;10:407–416. doi: 10.1038/nrmicro2797. [DOI] [PubMed] [Google Scholar]
- 9.Davies PD. The role of DOTS in tuberculosis treatment and control. Am J Respir Med. 2003;2:203–209. doi: 10.1007/BF03256649. [DOI] [PubMed] [Google Scholar]
- 10.Operation ASHA Technology: The eCompliance Project. 2013. http://www.opasha.org/our-work/ecompliance-innovation-and-health/ Available at. Accessed October 11, 2013.
- 11.Newell JN, Baral SC, Pande SB, Bam DS, Malla P. Family-member DOTS and community DOTS for tuberculosis control in Nepal: cluster-randomized controlled trial. Lancet. 2006;367:903–909. doi: 10.1016/S0140-6736(06)68380-3. [DOI] [PubMed] [Google Scholar]
- 12.Wright J, Walley J, Philip A, Pushpananthan S, Dlamini E, Newell J, Dlamini S. Direct observation of treatment for tuberculosis: a randomized controlled trial of community health workers versus family members. Trop Med Int Health. 2004;9:559–565. doi: 10.1111/j.1365-3156.2004.01230.x. [DOI] [PubMed] [Google Scholar]