Abstract
Purpose of review
The musculoskeletal system is largely regulated through dynamic physical activity and is compromised by cessation of physical loading. There is a need to recreate the anabolic effects of loading on the musculoskeletal system, especially in frail individuals who cannot exercise. Vibration therapy is designed to be a nonpharmacological analogue of physical activity, with an intention to promote bone and muscle strength.
Recent findings
Animal and human studies suggest that high-frequency, low-magnitude vibration therapy improves bone strength by increasing bone formation and decreasing bone resorption. There is also evidence that vibration therapy is useful in treating sarcopenia, which confounds skeletal fragility and fall risk in aging. Enhancement of skeletal and muscle strength involves regulating the differentiation of mesenchymal stem cells to build these tissues; mesenchymal stem cell lineage allocation is positively promoted by vibration signals.
Summary
Vibration therapy may be useful as a primary treatment as well as an adjunct to both physical and pharmacological treatments, but future studies must pay close attention to compliance and dosing patterns, and importantly, the vibration signal, be it low-intensity vibration (<1g) appropriate for treatment of frail individuals or high-intensity vibration (>1g) marketed as a training exercise.
Keywords: low-intensity vibration, mesenchymal stem cells, osteocyte, osteoporosis
INTRODUCTION
Conditions associated with reduced mobility and systemic decline lead to failure of the musculoskeletal system with loss of skeletal strength and muscle dysfunction, significantly compromising measures of life quality. Both bone and muscle perceive and respond to local dynamic loading, building form, and strength to support function [1]. The need to mechanically load the skeleton translates to ‘use it or lose it’. As such, pathological or occupational reductions in functional loading manifest as bone becomes more susceptible to fracture [2]. To avoid off-target pharmacological complications, it makes more sense to physically target the musculoskeletal system: mechanical signals present unique advantages in that effects are both self-targeting and self-optimizing. However, typical exercise regimens (running or walking) are difficult for frail individuals. Vibration therapy delivered as a low-magnitude, high-frequency stimulus [‘LIV’ defined as <1 gravity (g =acceleration of 9.81 m/s2, frequency >30 Hz)] offers a means to deliver relevant mechanical signals safely to patients who can not exercise to build musculoskeletal strength [3]. Low intensity vibration (LIV), as compared with devices that deliver high-magnitude signals (>1g), provides different levels of applicability and safety.
What types of mechanical signals does the skeleton perceive as anabolic? Bone formation requires dynamic mechanical loading, with varied time between loading bouts [4]. In contrast, static loads induce bone resorption [5]. Dynamic load has both magnitude and frequency components within the bone matrix [6]. Large-magnitude strain can induce tissue responses directly via matrix deformation or indirectly through fluid shear, pressure, or streaming potentials [7]. The skeleton experiences relatively few low-frequency (1–3 Hz), large-magnitude (2000–3000 microstrain) events throughout the day but is bombarded with persistent high-frequency (10–50 Hz), low-magnitude signals [8]. These constant small signals are generated from postural muscle contractions; these contractions decrease with sarcopenia or disuse muscle atrophy [9]. LIV therapy replicates these high-frequency, low-magnitude signals to improve bone strength [10].
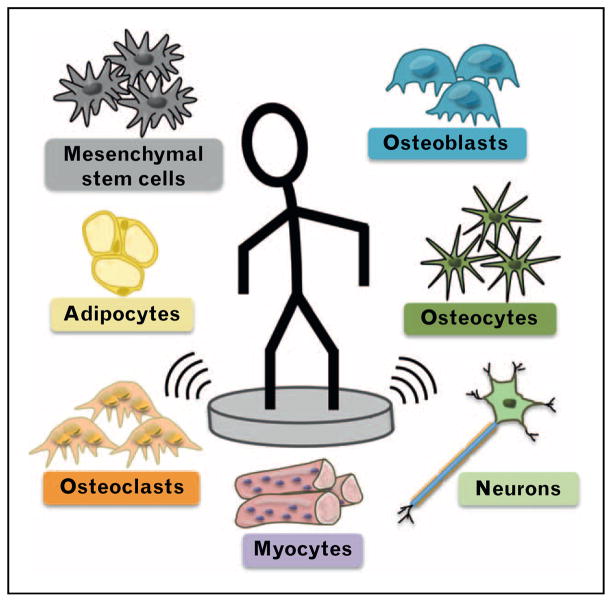
Vibration therapy is directed at processes activated by direct mechanical loading [7] (Fig. 1), including the progenitor for osteoblasts, osteocytes, and myocytes, the mesenchymal stem cell (MSC). Vibration directs osteogenic differentiation [11] while restricting MSC adipogenic commitment [12]. Although little is known regarding effects of vibration on osteocytes embedded in mineralized tissue, vibration of osteocytes in culture decreased expression of osteoclast-forming RANKL [13], which rises during unloading [14], and increased cell communication [15■]. Also, the hematopoietic-derived osteoclast is mechanically responsive [16] and vibration reduced osteoclast formation [17].
