Abstract
Evening chronotype, a correlate of delayed circadian rhythms, is associated with depression. Altered positive affect (PA) rhythms may mediate the association between evening chronotype and depression severity. Consequently, a better understanding of the relationship between chronotype and PA may aid in understanding the etiology of depression. Recent studies have found that individuals with evening chronotype show delayed and blunted PA rhythms, although these studies are relatively limited in sample size, representativeness, and number of daily affect measures. Further, published studies have not included how sleep timing changes on workday and non-workdays, or social jet lag (SJL), may contribute to the chronotype-PA rhythm link. Healthy non-depressed adults (n = 408) completed self-report affect and chronotype questionnaires. Subsequently, positive and negative affect were measured hourly while awake for at least two workdays and one non-workday by ecological momentary assessment (EMA). Sleep variables were collected via actigraphy and compared across chronotype groups. A cosinor variant of multilevel modeling was used to model individual and chronotype group rhythms and to calculate two variables, (1) amplitude of positive affect, or the absolute amount of daily variation from peak to trough during one period of the rhythm, and (2) acrophase, or the time at which the peak amplitude of affect rhythms occurred. On workdays, individuals with evening chronotype had significantly lower PA amplitudes and later workday acrophase times than their morning type counterparts. In contrast to predictions, SJL was not found to be a mediator in the relationship between chronotype and PA rhythms. The association of chronotype and PA rhythms in healthy adults may suggest the importance of daily measurement of PA in depressed individuals and would be consistent with the hypothesis that evening chronotype may create vulnerability to depression via delayed and blunted PA rhythms.
Introduction
A large body of evidence implicates abnormal circadian rhythms in mood disorders (Germain & Kupfer, 2008; McClung, 2013). Specifically, researchers have found blunted and delayed biological rhythms in those who are depressed (Claustrat et al., 1984; Daimon et al., 1992; Lewy et al., 2006; Posener et al., 2000). Although definitive evidence has yet to be reported, abnormal circadian rhythms may be etiologically important, with several hypotheses invoking shifted (Lewy et al., 2006) and blunted rhythms (Souętre et al., 1989; Murray et al., 2002) in the development of depression. Contributing to this body of literature, several studies have now focused on chronotype, a self-report measure of activity preference timing and a proxy for circadian phase (Duffy et al., 2001). Depression diagnosis and depressive symptoms are greatest in evening types, or those who are most active in the evening hours (Chelminski et al., 1999; Drennan et al., 1991; Hirata et al., 2007; Levandovski et al., 2011). However, the mechanism linking evening chronotype and depression remains poorly understood.
Recent research suggests that positive affect (PA) may mediate the relationship between evening chronotype and depression (Hasler et al., 2010). Although negative affect (NA) has been shown to be high in depression and other mood disorders, PA is low in depression and not anxiety (Watson et al., 1988), indicating its potential specificity in the development or maintenance of depression. Further, PA has been shown to have an endogenous circadian rhythm (Boivin et al., 1997; Murray et al., 2009), a pattern not consistently found in NA. This variation in PA has been proposed as a reflection of the underlying behavioral approach system (Watson et al., 1999), which may encourage engagement with the environment at adaptive times during the day. Therefore, individual variation in PA peak timing (acrophase) and maximal variation (amplitude) may directly impact one's likelihood to engage with the environment and acquire reward from their surroundings (e.g. social interaction). Given that behavioral models of depression implicate decreased engagement with the social environment (e.g. Lewinsohn & Atwood, 1969), abnormal delayed and blunted PA rhythms may occasion a lesser opportunity for behavioral activation and associated vulnerability to depression. Therefore, understanding predictors of variation in PA rhythms (see Figure 1A) in a healthy sample may inform our understanding depression vulnerability.
Figure 1.
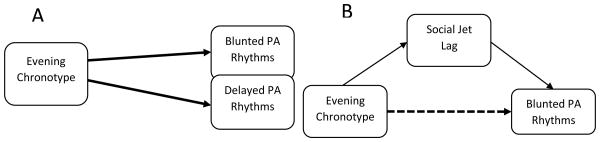
Depiction of the study model. A. Depicts the main hypotheses predicting evening chronotype leading to blunted and delayed positive affect (PA). B. Depicts additional study aim of investigating social jet lag as a potential mediator in the relationship between evening chronotype and blunted PA rhythms.
Preliminary evidence suggests that PA rhythms vary by chronotype, with evening chronotypes exhibiting more delayed acrophase (Hasler et al., 2012; Porto et al., 2006) and blunted amplitude (Hasler et al., 2012). However, previous studies are limited in generalizability, given their unique samples (insomnia, Hasler et al., 2012; medical students, Porto et al., 2006), sample size (N = 100, Hasler et al., 2012; N = 28, Porto et al., 2006), and fewer daily PA measurements, with some studies measuring PA every three hours of wakefulness (Porto et al., 2006) and others taking four daily measurements (Hasler et al., 2012). Therefore, the present study sought to use a larger, more generalizable sample, with PA measures every hour of wakefulness to more accurately estimate the acrophase and amplitude of the daily PA rhythm in an effort to better understand the relationship between chronotype and PA rhythms.
Although the connection between evening chronotype, representing delayed rhythms, and delayed PA rhythms may be more obvious, the current study additionally aims address an explanation for the association between evening chronotype and blunted PA rhythms. One potential mechanism may be social jet lag (SJL; Wittmann et al., 2006; Roenneberg et al., 2012), in which individuals who naturally have delayed rhythms experience a misalignment in circadian and sleep rhythms when they are forced to wake at earlier times on workdays. Social jet lag is indexed by the degree of discrepancy between work and non-work day sleep timing. Given that desynchronized rhythms are thought to create blunted rhythms (Souętre et al., 1989), the misalignment between circadian processes and sleep associated with SJL may be related to blunted PA rhythms. Those who have naturally delayed rhythms, like those with evening chronotype, tend to experience the greatest SJL and related circadian misalignment, and hypothetically would also exhibit the largest decrement in PA amplitude. Therefore, SJL may explain an association between chronotype and PA amplitude (see Figure 1B). In support of this theory, SJL has been associated with evening chronotype and increased vulnerability to depression (Wittmann et al., 2006; Levandovski et al., 2011; Hasler et al., 2012), though no study has previously investigated a potential SJL-PA rhythm association. Therefore, the current study additionally investigated whether SJL mediates the relationship between evening chronotype and blunted PA rhythms.
