Abstract
The uniform multidrug therapy clinical trial, Brazil (U-MDT/CT-BR), database was used to describe and report the performance of available tools to classify 830 leprosy patients as paucibacillary (PB) and multibacillary (MB) at baseline. In a modified Ridley and Jopling (R&J) classification, considering clinical features, histopathological results of skin biopsies and the slit-skin smear bacterial load results were used as the gold standard method for classification. Anti-phenolic glycolipid-I (PGL-I) serology by ML Flow test, the slit skin smear bacterial load, and the number of skin lesions were evaluated. Considering the R&J classification system as gold standard, ML Flow tests correctly allocated 70% patients in the PB group and 87% in the MB group. The classification based on counting the number of skin lesions correctly allocated 46% PB patients and 99% MB leprosy cases. Slit skin smears properly classified 91% and 97% of PB and MB patients, respectively. Based on U-MDT/CT-BR results, classification of leprosy patients for treatment purposes is unnecessary because it does not impact clinical and laboratories outcomes. In this context, the identification of new biomarkers to detect patients at a higher risk to develop leprosy reactions or relapse remains an important research challenge.
Introduction
Leprosy is a chronic and curable infectious disease, which affects mainly skin and peripheral nerves, presenting a spectrum of clinical manifestations associated with different immune responses to Mycobacterium leprae. Because of its broad spectrum of clinical manifestations, leprosy classification is complex and may include clinical, histopathological, microbiological, and immunological features as proposed by Ridley and Jopling (R&J).1 In one extreme of the spectrum lies the polar tuberculoid leprosy form (TT) with low bacterial load, predominant cell-mediated immunity, and low or absent production of specific antibodies. The polar lepromatous form (LL) is in the other extreme, in which patients show high bacterial load and respond to infection with high production of antibodies and lower or absent M. leprae–specific cell-mediated immunity. Between the polar forms, lie the immunological and clinical unstable forms known as borderline tuberculoid (BT), borderline borderline (BB), and borderline lepromatous (BL).1
The diagnosis of leprosy remains based on the presence of clinical signs and symptoms such as the presence of skin lesions (that can vary widely in form, appearance, and color), the degree of sensory loss, and the presence of thickened peripheral nerves.2 The identification of the causative organism by slit-skin smears, histopathology, or polymerase chain reaction is not included in the routine to support leprosy diagnosis. There is restricted availability of laboratory facilities for slit-skin smear and histopathology in many endemic countries, and the current polymerase chain reaction technology is still not applicable for diagnosis. Thus, leprosy diagnosis in the field continues to be based on epidemiological and clinical evidence.
In 1981, the World Health Organization (WHO) considered multibacillary (MB) patients as those who had a bacterial index (BI) of at least two at any site in the initial skin smear. From 1988 on, MB leprosy included all smear-positive patients, as well as patients with more than five skin lesions. For operational purposes, a simplified leprosy classification system based on the number of skin lesions was proposed in the late 1990s. According to this, patients showing up to five skin lesions were considered paucibacillary (PB) and were treated with six monthly supervised doses of rifampicin and dapsone plus daily self-administered doses of dapsone. Patients with six or more skin lesions were considered MB and were prescribed with 12 monthly supervised doses of rifampicin, clofazimine, and dapsone plus daily self-administered doses of clofazimine and dapsone.3
Proper treatment is considered the key for the success of leprosy control programs, and a uniform regimen for all patients would make classification for treatment purposes unnecessary, simplifying leprosy control and benefitting patients. The “Clinical Trial for Uniform Multidrug Therapy regimen for leprosy patients in Brazil (U-MDT/CT-BR)” is an ongoing, randomized, open-label clinical trial to compare the effectiveness of the regular WHO/MB MDT regimen of 12 doses with a uniform 6 doses MB-MDT regimen with rifampicin, clofazimine, and dapsone for all leprosy patients, regardless of classification.4 Primary results showed that there was no statistical difference in the frequency of leprosy reactions between MB patients under U-MDT regimen and under the regular MB/WHO regimen.5
Several studies have shown that the bacterial load of leprosy patients correlates with the presence of IgM antibodies to M. leprae–specific phenolic glycolipid-I (PGL-I): 15–40% PB patients were seropositive, compared with 80–100% positivity among MB patients.6,7 The detection of anti-PGL-I antibodies can be a useful adjunct method to identify patients with higher bacterial load and point-of-care serological tests such as ML Flow test, performed at diagnosis can contribute to the prompt identification of MB leprosy patients,6,8,9 which are the ones with higher risk to develop reactions.10–12 This study used the U-MDT/CT-BR database to describe and report the performance of available tools to classify PB and MB leprosy patients at baseline. Anti-PGL-I serology, bacterial load, and the number of skin lesions were evaluated considering a modified R&J classification criteria as the gold standard.