FIGURE 1.
Cellular targets of vibration. The physiological effects of vibration are mediated by individual cellular actions. Low-magnitude mechanical signals target many cell types including mesenchymal stem cells, osteoblasts, osteocytes, adipocytes, osteoclasts, myocytes, and neurons.
Anabolic effects of low-intensity mechanical signals on bone may be induced indirectly via extra-skeletal tissues. Enhanced muscle strength, size, and performance were observed in humans [18] and animals [19] following vibration, possibly due to increased neuromuscular efficiency [18]. Vibration enhances expression of anabolic genes in tendons [20]. Other studies suggest that bone density increases due to vibration’s repression of fat development [12,21]. The molecular mechanisms controlling these responses may be due to enhanced β-catenin [12] or enhanced gap junction communication [15■]. Signal activation may be induced via acceleration of the cell nucleus [15■], independently of matrix strain [12] or fluid shear [22,23].
HUMAN STUDIES
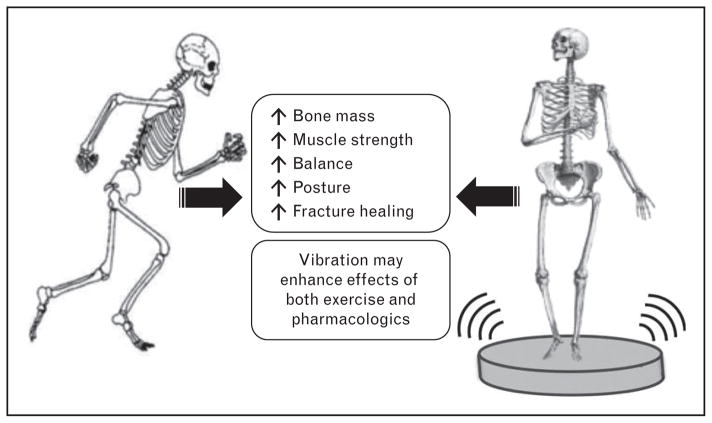
Whole-body vibration (WBV) is a promising non-pharmacological treatment strategy to improve bone quality, strength, and posture in patients who are unable to perform high-impact exercises (Fig. 2). Available human trials are complicated because of multiple vibration parameters ranging from high (3–5g) to low intensity (<1g). Although high-intensity vibration devices are frequently found in gyms and marketed as workout machines, it is unsuited for frail patients. WBV regimens need to be optimized for anabolic responses while minimizing adverse effects.
FIGURE 2.
Physiological responses of whole-body vibration. Delivery of low-magnitude mechanical signals mimic aspects of loading exercise, providing direct benefits to the skeleton, but also indirectly improves musculoskeletal outcomes including balance, posture, and muscle strength. These additional benefits feed back to further enhance skeletal strength.
A double-placebo controlled study evaluated the effects of low-intensity WBV on bone density in humans; postmenopausal women were randomized to receive 2 × 10 min LIV (0.2g, 30 Hz) or an inactive placebo plate [24]. Women with high adherence showed a benefit of LIV: the control group lost 2% femoral neck bone mineral density (BMD) whereas the treatment group gained 0.04%, a 2.17% relative BMD increase. Similar LIV signals were studied over 1 year in premenopausal women with low Z-scores and positive fracture history [25]. Quantitative computed tomography showed that women in the active LIV group gained 2% trabecular and cortical bone compared with the inactive group. Imaging also showed LIV caused a 4.9% increase in paraspinous muscle area.