To further elucidate the relationship between chronotype and PA rhythms, the current study aimed to extend existing literature on the association between chronotype and PA rhythms in a large, healthy non-depressed sample of middle-aged adults. Additionally we aimed to explore SJL as a potential mediator in the relationship between chronotype and PA amplitude. To this end, hourly measures of PA were collected over an average of 3 workdays and one non-workday and were used to estimate individual and chronotype-related PA rhythms. Additionally, actigraphy was used to measure sleep variables in order to calculate SJL. It was predicted that individuals with evening chronotype would exhibit decreased PA amplitude and delayed acrophase relative to their morning type counterparts and that SJL would mediate the relationship between chronotype and PA amplitude.
Materials and Methods
Participants
Participants were adults (N = 486) from the greater Pittsburgh, Pennsylvania area in the Adult Health and Behavior II project registry (AHAB II). Individuals were excluded if they were taking antidepressant medications or any psychoactive drugs that might alter their response to questionnaire measures, or if they reported a history of schizophrenia, major neurological disorders or other psychiatric illness. Further, all night shift workers were excluded from the study. Participants were excluded (n = 44) from the current analyses if they met DSM-IV (APA, 1994) criteria for current or past mood disorders as assessed with the Mini International Neuropsychiatric Interview, Version 6.0 (M.I.N.I. 6.0; Sheehan, et al., 1997). Additionally, 2 participants did not complete the chronotype questionnaire and 32 additional participants did not have actigraphy data due to non-compliance or lack of sufficient data (a workday and non-workday) to calculate SJL. The total sample consisted of 408 participants. Informed consent was obtained in accordance with the guidelines of the University of Pittsburgh Institutional Review Board and ethical guidelines established by the journal were met (Portaluppi et al., 2010).
Ecological Momentary Assessment (EMA) and Actigraphy Protocol
EMA and actigraphy data collected as part of the larger AHAB II study were used to assess diurnal variation in PA. Actigraphy data was collected in the field (i.e., non-laboratory, home and work environments) for seven to ten monitoring days via an Actiwatch-16 (Respironics, PA). Additionally, four of the days in the field were EMA monitoring days (three workdays and one non-workday). During waking hours on EMA measurement days, participants were signaled hourly to complete a 43-item questionnaire (including affect items described below) using a personal digital assistant (PDA; Palm Z22, software: Satellite Forms). Participants received extensive training and practice using the PDA and received feedback on compliance following a practice day. Additionally, participants were phoned four times throughout their time in the field for technical support. All participant data were used in analyses given that all completed at least two workdays and one non-workday worth of measurements. Importantly, 92% of the sample completed three workdays and one non-workday of EMA monitoring.
Measures
Positive and Negative Affect
PA and negative affect (NA) were collected in two distinct manners. First, the Positive Affect Negative Affect Schedule-Expanded Form (PANAS-X; Watson & Clark, 1999) trait measure of affect was administered during a laboratory visit. Second, an adapted version of the Positive Affect Negative Affect Schedule Short Form (PANAS-SF; Thompson, 2007) using a six point scale to measure hourly endorsement of 13 affect items was administered by EMA every hour since wake time during the participation period. The items “ashamed,” “active,” and “alert” were deleted a priori from the scale due to rotated principal components analysis performed on previous samples, which revealed low factor loading on these items. Additional items, “happy” and “cheerful” from the Profile Of Mood States scale (McNair et al., 1981) were added to represent PA terms with low arousal associations. The resulting survey included “inspired,” “determined,” “attentive,” “happy,” and “cheerful” items in the PA scale. Additional to the NA items “upset,” “hostile,” “nervous,” and “afraid” from PANAS-SF, “angry,” “lonely,” and “sad” items were added a priori from the PANAS-X in order to include items which measured sadness and anger as well as anxiety. Amplitude and phase data calculated based on hourly measures of PA were used as the dependent variable in the primary analyses.
Chronotype
The Composite Scale of Morningness (CSM; Smith et al., 1989) was used to assess chronotype during a laboratory visit. The CSM scale is a continuous measure based on 13 items assessing timing preferences for activity and sleep. Higher scores indicate a preference for morning activity and early bed and wake times (i.e., morningness) and lower scores indicate a preference for evening activity and later bed and wake times (i.e., eveningness). Group chronotype analyses were based on pre-established cutoffs (Natale & Alzani, 2001): 13–26 evening-type; 27–41 intermediate-type; 42–55 morning type. The scale has acceptable internal consistency (Cronbach's α = .83; Smith et al., 1989) and good test-retest reliability and predictive validity (Guthrie et al., 1995; Greenwood, 1994). CSM score was used as the independent variable in the primary analyses.
Sleep Measures
Actigraphy data were collected for at least 7 nights (including at least 4 workdays and 1 non-workday). All available nights were used in calculations. Actigraphy data were stored in 1-minute epochs and scored with Actiware software (v5.59) using automated, standard medium thresholds. Sleep onset was defined as a period with fewer than 40 activity counts per 1-minute epoch over 10 consecutive epochs. Wake onset was defined as 10 consecutive minutes of greater than or equal to 40 activity counts per epoch. Average workday and non-workday mid-sleep point and sleep duration were calculated. As described by Wittmann et al. (2006), the SJL variable was calculated by taking the absolute value of the difference between mid-sleep on non-workdays and mid-sleep on workdays. For the present study, mid-sleep on non-workdays (M = 5 days) was subtracted from the average mid-sleep time across workdays (M = 2 days).