Methods
Study groups.
This is a cross-sectional descriptive study that used the database of the U-MDT/CT-BR at baseline. Originally, 859 newly diagnosed, previously untreated PB and MB leprosy patients were included. Patients were recruited from March 2007 until February 2012 at two national leprosy referral centers from two Brazilian states: “Centro Dermatológico Dona Libânia” (Fortaleza, Ceará State, northeast Brazil) and “Fundação Alfredo da Matta” (Manaus, Amazonas State, north Brazil), according to the rationale and design of the study.4 In short, the number of skin lesions was registered at diagnosis, and patients were randomized and prescribed with specific treatment according to WHO classification criterion: patients presenting up to five skin lesions were considered PB leprosy and patients presenting more than five lesions were operationally classified as MB leprosy. The exact number of skin lesions was registered up to 10 lesions, and those with numerous lesions or presenting with diffuse infiltration were reported as having more than 10 lesions. In addition, slit-skin smears were taken to identify patients with a high BI. Punch skin biopsies were taken from leprosy skin lesions for histopathological analysis. A point-of-care serological test, the ML Flow,13 was also performed to detect IgM anti-PGL-I antibodies at baseline in all patients from both recruitment sites. Five patients were excluded from the analysis because their serology results were not available.
R&J classification.
For data analyses of this study, patients were classified according to a modified R&J classification system taking into account clinical features, histopathological results of skin biopsies, and the slit-skin smear bacterial load; Mitsuda test and BI of the skin biopsy were not performed. Patients classified according to this R&J system as TT or BT were merged as PB leprosy, whereas BB, BL, or LL patients were grouped as MB leprosy, and this classification was referred as MB or PB based on R&J classification. Twenty-four patients classified as indeterminate leprosy were excluded from the analyses. Therefore, after exclusions (5 because of unavailable ML Flow results and 24 indeterminate leprosy cases) a total of 830 leprosy patients were included in this study.
Statistical analyses.
Descriptive analyses of clinical and epidemiological variables and their frequencies were performed. The agreement between results of different classification methods was calculated by cross tabulation and results were expressed as percentage with the graduated kappa index (κ): low (0–0.5), moderate (0.51–0.75), and excellent (0.76–1.0).14 For these analyses, sensitivity was defined as the capacity to correctly detect MB cases among leprosy patients and the specificity was defined as the ability to correctly exclude PB patients. These parameters were calculated and the corresponding receiver operating characteristic (ROC) curves were plotted to assess the performance of each method to correctly classify leprosy patients compared with the R&J classification. Data analysis was performed with SPSS version 21.
Ethical aspects.
The U-MDT/CT-BR study was designed under the international (Helsinki) and Brazilian research regulations and was approved by three regional ethical committees from all the states involved and by the National Ethics Commission of Research (CONEP) of the National Health Council, Ministry of Health (protocol #12949, approval #631/2006). All individuals enrolled had signed the informed consent and were not exposed to any risk or danger as a result of the study. For patients under the age of 18 years, one of the parents or the legal guardian signed the informed consent.
Results
Main characteristics of enrolled leprosy patients at baseline.
A total of 830 leprosy patients were included: 660 (79.5%) were from Ceará State, northeast Brazil (Dona Libânia referral center) and 170 (20.5%) were from Amazonas State, north region (Alfredo da Matta referral center). The median age of participants was 40 years (range 6–75 years), and 60.2% (500/830) were male.