Several subsequent studies confirmed improvement in BMD with WBV; however, these studies are of varying quality and design. In some cases, high-intensity vibration was used and was linked to back and joint pain. A nonrandomized controlled study of 116 postmenopausal women with osteoporosis who received high-intensity vibration (30 Hz, 5 mm amplitude =18g) for 10 min 5 days a week increased lumbar and femoral neck BMD by 4.3 and 3.2%, respectively [26]. Another high-magnitude 30 Hz, 3.2g study dosed for 5 min thrice weekly showed a 2% significant increase in lumbar spine BMD in postmenopausal women in which controls lost bone over 6 months [27].
More recently, in a randomized controlled trial [28], 202 osteopenic postmenopausal women were randomized to LIV (0.3g, 37 or 90 Hz) for 20 min daily for 1 year. No significant differences were found in the primary outcome of tibial trabecular volumetric BMD or in secondary measures such as femoral neck, total hip, or lumbar spine BMD [28]. However, compliance with LIV of 65–79% was poor. Limitations included low adherence, lack of very low bone density, and the fact that the placebo group did not lose significant bone density over the year study.
Several studies evaluated the ability of WBV to augment the anabolic effects of dynamic exercise. Gomez-Cabello et al. [29] randomized 49 elderly men and women to receive either WBV (35 Hz, ~16g) while completing a trained squat three times a week for 11 weeks or to a control group receiving no vibration or exercise. At the end of the short study, there were no changes in dual-energy X-ray absorptiometry scan measures. An 18-month study showed that vibration therapy combined with low-impact activity enhanced the effect of training to increase lumbar BMD [30]. Interestingly, the vibration group had decreased falls as well. Another recent study [31] in seniors combined 6-month vibration therapy (44–55 Hz, 0.5g) with a tilting angle exercise for 20 min thrice weekly demonstrated LIV-induced BMD increases that were higher in women compared with men, and in participants with osteoporosis, compared with those without low bone density.
The effects of LIV have been studied in children with immobility-associated disability. Children with disabling conditions randomized to LIV (90 Hz, 0.3g, 10 min/day) demonstrated a 6.3% increase, whereas those in the control group had a decrease of 12% in BMD [32]. In children with osteogenesis imperfecta, high-intensity vibration (15–20 Hz, 1–2 mm amplitude, ~12g) combined with tilt-table exercise induced improvements in muscle and ground reaction forces [33]. Children with idiopathic scoliosis may also benefit: 149 girls, 15–25 years old with adolescent idiopathic scoliosis with Z-scores below −1, were randomized to low-magnitude, high-frequency WBV (32–37 Hz, 0.3g) for 20 min/day, 5 days weekly for 12 months [34]. The treatment group showed significant increases in femoral neck BMD from baseline (0.015–2.15g/cm2) and an increase in lumbar spine bone compared with controls. These studies suggest that vibration has greater anabolic potential in the growing skeleton, perhaps by altering the outcome of a more robust MSC pool.
The Gilsanz et al. study [25] reviewed above suggested that muscles in young females responded to LIV. In a post-hoc analysis of girls who used LIV for at least 2 min daily, an even higher influence on muscle and bone formation was seen. Even in postmenopausal women, LIV enhanced effects of a squat/lunge resistance training program; simultaneous treatment with LIV (35–40 Hz, 2–5g) induced 15% increases in knee strength [35]. Other studies support that WBV has the potential to increase effects of exercise training. Four months of high-intensity vibration (30–40 Hz, 2–2.8g) combined with resistance exercises in postmenopausal women enhanced muscular strength compared with resistance training alone at multiple sites [36]. Further, WBV training combined with exercise improved strength and balance in stroke patients. After 6 weeks, patients receiving WBV (35–40 Hz, 1.7–2.5 mm, ~16g) for 30–60 s, thrice weekly had significant improvements in lower limb strength and postural control compared with non-LIV individuals [37■]. At this point, however, we would caution that there is no evidence that high-intensity vibration performs better than low-intensity vibration, and may lead to adverse effects.
LIV may also be beneficial for postural instability due to sarcopenia or degraded neuromuscular control due to immobility [38]. LIV (39 Hz, 0.3–0.5g) was studied in healthy adults subjected to 90 days of head-down tilt bed rest. Postural stability, measured by plantar-based center displacement and velocity, showed LIV treatment defended against a loss of stability due to bed rest. Overall, LIV may limit musculoskeletal degeneration caused by physical inactivity, which leads to falls and fracture.
