Electrical synapses have a rich and sometimes controversial history. During the early 20th century, the question of whether chemical or electrical synapses underlie the main mode of signaling in the mammalian central nervous system (CNS) was hotly debated (1). However, after the discovery of neuronal inhibition in the early 1950s, it was accepted that transmission of information via neurotransmitter molecules (chemical synapses) represents the major form of signaling among CNS neurons. Although a consensus developed that electrical synapses are present in a subset of neuronal connections, it was unknown whether these synapses exhibit selectivity and plasticity as shown for most chemical synapses, or whether neuronal activity can result in long-term change of synaptic strength of electrical synapses (2) in the mammalian CNS. On page 389 of this issue, Haas et al. (3) demonstrate that the strength of electrical synapses among specific neurons in the thalamus of the mammalian brain affects long-term depression (LTD), a process important for learning and memory. Given the proposed role that electrical synapses play in synchronizing neuronal activity, these findings suggest a mechanism for controlling the coordination of neuronal activity.
Chemical synapses selectively connect different types of neurons to provide a specific path of communication among CNS neurons (4, 5). It was discovered more than 10 years ago that electrical synapses are formed with exquisite selectivity among specific classes of inhibitory neurons (6, 7). Moreover, another hallmark of synapses—modulation by neurotransmitters—has been also demonstrated in both the thalamus and hippocampus (8, 9).
To investigate the effects of neuronal activity on the strength of electrical synapses in the mammalian CNS, Haas et al. recorded electrical activity from pairs of neurons in the rat thalamic reticular nucleus (TRN). All sensory messages are conveyed to the cortex via the thalamus (see the figure) (10). The TRN is located between the thalamus and the cortex and, unlike other groups of thalamic neurons (thalamic nuclei), is composed of inhibitory neurons that are connected by electrical synapses (11). These electrical synapses, together with inhibitory chemical synapses (those that transmit γ-aminobutyric acid), can synchronize firing of TRN neurons. Because TRN neurons project to other thalamic nuclei, synchronized activity of TRN neurons can entrain the activity of other thalamic regions (10).
Figure. Depression of electrical synapses.
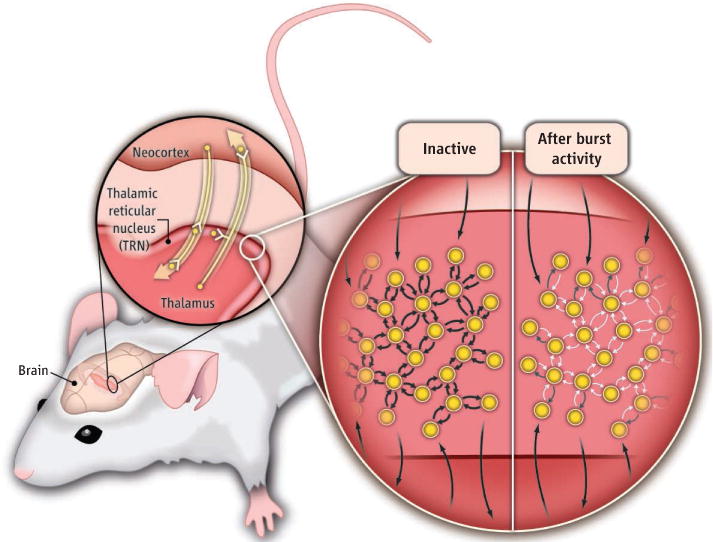
(Enlarged view, left side) Inhibitory neurons (circles) in the mammalian thalamic reticular nucleus (TRN) are connected via electrical (small black arrows) and inhibitory chemical synapses (not illustrated). External excitatory inputs impinge on TRN neurons and can activate them. (Enlarged view, right side) When TRN neurons generate bursts of action potentials, electrical synapse strength decreases. The decrease of strength can be asymmetrical between cells that do not generate bursts (white arrows) and those that do (gray arrows).