On the basis of the number of skin lesions, 78.8% (669/830) patients were considered to have MB leprosy, and 21.2% (161/830) were classified as PB leprosy patients. Among MB cases, 398 (46.6%) patients presented 11 or more skin lesions and 118 (13.8%) presented diffuse infiltration. On the basis of R&J classification, 52.2% (486/830) patients were merged as MB group (BB, BL, and LL forms) and 41.4% (344/830) were merged as PB group (BT and TT). When groups were merged, 187 BT patients with more than 5 skin lesions fell into the PB category. Slit-skin smears were positive in 59.2% (491/830) patients.
Performance of different methods of classification compared with R&J system.
Clinical and laboratory features of patients were compared with the R&J classification (Table 1). Serological method, number of skin lesions, and slit-skin smear results showed a tendency of higher positivity toward the MB pole. A gradual increase in the number of registered skin lesions, serology, and slit-skin smears positivity was observed from TT to BL/LL forms. BL and LL forms showed comparable results regarding seropositivity, number of skin lesions, and positive slit-skin smears. In the BT group, 63.4% presented 6 or more skin lesions whereas 10.5% had positive BI and 32.5% had positive ML Flow tests.
Table 1.
Clinical and laboratorial features according to R&J classification of leprosy patients
| R&J classification | Number of lesions (mean/SD) | > 6 lesions N (%) | Slit skin smear N positive (%) | ML Flow N positive (%) |
|---|---|---|---|---|
| TT | 1.2 (0.49) | 0 (0) | 0 (0) | 5 (10.2) |
| BT | 6.3 (3.78) | 187 (63.4) | 31 (10.5) | 96 (32.5) |
| BB | 9.9 (1.99) | 154 (97.5) | 134 (84.8) | 125 (79.1) |
| BL | 10.8 (0.80) | 189 (100) | 187 (98.9) | 172 (91.0) |
| LL | 10.8 (0.80) | 139 (100) | 139 (100) | 127 (91.4) |
BB = borderline borderline; BL = borderline lepromatous; BT = borderline tuberculoid; LL = lepromatous; SD = standard deviation; TT = tuberculoid.
Considering the R&J classification system as gold standard (Table 2), ML Flow tests correctly allocated 70% patients in the PB group and 87% in the MB group. The classification based on counting the number of skin lesions correctly allocated 46% PB patients and 99% MB leprosy cases. Slit-skin smears properly classified 91% and 97% PB and MB patients, respectively.
Table 2.
Positivity to different classification methods according to R&J classification
| R&J classification* | |||
|---|---|---|---|
| PB (%) | MB (%) | ||
| ML Flow test | Negative | 243 (70) | 62 (13) |
| Positive | 101 (30) | 424 (87) | |
| Operational classification | PB | 157 (46) | 4 (1) |
| MB | 187 (54) | 482 (99) | |
| Bacterial index | Negative | 313 (91) | 26 (5) |
| Positive | 31 (9) | 460 (95) | |
MB = multibacillary; PB = paucibacillary.
TT (tuberculoid) and BT (borderline tuberculoid) patients were grouped as PB. BB (borderline borderline), BL (borderline lepromatous), and LL (lepromatous) patients were grouped as MB. Indeterminate leprosy patients were excluded.
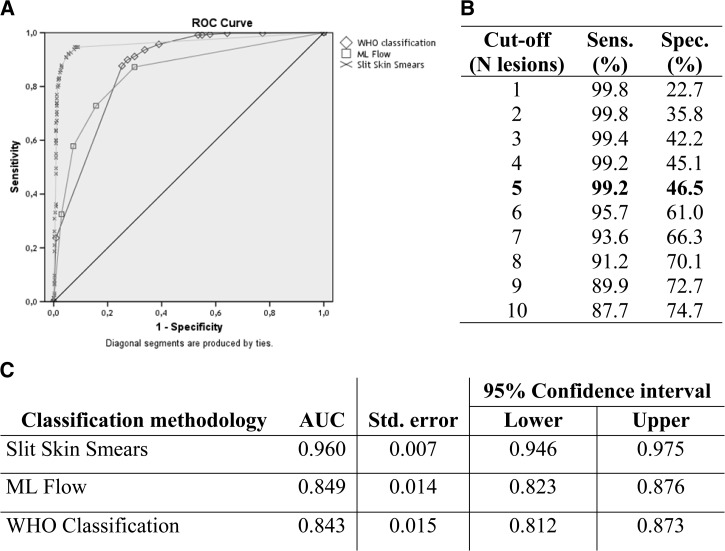
For the PB and MB classification, slit-skin smear results showed the highest area under the curve (AUC) followed by the ML Flow tests and by the number of skin lesions (Figure 1). The WHO classification criterion showed the highest sensitivity to detect MB cases but the lowest specificity among PB patients, which lowered the positive predictive value (PPV) of this criterion. All performance parameters are presented in Table 3. The ROC curve shows a sensitivity of 99.2% and specificity of 46.5% when using the cut-off of 5 lesions as the WHO recommendation. The best performance of the WHO classification criterion to discriminate MB and PB leprosy was seen using the threshold of 10 lesions with a sensitivity of 87.7% and specificity of 74.7%.