ANIMAL STUDIES
Animal studies proved the efficacy of LIV on bone endpoints by avoiding human study limitations of age, sex, hormonal status, and comorbidity. For instance, adult sheep exposed to daily LIV (30 Hz, 0.3g, 20 min) showed a 34% increase in femoral trabecular bone by micro-computed tomography and histology at 1 year [39,40]. In mice, only 3 weeks of LIV increased trabecular bone, with a greater response to the 0.3g, rather than 0.6g, parameter [41].
Interestingly, genetic variation within mice also modulates the sensitivity of the skeleton to mechanical stimuli. Mouse strains display varying responses to anabolic LIV treatment and the catabolic effects of disuse (unloading). In the ‘low density’ C57BL/6J mouse, hind limb unloading did not affect bone formation rate (BFR), and LIV induced significant increases in the BFR of both loaded and unloaded hind limbs. Unloading in the ’mid-density’ BALB/cByJ mouse significantly reduced BFR and this was ameliorated by LIV. Finally, the ‘high-density’ C3H/HeJ mice did not respond to either disuse or LIV [42]. This suggests that genetics influence the skeletal response to physical cues and may partially explain why vibration is not universally effective in humans.
Can LIV prevent estrogen deficiency bone loss? Treatment with LIV for 28 days following ovariectomy in mature rats led to a 159% increase in trabecular bone formation [6]. Further, in ovariectomized rat bone, LIV not only increased periosteal BFR but also decreased endocortical resorption, resulting in improved biomechanical strength [43].
Animal studies reinforce that specific genetic mutations leading to skeletal fragility also might be amenable to vibration therapy. The severe bone fragility of osteogenesis imperfecta is associated with overactive bone remodeling with disorganized woven bone, reduced BMD, and decreased mechanical properties [44]. Some improvements in skeletal endpoints have been achieved with bisphosphonates [45], but long-term therapy is concerning in growing children [46]. An osteogenesis imperfecta mouse model provides promising results for the use of vibration to improve bone properties. LIV treatment (0.3g, 45 Hz, 15 min, 5 day/week) resulted in significantly improved femoral and tibial cortical area and thickness compared with sham controls after 5 weeks, with improved trends in trabecular bone [47■■].
Vibration therapy may also be an effective adjunct to pharmacological interventions aimed at improving low bone mass. A recent study compared alendronate treatment in combination with LIV (0.3g, 45–55 Hz, 20 min/day, five times/week). After 3 months, alendronate alone induced greater improvements in trabecular bone compared with LIV alone; however, combining alendronate and LIV resulted in the greatest anabolic response [48■■]. This study demonstrates the potential for LIV to augment drug treatments targeting bone.
Fracture healing may also be improved by LIV. Individuals with low bone density or poor bone quality resulting from hormonal imbalances or genetic mutations have higher risk for fracture [49]. Bone fractures, especially in the elderly, lead to severe functional and economic burdens [50]. LIV can be delivered to bed-bound patients, at risk for fracture disunion, as it is well known that loading is critical to achieving successful fracture remodeling. Fracture repair was studied in ovariectomy (OVX) rats: LIV (35–90 Hz, 15 min/daily) resulting in improved callus density, enlarged callus area and width, accelerated osteotomy bridging, upregulated osteocalcin expression, and suppressed osteoclast activity at 30 days [51]. Another OVX rat tibial osteotomy fracture model combined LIV with either estrogen or raloxifene treatment; combination therapy with LIV and estrogens resulted in improved stiffness and increased endosteal and trabecular bone densities compared with LIV or raloxifene alone [52]. These studies suggest that LIV can enhance current pharmacological interventions for fracture healing.
DEVICES
A significant problem with the scientific literature of vibration therapy, as well as practitioner and patient comprehension, is the availability of multiple devices from which clinical information has been collected. These devices deliver different directionality (horizontal displacement from side-to-side or vertically), amplitudes (displacements resulting in gravitational force from less than 1 to greater than 15g), and frequency (5–90 Hz) [53] (Fig. 3). Some high-intensity devices are marketed as workout or slimming devices, and can increase muscle damage and even generate unwanted rotational movements in joints, which buffer impacts of loading [54]. In contrast, low-intensity vertical vibration devices are well tolerated [32,34].