To measure the strength of electrical coupling, Haas et al. injected depolarizing current into one TRN neuron and measured the response in both the injected cell and the noninjected neuron. The ratio of the two electrical responses is defined as the coupling coefficient (cc). The coupling coefficient among TRN neurons is large (indicating strong electrical coupling), and often a burst of electrical activity (action potential) in one neuron will produce action potential in the coupled neuron. The coupling coefficient can be estimated in two ways: by injecting current in cell 1 (i.e., cc12) or by injecting current in cell 2 (i.e., cc21). Interestingly, Haas et al. found that a substantial number of neuron pairs exhibited asymmetrical coupling. That is, the distribution of the ratio cc1/cc2 was skewed. In vivo, TRN neurons often fire bursts of electrical activity (spikes). The authors found that bursts of spikes in pairs of coupled TRN neurons resulted in LTD of the strength of electrical synapses. Burst activity in both neurons produced symmetrical LTD such that cc12 = cc21. However, when cell 1 was induced to burst while the coupled cell (cell 2) was prevented from bursting (by current injection), the resulting LTD was asymmetrical. Under these conditions, the coupling measure from cell 1 to cell 2 (cc12) decreased much less than the coupling from the inactive cell (cc21) that was prevented from bursting. Although Hass et al. observed a modest decrease of synaptic strength, they show that LTD of electrical coupling is sufficient to prevent propagation of bursting activity among neurons.
What are the mechanisms underlying activity-dependent LTD of electrical synapses? The results of Haas et al. suggest that sodium-dependent action potentials may play a role (in response to a stimulus, sodium channels open and allow sodium into the neuron, which triggers the firing of an action potential). In addition, an increase in cytoplasmic calcium concentration could activate a protein kinase that modifies (by phosphorylation) gap junction channels, which bridge electrically coupled neurons. Alternatively, partial replacement of nonrectifying gap junctions with rectifying gap junctions (thus allowing electrical current to pass preferentially in one direction) is a possible mechanism.
The functional consequences of LTD produced by electrical synapses are of great interest. By producing asymmetrical changes of electrical coupling, the outbound connection of a group of bursting neurons will be stronger than the inbound connection from coupled neurons that did not burst. The functional consequences resulting from the generation of asymmetry of electrical synapses within the TRN remains to be explored. The impact of reducing the strength of electrical synapses at the circuit level and their potential effect on rhythmic activity in the TRN is also of interest. In vivo, TRN neurons can switch between burst and tonic firing modes (10). If these activities can induce LTD, one may expect to find changes in the strength of electrical coupling in the TRN that correlate with different behavioral states during sleep and wakefulness.
Footnotes
The strength of electrical synapses in the mammalian brain can be modulated by neuronal ativity.
References
- 1.Cowan WM, Kandel ER. In: Synapses. Cowan WM, Südhof TC, Stevens CF, editors. Johns Hopkins Univ Press; Baltimore: 2001. pp. 1–87. [Google Scholar]
- 2.Pereda AE, et al. Proc Natl Acad Sci USA. 1998;95:13272. doi: 10.1073/pnas.95.22.13272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haas JS, Zavala B, Landisman CE. Science. 2011;334:389. doi: 10.1126/science.1207502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown SP, Hestrin S. Curr Opin Neurobiol. 2009;14:415. doi: 10.1016/j.conb.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ko H, et al. Nature. 2011;473:87. doi: 10.1038/nature09880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galarreta M, Hestrin S. Nature. 1999;402:72. doi: 10.1038/47029. [DOI] [PubMed] [Google Scholar]
- 7.Gibson JR, Beierlein M, Connors BW. Nature. 1999;402:75. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- 8.Landisman CE, Connors BW. Science. 2005;310:1809. doi: 10.1126/science.1114655. [DOI] [PubMed] [Google Scholar]
- 9.Zsiros V, Maccaferri G. J Neurosci. 2008;28:1804. doi: 10.1523/JNEUROSCI.4616-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sherman SM, Guillery RW. In: The Synaptic Orgnaization of the Brain. 5. Shepherd GM, editor. Oxford Univ Press; Oxford: 2004. pp. 311–359. [Google Scholar]
- 11.Landisman CE, et al. J Neurosci. 2002;22:1002. doi: 10.1523/JNEUROSCI.22-03-01002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