Figure 1.
Receiver operating characteristic (ROC) curve for all classification methods evaluated. In (A), plotted ROC curve for World Health Organization (WHO) classification criteria (diamonds), slit skin smears (crosses), and ML Flow test (squares). In (B), parameters of sensitivity and specificity for different cut-off values for WHO classification criterion and in (C), area under the curve and standard error for all classification methods.
Table 3.
Performance parameters for classification methods compared with the R&J classification
| ML Flow | WHO classification | Bacterial index | |
|---|---|---|---|
| Sensitivity | 87.2 | 99.2 | 94.7 |
| Specificity | 70.6 | 45.7 | 91.0 |
| PPV | 80.8 | 72.0 | 93.7 |
| NPV | 79.7 | 97.5 | 92.3 |
| LR+ | 2.97 | 1.82 | 10.5 |
| LR− | 0.18 | 0.02 | 0.06 |
LR+ = positive likelihood ratio; LR− = negative likelihood ratio; NPV = negative predictive value; PPV = positive predictive value; WHO = World Health Organization.
Discussion
This study used a well-characterized databank of baseline characteristics of patients enrolled in the U-MDT/CT-BR trial to describe the performance of different tools to classify leprosy patients. At present, the classification of leprosy patients into PB and MB orients both the duration of treatment and the drug regimen. However, there is a concern that misclassification might lead to increased risk of relapse because of insufficient treatment of MB patients, moreover misclassification may also lead to overtreatment of PB patients with drugs that present severe side effects. In this context, implementation of a uniform regimen for all leprosy patients would make classification for treatment purposes unnecessary, simplifying leprosy control and benefitting patients.4 Nevertheless, it is known that patients with high BI have a higher risk of developing reactions,10–12 but besides the high BI other unknown factors play a role in the susceptibility to leprosy reactions. Previous results of U-MDT/CT-BR trial showed that there is no statistical difference between U-MDT or regular-MDT (R-MDT) treatment regimen regarding frequency of reactions among MB patients, even when BI was stratified as BI ≥ or < 3.0.5
When the results were analyzed taking into account the ROC curve values to asses sensitivity and specificity of different approaches to properly allocate PB and MB leprosy, the highest AUC was obtained by slit-skin smear's BI, but this finding was expected by definition because MB and PB classification depends on the BI. However, reliable services with enough well-trained staff and using high standards for collecting, staining, and reading smears were hardly available. Considering the public health perspective, to reduce the number of MB patients misclassified as PB, the classification methods must prioritize sensitivity rather than specificity. In this study, the most sensitive approach to identify MB leprosy was represented by counting the number of skin lesions, which identified 99% MB leprosy patients, but this method showed the lowest specificity (46%). Nevertheless, there are benefits and limitations in counting the number of skin lesions for classification of PB and MB leprosy. Although it is easy and simple to apply it in the field by general health workers, there is always the risk of a certain proportion of patients being either under- or overtreated.