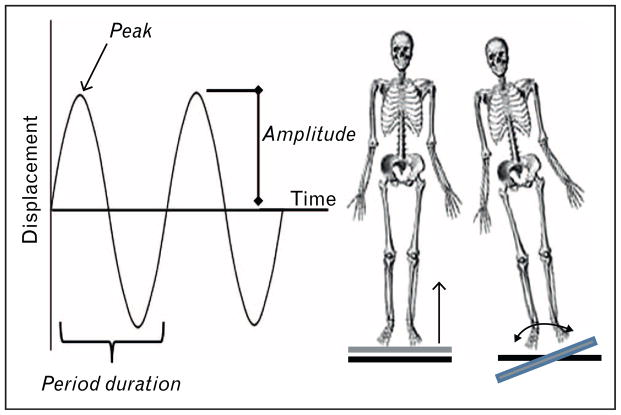
FIGURE 3.
Vibration dynamics. Displacement (amplitude) of the vibration plate, which when combined with the sinusoidal period duration (frequency, Hz) can be translated into acceleration (g-force). Vibration can be dosed horizontally with acceleration directed upward through the hips, or in side-to-side alternating vibrations in which forces are buffered by joints.
A Google search brings up more than 50 devices that deliver WBV. Most of these provide information regarding displacement and frequency. Importantly, the key index of safety (determined by occupational safety and health administration) is acceleration, or g-force. g-force is derived from a complex product of displacement and frequency (for example, displacing 1 mm at 10 Hz results in 0.4g, but increasing frequency to 50 Hz results in 10g acceleration). Most vibration devices can provide both high-magnitude (>1g) and low-magnitude (<1g) forces, but those marketed as workout adjuncts generally deliver forces greater than 4g. Such exercise devices are not appropriate for elderly or frail patients in whom the endpoint is improving bone strength [55]. When selecting a treatment regimen, we would recommend that physicians and rehabilitation specialists use devices that clearly report the vibration parameters and that deliver low-intensity (<1g), horizontal displacements at high frequencies (30–100 Hz).
CONCLUSION
Skeletal disuse leads to a wide array of consequences in the musculoskeletal system. Providing noninvasive, anabolic mechanical signals to mimic exercise in bone presents an attractive alternative to pharmacological treatments for osteoporosis. Although drug interventions have relied almost exclusively on preventing bone resorption, low-intensity vibration initiates anabolic responses and counteracts catabolic signals. Furthermore, the musculoskeletal system’s self-targeting response to mechanical signals avoids off-target effects and bestows additional benefits, including improved postural control and neuromuscular activation. These positive influences are at least partly conveyed through mechanical regulation of mesenchymal stem cells, which provide progenitors for bone and muscle growth. Although a uniform consensus regarding the most effective anabolic treatment regimen has not been reached, delivering low-magnitude mechanical signals is an appealing method to supply an exercise surrogate for those who are otherwise unable to load their skeletons.
KEY POINTS.
Low-intensity, high-frequency vibration therapy is a promising exercise analogue to stimulate anabolic responses of the musculoskeletal system.
Until it is clear that the benefits of high-intensity vibration outweigh its adverse effect consequences, physicians and other healthcare providers should consider low-intensity (<1g) treatments for their patients.
Additional studies are needed to identify the most effective intensity, frequency, and duration of the vibration treatments and to investigate its use to enhance other physical/exercise and pharmacological therapies.
Vibration therapy may cause a more pronounced anabolic effect in children, possibly due to stimulation of osteogenesis from the increased pool of mesenchymal progenitor cells in younger individuals.
Low-intensity vibration therapy likely targets multiple tissues within the neuromuscular and musculoskeletal systems, leading to an additive anabolic effect, thus improving overall musculoskeletal health.
Acknowledgments
We would like to thank Dr Gunes Uzer and Dr Clinton Rubin for their constructive feedback in the preparation of this manuscript.
Funding support: Author’s efforts were supported by AR042360 (J.R.), AR056655 (J.R.), EB014351 (J.R.), AR064133 (W.R.T.).