Primary Analyses
Chronotype Group Analysis of PA Rhythms
Because of the effects of work constraints on activity rhythms, all PA analyses were conducted separately for workdays and non-workdays. Preliminary analyses using a cosinor analysis variant of multilevel modeling (MLM) was used to confirm the presence of diurnal rhythms in PA and to quantify the effect of chronotype group (morning, intermediate, or evening chronotype group) on PA rhythm amplitude and acrophase. In this type of MLM, cosinor analysis was used at level one of the model to fit PA data to a sinusoidal curve with a 24 hour period through regression on a sine wave. A cosine term was added to the model to account for phase shifts in the diurnal rhythms resulting in the following equation:
To complete the MLM, chronotype, age, and gender were included as fixed effects, along with random effects for individual participants within chronotype groups. Chronotype group-based estimates of PA rhythm amplitude and acrophase were obtained via transformations of the fitted MLM parameter estimates. The delta method was then used to approximate standard errors of estimated amplitudes and acrophases among chronotype groups, as well as standard errors of differences in estimated amplitudes and acrophases between chronotype groups (Mikulich et al., 2003). This approach allowed for Wald-based inference on the mean 24-hour diurnal rhythmic parameters (amplitude and acrophase) for each group, in addition to Wald-based inference on the difference in mean 24-hour diurnal rhythmic parameters between groups. The cosinor MLM was fit using maximum likelihood estimation, and all analyses were performed using SAS, Version 9.3 (SAS Institute Inc., Cary, NC). The morning chronotype was chosen as the reference group to which all other group effects are compared. Subsequent analyses using individual level PA amplitude and acrophase values are described next.
Individual Analyses of PA Rhythms
In order to test for the impact of SJL on chronotype and to better understand how rhythm estimates contribute to our understanding of chronotype, individual PA amplitude and acrophase were also extracted from the EMA data for each study participant using similar statistics as in the above group analyses. Two Ordinary Least Squares (OLS) regression models were constructed using chronotype score to predict individual PA amplitude and individual PA acrophase to investigate whether the individual acrophase and amplitude analysis replicated chronotype group comparisons.
Social Jet Lag Analysis
Given the prediction that SJL may mediate the relationship between chronotype and PA rhythm estimates, regression models, including study covariates, were created testing each pathway. In the instances in which pathways were significant, we tested the direct effects of chronotype on PA rhythm through social jetlag effects.
Secondary Analyses
Chronotype Groups and NA Rhythms
The current literature is inconsistent in finding significant NA diurnal rhythms, with some studies detecting NA rhythmicity (Stone et al., 2006; Vittengl et al., 1998, Golder & Macy, 2011) and others not (Murray, 2007; Murray et al., 2002; Wood & Magnello, 1992; Clark et al., 1989). Therefore, a secondary analysis used a similar MLM model described above to test for potential diurnal rhythms in NA by chronotype group.
Results
Of the total sample (n = 408, 51.7% female), 82.1% identified as Caucasian, while the remaining sample identified as African American (15.7%), Asian (1.5%), Mixed (0.5%) or other race (0.5%). The average age was 42.89 years (SD = 7.33). The sample had an average education level of 16.97 years (SD = 2.84) and the median reported income was between $65,000 and $79,999 USD. The average CSM score in the sample was 39.52 (SD = 7.13), in the intermediate chronotype range. Using Natale & Alzani (2001) chronotype cut-off ranges, individuals were grouped into morning (43.3%), intermediate (52.2%), and evening groups (4.4%). Table 1 depicts sample descriptives by chronotype group. Consistent with previous research, age and trait PA differed significantly by chronotype group (see Table 1).
Table 1.
Demographic variables by chronotype group.
| Variable | Chronotype Group | |||
|---|---|---|---|---|
|
| ||||
| Morning (n=177) | Intermediate (n=213) | Evening (n=18) | p-value | |
| Female n (%) | 94 (53%) | 110 (52%) | 7 (39%) | 0.516 |
| Caucasian n (%) | 146 (82%) | 172 (81%) | 17 (94%) | 0.740 |
| Age | 44.08 (6.81) | 42.38 (7.52) | 36.50 (6.32) | 0.001** |
| Years of Education | 17.19 (2.75) | 16.80 (2.94) | 16.78 (2.65) | 0.388 |
| CSM | 45.88 (3.16) | 35.65 (4.00) | 22.78 (2.76) | 0.001** |
| PA | 35.24 (5.67) | 33.40 (5.71) | 30.94 (6.18) | 0.001** |
| NA | 14.43 (4.79) | 15.60 (5.09) | 14.61 (3.58) | 0.061 |
| Mean Workday Mid-sleep | 2:51 (00:33) | 3:11 (00:32) | 3:52 (00:44) | 0.001** |
| Mean Workday Sleep Duration | 6:43 (1:01) | 6:35 (0:58) | 6:28 (1:11) | 0.291 |
| Mean Non-Workday Mid-sleep | 3:28 (00:43) | 3:50 (00:41) | 4:23 (1:01) | 0.001** |
| Mean Non-Workday Sleep Duration | 7:17 (1:21) | 7:19 (1:20) | 7:07 (2:11) | 0.847 |
| Social Jet Lag | 41.92 (35.07) | 45.95 (37.00) | 58.19 (44.84) | 0.157 |
Note: Positive and Negative Affect means are from the one time PANAS measure. Social Jet Lag is listed in minutes. Chi-square test was used for group comparisons of gender and race. All other group comparisons were performed with ANOVA. Standard deviations are listed in parentheses except where indicated. CSM = Composite Scale of Morningness, PA= Positive Affect, NA= Negative Affect.
p <0.001.