In this study, 63.4% patients classified as BT presented more than 5 skin lesions and therefore would be considered over treated as MB by WHO classification. INFIR, a cohort study from India designed to study risk factors for leprosy reactions among 303 newly diagnosed MB leprosy patients, showed that 43%MB patients were classified as BT leprosy by clinical signs. However, when clinical and histopathological data were compared, 20% of these biopsies showed only minimal inflammation pointing out that WHO MB classification is very heterogeneous including either patients immunologically active with no detectable bacilli or patients with high bacillary load. The INFIR study pointed out the important role that pathologists in leprosy centers play supporting and helping clinicians with difficult cases.10
This study indicated that slit-skin smear results, serological tests, and the WHO classification criterion were consistent with the R&J classification. We acknowledge that all classification approaches investigated herein had limitations. However, the bacillary load, the number of skin lesions, and the humoral immune response were directly correlated as they gradually increased toward the LL pole of the spectrum of the disease. Similar results have been reported by other groups.6
MB and PB leprosy classification has been used to guide treatment and to alert physicians regarding risks of reaction, that is, which patients to monitor more closely enabling earlier identification of reaction and nerve injury, which were crucial for earlier interventions and better outcomes. However, previous results of U-MDT/CT-BR have strongly shown that both MB and PB patients have had similar clinical (reactions and relapses/reinfection) and laboratories (BI decrease) outcomes regarding the frequency of leprosy reactions. Moreover, similar BI decrease has been observed among MB patients under U-MDT and regular MDT, and relapse rates in both groups were being monitored by longer follow-up.15 It is important to point out that U-MDT results have shown high efficacy for both PB and MB patients. In leprosy, the risk of clinical complications may also be associated with host genetic susceptibility. From a public health perspective, it remains important to identify, among PB and MB leprosy patients, the ones at a higher risk to develop reactions and nerve injuries. In this context, new biomarkers capable of detecting patients with higher risk of complications are needed.
ACKNOWLEDGMENTS
We thank all patients for donating their blood and information for these studies and all medical staff involved in sample and data collection.
Disclaimer: The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The NT-P-BSA used for the production of the ML Flow tests was kindly provided by Fujiwara, Institute for Natural Science, Nara University, Nara, Japan.
Footnotes
Financial support: Rodrigo Scaliante Moura was a recipient of a scholarship from the National Council for Scientific and Technological Development/CNPq, Brazil (grant #152678/2011-5) and Ludimila Paula Vaz Cardoso was supported by a fellowship from the Coordination for the Improvement of Higher Education Personnel/CAPES, Brazil (grant #02479/09-5). Mariane Martins De Araújo Stefani is a recipient of a fellowship from CNPq (grant # 304869/2008-2). U-MDT/CT-BR was funded by the Department of Science and Technology (DECIT) of Brazilian Ministry of Health and CNPq (grant # 40.3293/2005-7).
Authors' addresses: Rodrigo Scaliante Moura, Ludimila Paula Vaz Cardoso, Mariane Martins de Araújo Stefani, and Samira Bührer-Sékula, Departamento de Imunologia, Instituto de Patologia Tropical e Saúde Pública, Sala 335, Rua 235 s/n, Setor Leste Universitário Goiânia, Goias, Brazil, E-mails: rodrigoscaliant@gmail.com, ludimilacardoso@gmail.com, mmastefani@gmail.com, samira@buhrer.net, and samira.buhrer@gmail.com. Gerson Oliveira Penna, Núcleo de Medicina Tropical, Campus Universitário Darci Ribeiro, Universidade de Brasília, Brasília–DF, E-mail: gpenna@gpenna.net. Maria Araci de Andrade Pontes and Heitor de Sá Gonçalves, Centro de Referência Nacional Dona Libânia–CDERM, Rua Pedro I, Centro, Fortaleza, Brazil–CE, E-mails: maracipontes@gmail.com and heitorsg@terra.com.br. Rossilene Cruz, Centro de Referência Nacional Alfredo da Matta–FUAM, Avenida Codajás, Cachoeirinha, Manaus–AM. Maria Lúcia Fernandes Penna, Centro de Ciências Médicas, Instituto de Saúde da Comunidade, Universidade Federal Fluminense, Rua Marques do Paraná, Centro, Niteroi, Brazil–RJ, E-mail: mlfpenna@gmail.com.