Footnotes
Author Contributions: W.R.T., S.S.Y., and J.R.: concept/design, manuscript writing, final approval of manuscript.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.Wolff J. The law of transformation of bone. Kirschwald: 1892. in German. [Google Scholar]
- 2.Sievanen H. Immobilization and bone structure in humans. Arch Biochem Biophys. 2010;503:146–152. doi: 10.1016/j.abb.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Chan ME, Uzer G, Rubin CT. The potential benefits and inherent risks of vibration as a nondrug therapy for the prevention and treatment of osteoporosis. Curr Osteoporos Rep. 2013;11:36–44. doi: 10.1007/s11914-012-0132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robling AG, Duijvelaar KM, Geevers JV, et al. Modulation of appositional and longitudinal bone growth in the rat ulna by applied static and dynamic force. Bone. 2001;29:105–113. doi: 10.1016/s8756-3282(01)00488-4. [DOI] [PubMed] [Google Scholar]
- 5.Lanyon LE, Rubin CT. Static vs dynamic loads as an influence on bone remodelling. J Biomech. 1984;17:897–905. doi: 10.1016/0021-9290(84)90003-4. [DOI] [PubMed] [Google Scholar]
- 6.Judex S, Lei X, Han D, Rubin C. Low-magnitude mechanical signals that stimulate bone formation in the ovariectomized rat are dependent on the applied frequency but not on the strain magnitude. J Biomech. 2007;40:1333–1339. doi: 10.1016/j.jbiomech.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Thompson WR, Rubin CT, Rubin J. Mechanical regulation of signaling pathways in bone. Gene. 2012;503:179–193. doi: 10.1016/j.gene.2012.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fritton SP, McLeod KJ, Rubin CT. Quantifying the strain history of bone: spatial uniformity and self-similarity of low-magnitude strains. J Biomech. 2000;33:317–325. doi: 10.1016/s0021-9290(99)00210-9. [DOI] [PubMed] [Google Scholar]
- 9.Huang RP, Rubin CT, McLeod KJ. Changes in postural muscle dynamics as a function of age. J Gerontol A Biol Sci Med Sci. 1999;54:B352–B357. doi: 10.1093/gerona/54.8.b352. [DOI] [PubMed] [Google Scholar]
- 10.Ozcivici E, Luu YK, Adler B, et al. Mechanical signals as anabolic agents in bone. Nat Rev Rheumatol. 2010;6:50–59. doi: 10.1038/nrrheum.2009.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pre D, Ceccarelli G, Gastaldi G, et al. The differentiation of human adipose-derived stem cells (hASCs) into osteoblasts is promoted by low amplitude, high frequency vibration treatment. Bone. 2011;49:295–303. doi: 10.1016/j.bone.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 12.Sen B, Xie Z, Case N, et al. Mechanical signal influence on mesenchymal stem cell fate is enhanced by incorporation of refractory periods into the loading regimen. J Biomech. 2011;44:593–599. doi: 10.1016/j.jbiomech.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lau E, Al-Dujaili S, Guenther A, et al. Effect of low-magnitude, high-frequency vibration on osteocytes in the regulation of osteoclasts. Bone. 2010;46:1508–1515. doi: 10.1016/j.bone.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiong J, Onal M, Jilka RL, et al. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17:1235–1241. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15■.Uzer G, Pongkitwitoon S, Ian C, et al. Gap junctional communication in osteocytes is amplified by low intensity vibrations in vitro. PLoS One. 2014;9:e90840. doi: 10.1371/journal.pone.0090840. This is the first study demonstrating regulation of gap junctional communication by low-intensity vibration in bone cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurata K, Uemura T, Nemoto A, et al. Mechanical strain effect on bone-resorbing activity and messenger RNA expressions of marker enzymes in isolated osteoclast culture. J Bone Miner Res. 2001;16:722–730. doi: 10.1359/jbmr.2001.16.4.722. [DOI] [PubMed] [Google Scholar]