Chronotype Groups and PA Rhythms
Workday PA Diurnal Rhythms
In fitting the MLM, the morning chronotype was chosen as the reference group to which all other group effects are compared. Workday PA showed diurnal rhythmicity in the morning chronotype group, with a significant fit to both sine (β = -0.15, t16929 = -2.75, p = 0.01) and cosine (β = -1.08, t169297= -18.18, p < 0.0001) terms (Table 2). The intermediate chronotype main effect term was significant (β = -0.84, t418 = -2.47, p = 0.01), as well as the sine*intermediate interaction term (β = -0.28, t16929 = -3.76, p < 0.001) and cosine*intermediate term (β = 0.17, t16929 = -3.76, p = 0.03), indicating a difference in diurnal PA rhythm between intermediate and morning chronotype groups. Although the evening chronotype main effect term was not significant, the cosine*evening interaction term was (β = 0.65, t16929 = 4.09, p < 0.0001), indicating a significant difference in diurnal PA rhythm in evening types compared to morning types. See Figure 2 for a plot of the fitted diurnal rhythms by chronotype group.
Table 2.
Chronotype group cosinor MLM results for PA on both workdays and non-workdays.
| Variable | Workdays | Non-Workdays | ||
|---|---|---|---|---|
|
|
|
|||
| β | SE | β | SE | |
| Sine | -0.15* | 0.05 | -0.40** | 0.11 |
| Cosine | -1.08*** | 0.06 | -0.99*** | 0.10 |
| Intermediate | -0.84* | 0.34 | -0.68 | 0.38 |
| Sine*Intermediate | -0.28** | 0.08 | -0.17 | 0.15 |
| Cosine*Intermediate | 0.17* | 0.08 | 0.25* | 0.13 |
| Evening | -0.84 | 0.84 | -0.79 | 0.92 |
| Sine*Evening | -0.26 | 0.19 | 0.75* | 0.38 |
| Cosine*Evening | 0.65*** | 0.16 | 0.15 | 0.29 |
| Age | 0.03 | 0.02 | 0.04 | 0.03 |
| Gender | -0.79* | 0.33 | -0.71* | 0.36 |
Note: Morning is the reference group, therefore, morning type estimates of PA rhythm components are represented by the Sine and Cosine main effect terms. The Intermediate and Evening main effect terms represent differences in chronotype group PA means with respect to the morning group. The interaction terms represent differences in sine and cosine fits for the intermediate and evening chronotype groups with respect to the morning group.
p < 0.05,
p < 0.001,
p < 0.0001
Figure 2.
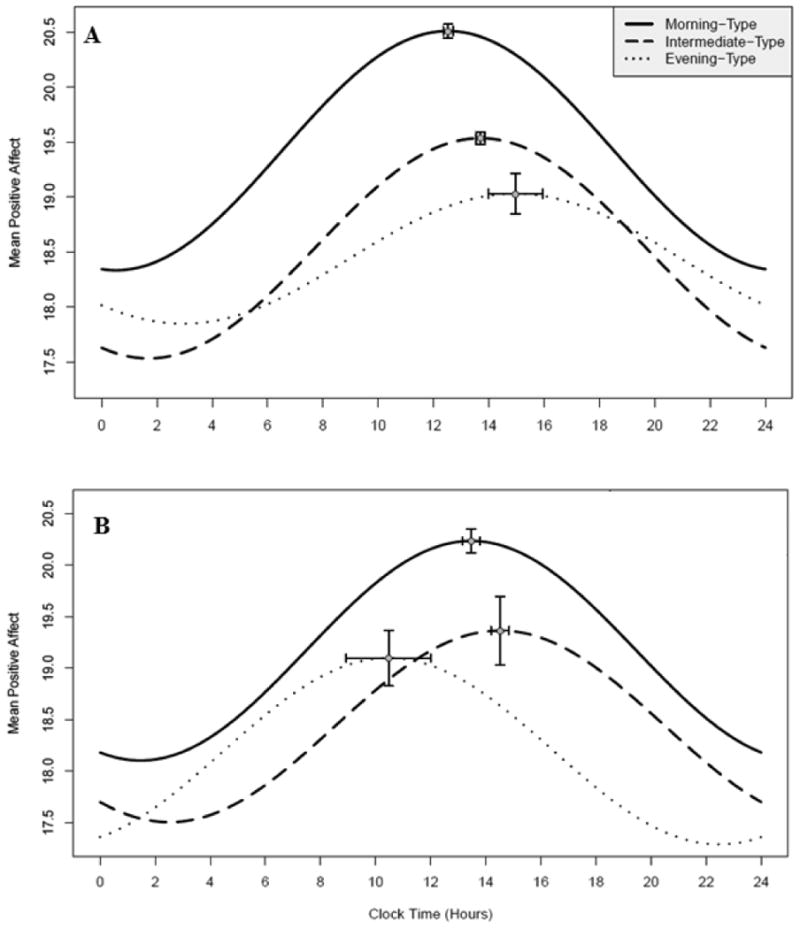
A. Modeled workday PA rhythm by chronotype group. B. Modeled non-workday PA rhythm by chronotype group. Horizontal error bars represent chronotype group standard errors for acrophase. Vertical error bars represent chronotype group standard errors for amplitude.
Estimates for amplitude and acrophase by chronotype group (Table 3) were extracted from regression results to allow for statistical group comparisons utilizing the delta method (Mikulich et al., 2003). Amplitude estimates were similar for both the morning (1.09, SE = 0.06) and the intermediate groups (1.00, SE = 0.06, p = 0.31). In contrast, the amplitude estimate for the evening group (0.59, SE = 0.18) was significantly lower compared to both the intermediate (z = -2.13, p = 0.03) and morning groups (z = -2.56, p = 0.01). Acrophase estimates occurred in the predicted order, such that the acrophase of morning types occurred at 12:31 hours (SE = 0:11), intermediate acrophase occurred at 13:42 hours (SE = 0:10), and evening acrophase occurred at 14:57 hours (SE = 0:59). Acrophase estimates differed significantly between the morning and evening groups (z = 2.43, p = 0.02) as well as between morning and intermediate groups (z = 1.17, p < 0.0001), although there was no statistically significant difference in acrophase between intermediate and evening groups (z = 1.26, p = 0.21).