References
- 1.Ridley DS, Jopling WH. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966;34:255–273. [PubMed] [Google Scholar]
- 2.WHO . WHO Expert Committee on Leprosy: Eighth Report. Geneva, Switzerland: WHO; 2012. [Google Scholar]
- 3.ILA Report of the International Leprosy Association Technical Forum. Paris, France, February 22–28, 2002: the diagnosis and classification of leprosy. Int J Lepr Other Mycobact Dis. 2002;70((Suppl 1)):S23–S31. [PubMed] [Google Scholar]
- 4.Penna GO, Pontes MAA, Cruz R, Goncalves HS, Penna MLF, Bührer-Sékula S. A clinical trial for uniform multidrug therapy for leprosy patients in Brazil: rationale and design. Mem Inst Oswaldo Cruz. 2012;107((Suppl 1)):22–27. doi: 10.1590/s0074-02762012000900005. [DOI] [PubMed] [Google Scholar]
- 5.Penna MLF, Buhrer-Sékula S, Pontes MAA, Cruz R, Gonçalves HDS, Penna GO. Primary results of clinical trial for uniform multidrug therapy for leprosy patients in Brazil (U-MDT/CT-BR): reactions frequency in multibacillary patients. Lepr Rev. 2012;83:308–319. [PubMed] [Google Scholar]
- 6.Moura RS, Calado KL, Oliveira ML, Bührer-Sékula S. Leprosy serology using PGL-I: a systematic review. Rev Soc Bras Med Trop. 2008;41((Suppl 2)):11–18. doi: 10.1590/s0037-86822008000700004. [DOI] [PubMed] [Google Scholar]
- 7.Oskam L, Slim E, Bührer-Sékula S. Serology: recent developments, strengths, limitations and prospects: a state of the art overview. Lepr Rev. 2003;74:196–205. [PubMed] [Google Scholar]
- 8.Parkash O, Kumar A, Pandey R, Nigam A, Girdhar BK. Performance of a lateral flow test for the detection of leprosy patients in India. J Med Microbiol. 2008;57:130–132. doi: 10.1099/jmm.0.47488-0. [DOI] [PubMed] [Google Scholar]
- 9.Bobosha K, Tjon Kon Fat EM, van den Eeden SJF, Bekele Y, van der Ploeg-van Schip JJ, de Dood CJ, Dijkman K, Franken KLMC, Wilson L, Aseffa A, Spencer JS, Ottenhoff THM, Corstjens PLAM, Geluk A. Field-evaluation of a new lateral flow assay for detection of cellular and humoral immunity against Mycobacterium leprae. PLoS Negl Trop Dis. 2014;8:e2845. doi: 10.1371/journal.pntd.0002845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lockwood DNJ, Nicholls P, Smith WCS, Das L, Barkataki P, van Brakel W, Suneetha S. Comparing the clinical and histological diagnosis of leprosy and leprosy reactions in the INFIR cohort of Indian patients with multibacillary leprosy. PLoS Negl Trop Dis. 2012;6:e1702. doi: 10.1371/journal.pntd.0001702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar B, Dogra S, Kaur I. Epidemiological characteristics of leprosy reactions: 15 years experience from north India. Int J Lepr Other Mycobact Dis. 2004;72:125–133. doi: 10.1489/1544-581X(2004)072<0125:ECOLRY>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 12.Baohong J. Does there exist a subgroup of MB patients at greater risk of relapse after MDT? Lepr Rev. 2001;72:3–7. doi: 10.5935/0305-7518.20010003. [DOI] [PubMed] [Google Scholar]
- 13.Bührer-Sékula S, Smits HL, Gussenhoven GC, van Leeuwen J, Amador S, Fujiwara T, Klatser PR, Oskam L. Simple and fast lateral flow test for classification of leprosy patients and identification of contacts with high risk of developing leprosy. J Clin Microbiol. 2003;41:1991–1995. doi: 10.1128/JCM.41.5.1991-1995.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 15.Penna MLF, Buhrer-Sékula S, Pontes MAA, Cruz R, Gonçalves HS, Penna GO. Results from the clinical trial of uniform multidrug therapy for leprosy patients in Brazil (U-MDT/CT-BR): decrease in bacteriological index. Lepr Rev. 2014;85:262–266. [PubMed] [Google Scholar]