- 17.Kulkarni RN, Voglewede PA, Liu D. Mechanical vibration inhibits osteoclast formation by reducing DC-STAMP receptor expression in osteoclast precursor cells. Bone. 2013;57:493–498. doi: 10.1016/j.bone.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blottner D, Salanova M, Puttmann B, et al. Human skeletal muscle structure and function preserved by vibration muscle exercise following 55 days of bed rest. Eur J Appl Physiol. 2006;97:261–271. doi: 10.1007/s00421-006-0160-6. [DOI] [PubMed] [Google Scholar]
- 19.Xie L, Rubin C, Judex S. Enhancement of the adolescent murine musculoskeletal system using low-level mechanical vibrations. J Appl Physiol. 2008;104:1056–1062. doi: 10.1152/japplphysiol.00764.2007. [DOI] [PubMed] [Google Scholar]
- 20.Keller BV, Davis ML, Thompson WR, et al. Varying whole body vibration amplitude differentially affects tendon and ligament structural and material properties. J Biomech. 2013;46:1496–1500. doi: 10.1016/j.jbiomech.2013.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubin CT, Capilla E, Luu YK, et al. Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proc Natl Acad Sci U S A. 2007;104:17879–17884. doi: 10.1073/pnas.0708467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uzer G, Manske SL, Chan ME, et al. Separating fluid shear stress from acceleration during vibrations in vitro: identification of mechanical signals modulating the cellular response. Cell Mol Bioeng. 2012;5:266–276. doi: 10.1007/s12195-012-0231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uzer G, Pongkitwitoon S, Ete Chan M, Judex S. Vibration induced osteogenic commitment of mesenchymal stem cells is enhanced by cytoskeletal remodeling but not fluid shear. J Biomech. 2013;46:2296–2302. doi: 10.1016/j.jbiomech.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubin C, Recker R, Cullen D, et al. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res. 2004;19:343–351. doi: 10.1359/JBMR.0301251. [DOI] [PubMed] [Google Scholar]
- 25.Gilsanz V, Wren TA, Sanchez M, et al. Low-level, high-frequency mechanical signals enhance musculoskeletal development of young women with low BMD. J Bone Miner Res. 2006;21:1464–1474. doi: 10.1359/jbmr.060612. [DOI] [PubMed] [Google Scholar]
- 26.Ruan XY, Jin FY, Liu YL, et al. Effects of vibration therapy on bone mineral density in postmenopausal women with osteoporosis. Chin Med J. 2008;121:1155–1158. [PubMed] [Google Scholar]
- 27.Lai CL, Tseng SY, Chen CN, et al. Effect of 6 months of whole body vibration on lumbar spine bone density in postmenopausal women: a randomized controlled trial. Clin Interv Aging. 2013;8:1603–1609. doi: 10.2147/CIA.S53591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slatkovska L, Alibhai SM, Beyene J, et al. Effect of 12 months of whole-body vibration therapy on bone density and structure in postmenopausal women: a randomized trial. Ann Intern Med. 2011;155:668–679. doi: 10.7326/0003-4819-155-10-201111150-00005. [DOI] [PubMed] [Google Scholar]
- 29.Gomez-Cabello A, Gonzalez-Aguero A, Morales S, et al. Effects of a short-term whole body vibration intervention on bone mass and structure in elderly people. J Sci Med Sport. 2014;17:160–164. doi: 10.1016/j.jsams.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 30.von Stengel S, Kemmler W, Engelke K, Kalender WA. Effects of whole body vibration on bone mineral density and falls: results of the randomized controlled ELVIS study with postmenopausal women. Osteoporos Int. 2011;22:317–325. doi: 10.1007/s00198-010-1215-4. [DOI] [PubMed] [Google Scholar]
- 31.Zha DS, Zhu QA, Pei WW, et al. Does whole-body vibration with alternative tilting increase bone mineral density and change bone metabolism in senior people? Aging Clin Exp Res. 2012;24:28–36. doi: 10.3275/7517. [DOI] [PubMed] [Google Scholar]
- 32.Ward K, Alsop C, Caulton J, et al. Low magnitude mechanical loading is osteogenic in children with disabling conditions. J Bone Miner Res. 2004;19:360–369. doi: 10.1359/JBMR.040129. [DOI] [PubMed] [Google Scholar]
- 33.Semler O, Fricke O, Vezyroglou K, et al. Results of a prospective pilot trial on mobility after whole body vibration in children and adolescents with osteogenesis imperfecta. Clin Rehabil. 2008;22:387–394. doi: 10.1177/0269215507080763. [DOI] [PubMed] [Google Scholar]