Table 3.
Chronotype group estimates and group comparisons for PA amplitude and acrophase on workdays and non-workdays.
| Workdays | ||||
|---|---|---|---|---|
|
| ||||
| Group Estimations | PA Amplitude | Acrophase in Clock Time | ||
|
| ||||
| Estimate | SE | Estimate | SE | |
|
| ||||
| Morning | 1.09**** | 0.06 | 12:31**** | 0:11 |
| Intermediate | 1.00**** | 0.06 | 13:42**** | 0:10 |
| Evening | 0.59*** | 0.18 | 14:57**** | 0:59 |
|
| ||||
| Estimate Differences | PA Amplitude Differences | Acrophase Differences in Hours | ||
|
|
|
|||
| Estimate | SE | Estimate | SE | |
|
| ||||
| Evening vs. Morning | -0.50** | 0.19 | 2:26* | 1:00 |
| Evening vs. Intermediate | -0.41* | 0.19 | 1:15 | 1:00 |
| Intermediate vs. Morning | -0.09 | 0.08 | 1:10**** | 0:14 |
| Non-Workdays | ||||
|---|---|---|---|---|
|
| ||||
| PA Amplitude | Acrophase in Clock Time | |||
|
|
|
|||
| Estimate | SE | Estimate | SE | |
|
| ||||
| Morning | 1.06**** | 0.12 | 13:28**** | 0:14 |
| Intermediate | 0.93**** | 0.11 | 14:31**** | 0:20 |
| Evening | 0.90*** | 0.27 | 10:28**** | 1:32 |
|
| ||||
| Estimate Differences | PA Amplitude Differences | Acrophase Differences in Hours | ||
|
| ||||
| Estimate | SE | Estimate | SE | |
|
|
|
|||
| Evening vs. Morning | -0.16 | 0.29 | -3:00 | 1:35 |
| Evening vs. Intermediate | -0.03 | 0.29 | -4:03** | 0:35 |
| Intermediate vs. Morning | -0.13 | 0.16 | 1:03* | 0:28 |
Note:
p < 0.05,
p < 0.01
p < 0.001,
p < 0.0001
Non-Workday PA Diurnal Rhythms
MLM analysis of PA on non-workdays revealed a diurnal PA rhythm for morning-type participants, with significant sine (β = -0.40, t5113 = -3.68, p < 0.001) and cosine (β = -0.99, t5113 = -9.77, p < 0.0001) terms (Table 2). Additionally, the cosine*intermediate interaction term (β = 0.25, t5113 = 1.92, p = 0.05) and sine*evening term (β = 0.75, t5113 = 1.99, p = 0.04) were significant, suggesting PA rhythms differed by chronotype group on non-workdays. Neither the main effect term for intermediate-types nor the main effect for evening-types were statistically significant suggesting overall group mean PA did not differ on non-workdays. See Figure 2 for a depiction of estimated rhythms by chronotype group.
Estimates for chronotype group amplitude and acrophase on non-workdays did not follow predictions. Morning chronotype demonstrated a peak at 13:28 hours (SE = 0:19) and intermediate at 14:31 hours (SE = 0:20) on non-workdays as predicted, but evening chronotypes had the earliest acrophase time at 10:28 hours (SE = 1:32). Group comparisons of estimates showed a significant phase difference between morning and intermediate (z = 2.26, p = 0.02) as well as intermediate and evening groups (z = -2.57, p = 0.01; Table 3).
Individual PA Rhythm Analyses
In order to replicate group comparisons with individuals, an Ordinary Least Squares regression model was used including age, gender, and chronotype as a continuous measure to predict workday individual PA amplitude and acrophase. The model predicting acrophase was statistically significant (3.1% of variance explained; p < 0.01), although the model predicting amplitude was not significant. Also consistent with hypotheses, greater eveningness was associated with relatively delayed PA acrophase on workdays (β = -0.11). Comparable models predicting non-workday PA amplitude and acrophase were not significant.
Social Jet Lag
Mid-sleep times on workdays and non-workdays differed significantly by chronotype. Evening individuals had the latest mid-sleep times on both workdays and non-workdays and morning individuals showed the earliest times (Table 1). However, neither sleep duration nor SJL differed significantly by chronotype group. Further, the regression models using continuous chronotype to predict SJL (R2 = 0.01, p = 0.14) and models using SJL to predict PA acrophrase (R2 = 0.01, p = 0.25) and amplitude (R2 = 0.01, p = 0.31) were not significant. Given the non-significant results of the pathways, mediation of SJL in the relationship between chronotype and amplitude/acrophase was not examined.
Chronotype Groups and NA Rhythms
A diurnal rhythm in workday NA was evidenced by a significant cosine term (β = -0.33, t1629 = -4.84, p <0.0001). However, chronotype terms and interaction terms were not significant, indicating NA rhythms did not differ significantly by chronotype group. Non-workday NA analyses were not significant (data not shown).
Discussion
The present study extended previous findings that chronotype is associated with PA rhythm estimates in a large healthy sample, while using hourly measures of PA. The evening group experienced peak PA significantly later and exhibited lower amplitude values compared to the morning chronotype group on workdays. Consistent with the delayed PA rhythms findings, mid-sleep times were also significantly associated with chronotype, with evening individuals exhibiting the latest mid-sleep times on both workdays and non-workdays. Importantly, sleep duration, a factor not directly influenced by the circadian clock (for review see: Dijk et al., 2002), did not differ by chronotype group. Together, the sleep findings suggest that individuals with evening chronotype exhibit delayed circadian rhythms in at least one biological process. Further, trait measures of PA varied significantly by chronotype, with evening types reporting lowest overall PA scores. In contrast to predictions, SJL was not statistically associated with chronotype or PA rhythms in this sample, although evening types evidenced later sleep times on both work day and non-work days. This suggests that sleep and circadian misalignment due to the magnitude of difference in weekday vs. weekend schedules (i.e., SJL) does not explain the association between evening chronotype and decreased PA amplitude. Overall, our findings suggest PA rhythms differ as expected by chronotype group.
In our main findings, workday acrophase times demonstrated the predicted pattern, such that the morning chronotype group exhibited the earliest time of peak PA (12:31), the intermediate group peaked in the middle (13:42), and the evening group peaked last (14:57). Therefore, along the continuum of chronotype scores from morningness to eveningness, PA amplitude decreases and there is a delay in acrophase. Because the same pattern of delay and decrease in PA is posited to increase vulnerability to depression through an associated decline in appetitive behaviors, our findings, even though based on a healthy sample without history of depression, may bear on the already established link between evening chronotype and depression. For example, individuals who display adaptive PA rhythms may sleep through their low point in PA and experience their peak PA during hours in which social interaction is most likely. In contrast, evening types may awake while still in the trough of PA, exhibit a delayed peak PA relative to availability of environmental reward, and experience lower overall PA. Therefore, evening chronotype individuals may be more vulnerable to developing depression due to their higher likelihood to exhibit delayed and blunted PA rhythms.
Interestingly, acrophase timing did not occur in the predicted order on non-workdays with evening individuals exhibiting the earliest peak. One possible explanation for this discrepancy may be the tendency for evening types to shift drastically from workday schedules to unconstrained non-workday schedules (Wittmann et al., 2006). In the context of this study, peak PA may occur during times in which the PDA was turned off (sleep time) and rhythm analyses may then statistically identify peak PA as occurring the following morning. A more likely explanation concerns the discrepancy between the number of affect measurement days between workday and non-workday. In the current study, workday estimates were created with an average of several day's affect measurement while non-workday estimates were created based on a single day's affect collection, potentially making non-workday analyses less reliable. To increase confidence in our non-workday measures, future studies could include multiple non-workdays over several weeks, or a vacation period of consecutive non-workdays.
Contrary to study predictions, SJL was not found to be associated with chronotype or with PA rhythm estimates, and therefore was not found to be a mediator in the relationship between evening chronotype and blunted PA rhythms. Although SJL has been linked with evening chronotype in previous studies (Levandovski et al., 2011; Wittmann et al., 2006), the association was not significant in the current sample. This is surprising due to the likelihood that evening individuals would need to make the largest circadian “correction” on workdays and therefore have the largest difference between non-workday and workday mid-sleep points. Of note, however, SJL did vary in the predicted direction, with evening individuals showing the greatest SJL and suggesting that lack of significant findings may reflect the fewer sleep measurements available on the single non-workday, relative to work-days. Additionally, SJL was not significantly associated with PA amplitude, in contrast to prior reports linking circadian misalignment to blunting in various physiological rhythms (Souętre et al., 1989). However, the null findings in the current sample may be due to the lack of longer SJL durations in the current sample. Specifically, previous reports document a large range of SJL (0 to 4+ hours; Roenneberg, 2012; Wittman, 2006), though the majority of the current sample (n = 300; 65%) exhibited less than an hour difference in workday and non-workday mid-sleep times. Further, it is important to note that the restricted variance in chronotype (see discussion below) may have limited our ability to detect an association with SJL and warrants further investigation. Together, results indicate that SJL may not play an important role in the chronotype-PA amplitude association.
In contrast with several studies (Clark et al., 1989; Murray et al., 2009; Murray et al., 2002; Murray, 2007; Wood & Magnello, 1992) but consistent with three (Golder & Macy, 2011; Stone et al., 2006; Vittengl et al., 1998), the current sample exhibited significant diurnal variation in NA. Interestingly, the largest studies investigating diurnal variation in affect (Golder & Macy, 2011; Stone et al., 2006) found a significant NA rhythm, suggesting that larger sample sizes such as ours are needed to detect a diurnal variation in NA. However, the current results found no significant impact of chronotype on NA rhythms, indicating that individual differences in circadian rhythms are not associated with the daily variation in NA. This lack of NA-chronotype relationship suggests a more stable, non-varying source of NA and suggests specificity for the PA-chronotype association.
Although the current study provided the first healthy population of this magnitude with multiple workday measurements, several study limitations may impact results and interpretation. First, the CSM self-report measure is vulnerable to reporting bias in that individuals may not accurately report their activity timing preference (Podsakoff et al., 2003) and similar studies would be improved by using direct measures of circadian phase such as dim light melatonin onset (DLMO, Pandi-Perumal et al., 2007). Further, modifications to the PANAS PA scale and the addition of two POMS items in our study makes the PA scales inconsistent with previous literature. However, the replication of previous results using our unique PA measures may provide stronger evidence that findings are a real phenomenon not based on measurement artifact. Additionally, a smaller number of participants in the current sample were classified in the evening chronotype group (n = 18) compared to the morning and intermediate groups and therefore interpretation of the group results is limited. Nonetheless, the fact that using chronotype as a continuous variable revealed similar results as in the group analyses increases confidence that our sample included enough variance in chronotype to predict PA rhythms. Lastly, the cross sectional study includes only healthy non-depressed individuals and cannot directly elucidate the association between chronotype and PA rhythms in the context of depression vulnerability. Indeed, the exclusion of participants with a history of depression in the current study may have created an especially depression-resistant sample and, perhaps, could further explain the low number of evening-type individuals. Future longitudinal research, including those at high risk for depression, will be helpful in establishing a causal link between delayed and blunted PA rhythms and depression vulnerability.
To date, the current study is the largest EMA sample including hourly affect measures to find that chronotype predicts daily variation in PA rhythms. Study results suggest that evening chronotype may lead to delayed timing of peak PA and lower PA amplitude in evening types, which has been hypothesized as a potential vulnerability to depression. Further, the lack of a link between chronotype and NA may point to the specificity of PA in the association between circadian timing and mood disorder vulnerability. Future longitudinal studies which establish abnormal PA rhythms as a vulnerability to developing depression may provide additional support and a potential mechanism for the efficacy of chronobiological treatments, such as light therapy (Terman et al., 2001). Specifically, treatments that advance PA rhythms so that they are more adaptively aligned with environmental reward may decrease depressive symptoms. To test such hypotheses, the PA rhythm before and after chronotherapeutic interventions could be assessed to further determine the validity of this mechanistic explanation for the link between evening chronotype and depression.
Acknowledgments
Current research was funded by P01 HL040962 (T. W.K., S.B.M.) and NSF DGE 124-7842 (M.A.M.).
Footnotes
Declaration of Interest Statement: The authors report no conflicts of interest.
References
- Boivin DB, Czeisler CA, Dijk DJ, Duffy JF, Folkard S, Minors DS, Totterdell P, Waterhouse JM. Complex interaction of the sleep-wake cycle and circadian phase modulates mood in healthy subjects. Arch Gen Psychiat. 1997;54:145–52. doi: 10.1001/archpsyc.1997.01830140055010. [DOI] [PubMed] [Google Scholar]
- Chelminski I, Ferraro FR, Petros TV, Plaud JJ. An analysis of the “eveningness–morningness” dimension in “depressive” college students. J Affect Disorders. 1999;52:19–29. doi: 10.1016/s0165-0327(98)00051-2. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D, Leeka J. Diurnal variation in the positive affects. Motiv Emotion. 1989;13:205–34. [Google Scholar]
- Claustrat B, Chazot G, Brun J, Jordan D, Sassolas G. A chronobiological study of melatonin and cortisol secretion in depressed subjects: plasma melatonin, a biochemical marker in major depression. Biol Psychiat. 1984;19:1215–28. [PubMed] [Google Scholar]
- Daimon K, Yamada N, Tsujimoto T, Takahashi S. Circadian rhythm abnormalities of deep body temperature in depressive disorders. J Affect Disorders. 1992;26:191–98. doi: 10.1016/0165-0327(92)90015-x. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Lockley SW. Invited Review: Integration of human sleep-wake regulation and circadian rhythmicity. J Appl Physiol. 2002;92:852–862. doi: 10.1152/japplphysiol.00924.2001. [DOI] [PubMed] [Google Scholar]
- Drennan MD, Klauber MR, Kripke DF, Goyette LM. The effects of depression and age on the Horne-Ostberg morningness-eveningness score. J Affect Disorders. 1991;23:93–98. doi: 10.1016/0165-0327(91)90096-b. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Rimmer DW, Czeisler CA. Association of intrinsic circadian period with morningness–eveningness, usual wake time, and circadian phase. Behav Neurosci. 2001;115:895–99. doi: 10.1037//0735-7044.115.4.895. [DOI] [PubMed] [Google Scholar]
- Germain A, Kupfer DJ. Circadian rhythm disturbances in depression. Hum Psychopharm Clin. 2008;23:571–85. doi: 10.1002/hup.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder SA, Macy MW. Diurnal and seasonal mood vary with work, sleep, and daylength across diverse cultures. Science. 2011;333:1878–81. doi: 10.1126/science.1202775. [DOI] [PubMed] [Google Scholar]
- Greenwood K. Long-term stability and psychometric properties of the Composite Scale of Morningness. Ergonomics. 1994;37:377–83. doi: 10.1080/00140139408963653. [DOI] [PubMed] [Google Scholar]
- Guthrie JP, Ash RA, Bendapudi V. Additional validity evidence for a measure of morningness. J Appl Psychol. 1995;80:186–90. [Google Scholar]
- Hasler BP, Allen JB, Sbarra DA, Bootzin RR, Bernert RA. Morningness-eveningness and depression: Preliminary evidence for the role of the behavioral activation system and positive affect. Psychiat Res. 2010;176:166–73. doi: 10.1016/j.psychres.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Dahl RE, Holm SM, Jakubcak JL, Ryan ND, Silk JS, Phillips ML, Forbes EE. Weekend—weekday advances in sleep timing are associated with altered reward-related brain function in healthy adolescents. Biol Psychol. 2012;91:334–341. doi: 10.1016/j.biopsycho.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Germain A, Nofzinger EA, Kupfer DJ, Krafty RT, Rothenberger SD, James AJ, Bi W, Buysse DJ. Chronotype and diurnal patterns of positive affect and affective neural circuitry in primary insomnia. J Sleep Res. 2012;21:515–26. doi: 10.1111/j.1365-2869.2012.01002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata FC, Lima MCO, de Bruin VMS, Nóbrega PR, Wenceslau GP, de Bruin PFC. Depression in medical school: the influence of morningness-eveningness. Chronobiol Int. 2007;24:939–46. doi: 10.1080/07420520701657730. [DOI] [PubMed] [Google Scholar]
- Levandovski R, Dantas G, Fernandes LC, Caumo W, Torres I, Roenneberg T, Hidalgo MPL, Allebrandt KV. Depression scores associate with chronotype and social jetlag in a rural population. Chronobiol Int. 2011;28:771–78. doi: 10.3109/07420528.2011.602445. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Atwood GE. Depression: A clinical-research approach. Psychotherapy: Theory, Research & Practice. 1969;6:166–71. [Google Scholar]
- Lewy AJ, Lefler BJ, Emens JS, Bauer VK. The circadian basis of winter depression. P of Natl Acad Sci USA. 2006;103:7414–19. doi: 10.1073/pnas.0602425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA. How Might Circadian Rhythms Control Mood? Let Me Count the Ways. Biol Psychiat. 2013;74:242–49. doi: 10.1016/j.biopsych.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair DM, Loor M, Droppleman LF. Profile of mood states. San Diego, CA: Educational and Industrial Testing Service; 1981. [Google Scholar]
- Mikulich SK, Zerbe GO, Jones RH, Crowley TJ. Comparing linear and nonlinear mixed model approaches to cosinor analysis. Stat Med. 2003;22:3195–211. doi: 10.1002/sim.1560. [DOI] [PubMed] [Google Scholar]
- Mongrain V, Lavoie S, Selmaoui B, Paquet J, Dumont M. Phase relationships between sleep-wake cycle and underlying circadian rhythms in morningness-eveningness. J Biol Rhythms. 2004;19:248–57. doi: 10.1177/0748730404264365. [DOI] [PubMed] [Google Scholar]
- Murray G. Diurnal mood variation in depression: A signal of disturbed circadian function? J Affect Disorders. 2007;102:47–53. doi: 10.1016/j.jad.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Murray G, Allen NB, Trinder J. Mood and the circadian system: Investigation of a circadian component in positive affect. Chronobiol Int. 2002;19:1151–69. doi: 10.1081/cbi-120015956. [DOI] [PubMed] [Google Scholar]
- Murray G, Allen NB, Trinder J, Burgess H. Is weakened circadian rhythmicity a characteristic of neuroticism? Affect Disorders. 2002;72:281–289. doi: 10.1016/s0165-0327(01)00465-7. [DOI] [PubMed] [Google Scholar]
- Murray G, Nicholas CL, Kleiman J, Dwyer R, Carrington MJ, Allen NB, Trinder J. Nature's clocks and human mood: The circadian system modulates reward motivation. Emotion. 2009;9:705–16. doi: 10.1037/a0017080. [DOI] [PubMed] [Google Scholar]
- Natale V, Alzani A. Additional validity evidence for the composite scale of morningness. Pers Indiv Differ. 2000;30:293–301. [Google Scholar]
- Pandi-Perumal SR, Smits M, Spencec W, Srinivasand V, Cardinalie DP, Lowef AD, Kayumovf L. Dim light melatonin onset (DLMO): A tool for the analysis of circadian phase in human sleep and chronobiological disorders. Prog Neuro-Psychoph. 2007;31:1–11. doi: 10.1016/j.pnpbp.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Podsakoff PM, MacKenzie SB, Lee JY, Podsakoff NP. Common method biases in behavioral research: a critical review of the literature and recommended remedies. J Appl Psychol. 2003;88:879–903. doi: 10.1037/0021-9010.88.5.879. [DOI] [PubMed] [Google Scholar]
- Portaluppi F, Smolensky MH, Touitou Y. Ethics and methods for biological rhythm research on animals and human beings. Chronobiol Int. 2010;27:1911–29. doi: 10.3109/07420528.2010.516381. [DOI] [PubMed] [Google Scholar]
- Porto R, Duarte L, Menna-Barreto L. Circadian variation of mood: comparison between different chronotypes. Biol Rhythm Res. 2006;37:425–31. [Google Scholar]
- Posener JA, DeBattista C, Williams GH, Kraemer HC, Kalehzan BM, Schatzberg AF. 24-Hour monitoring of cortisol and corticotropin secretion in psychotic and nonpsychotic major depression. Arch Gen Psychiat. 2000;57:755–60. doi: 10.1001/archpsyc.57.8.755. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Curr Biol. 2012;22:939–943. doi: 10.1016/j.cub.2012.03.038. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Harnett-Sheehan K, Janavs J, Weiller E, Bonara LI, Keskiner A, Schinka J, Knapp E, Sheehan MF, Dunbar GC. Reliability and Validity of the M.I.N.I. International Neuropsychiatric Interview (M.I.N.I.): According to the SCID-P. Eur Psychiat. 1997;12:232–41. [Google Scholar]
- Smith CS, Reilly C, Midkiff K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J Appl Psychol. 1989;74:728–38. doi: 10.1037/0021-9010.74.5.728. [DOI] [PubMed] [Google Scholar]
- Souętre E, Salvati E, Belugou JL, Pringuey D, Candito M, Krebs B, Ardisson JL, Darcourt G. Circadian rhythms in depression and recovery: evidence for blunted amplitude as the main chronobiological abnormality. Psychiat Res. 1989;28:263–78. doi: 10.1016/0165-1781(89)90207-2. [DOI] [PubMed] [Google Scholar]
- Stone AA, Schwartz JE, Schkade D, Schwarz N, Krueger A, Kahneman D. A population approach to the study of emotion: Diurnal rhythms of a working day examined with the day reconstruction method. Emotion. 2006;6:139–49. doi: 10.1037/1528-3542.6.1.139. [DOI] [PubMed] [Google Scholar]
- Terman JS, Terman M, Lo ES, Cooper TB. Circadian time of morning light administration and therapeutic response in winter depression. Arch Gen Psychiatry. 2001;58:69–75. doi: 10.1001/archpsyc.58.1.69. [DOI] [PubMed] [Google Scholar]
- Thompson ER. Development and validation of an internationally reliable short-form of the positive and negative affect schedule (PANAS) J Cross Cult Psychol. 2007;38:227–42. [Google Scholar]
- Vittengl JR, Holt CS. A time-series diary study of mood and social interaction. Motiv Emotion. 1998;22:255–75. [Google Scholar]
- Watson D, Clark LA, Carey G. Positive and negative affectivity and their relation to anxiety and depressive disorders. J Abnorm Psychol. 1988;97:346–53. doi: 10.1037//0021-843x.97.3.346. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA. The PANAS-X: Manual for the positive and negative affect schedule-expanded form. Iowa City, IA: The University of Iowa; 1999. [Google Scholar]
- Watson D, Wiese D, Vaidya J, Tellegen A. The two general activation systems of affect: Structural findings, evolutionary considerations, and psychobiological evidence. J Pers Soc Psychol. 1999;76:820–38. [Google Scholar]
- Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol Int. 2006;23:497–509. doi: 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- Wood C, Magnello M. Diurnal changes in perceptions of energy and mood. J Roy Soc Med. 1992;85:191–94. doi: 10.1177/014107689208500404. [DOI] [PMC free article] [PubMed] [Google Scholar]