- 34.Lam TP, Ng BK, Cheung LW, et al. Effect of whole body vibration (WBV) therapy on bone density and bone quality in osteopenic girls with adolescent idiopathic scoliosis: a randomized, controlled trial. Osteoporos Int. 2013;24:1623–1636. doi: 10.1007/s00198-012-2144-1. [DOI] [PubMed] [Google Scholar]
- 35.Verschueren SM, Roelants M, Delecluse C, et al. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study. J Bone Miner Res. 2004;19:352–359. doi: 10.1359/JBMR.0301245. [DOI] [PubMed] [Google Scholar]
- 36.Bemben DA, Palmer IJ, Bemben MG, Knehans AW. Effects of combined whole-body vibration and resistance training on muscular strength and bone metabolism in postmenopausal women. Bone. 2010;47:650–656. doi: 10.1016/j.bone.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 37■.Tankisheva E, Bogaerts A, Boonen S, et al. Effects of intensive whole-body vibration training on muscle strength and balance in adults with chronic stroke: a randomized controlled pilot study. Arch Phys Med Rehabil. 2014;95:439–446. doi: 10.1016/j.apmr.2013.09.009. This randomized, 12-month long study in postmeopausal women revealed no significant differences on bone outcomes among individuals treated with WBV compared with controls. This work contrasts findings of other clinical trials but provides critical insight into the heterogeniety of the responses to vibration therapy. [DOI] [PubMed] [Google Scholar]
- 38.Muir J, Judex S, Qin YX, Rubin C. Postural instability caused by extended bed rest is alleviated by brief daily exposure to low magnitude mechanical signals. Gait Posture. 2011;33:429–435. doi: 10.1016/j.gaitpost.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rubin C, Turner AS, Bain S, et al. Anabolism. Low mechanical signals strengthen long bones. Nature. 2001;412:603–604. doi: 10.1038/35088122. [DOI] [PubMed] [Google Scholar]
- 40.Rubin C, Turner AS, Muller R, et al. Quantity and quality of trabecular bone in the femur are enhanced by a strongly anabolic, noninvasive mechanical intervention. J Bone Miner Res. 2002;17:349–357. doi: 10.1359/jbmr.2002.17.2.349. [DOI] [PubMed] [Google Scholar]
- 41.Garman R, Gaudette G, Donahue LR, et al. Low-level accelerations applied in the absence of weight bearing can enhance trabecular bone formation. J Orthop Res. 2007;25:732–740. doi: 10.1002/jor.20354. [DOI] [PubMed] [Google Scholar]
- 42.Judex S, Donahue LR, Rubin C. Genetic predisposition to low bone mass is paralleled by an enhanced sensitivity to signals anabolic to the skeleton. FASEB J. 2002;16:1280–1282. doi: 10.1096/fj.01-0913fje. [DOI] [PubMed] [Google Scholar]
- 43.Oxlund BS, Ortoft G, Andreassen TT, Oxlund H. Low-intensity, high-frequency vibration appears to prevent the decrease in strength of the femur and tibia associated with ovariectomy of adult rats. Bone. 2003;32:69–77. doi: 10.1016/s8756-3282(02)00916-x. [DOI] [PubMed] [Google Scholar]
- 44.Forlino A, Cabral WA, Barnes AM, Marini JC. New perspectives on osteogenesis imperfecta. Nat Rev Endocrinol. 2011;7:540–557. doi: 10.1038/nrendo.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bishop N, Adami S, Ahmed SF, et al. Risedronate in children with osteogenesis imperfecta: a randomised, double-blind, placebo-controlled trial. Lancet. 2013;382:1424–1432. doi: 10.1016/S0140-6736(13)61091-0. [DOI] [PubMed] [Google Scholar]
- 46.Ward LM, Rauch F. Oral bisphosphonates for paediatric osteogenesis imperfecta? Lancet. 2013;382:1388–1389. doi: 10.1016/S0140-6736(13)61531-7. [DOI] [PubMed] [Google Scholar]
- 47■■.Vanleene M, Shefelbine SJ. Therapeutic impact of low amplitude high frequency whole body vibrations on the osteogenesis imperfecta mouse bone. Bone. 2013;53:507–514. doi: 10.1016/j.bone.2013.01.023. This is the first study demonstrating the anabolic effects of vibration therapy in a mouse model of osteogenesis imperfecta, providing critical information to design mechanistic studies capable of elucidating the effects vibration on this debilitating genetic condition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48■■.Chen GX, Zheng S, Qin S, et al. Effect of low-magnitude whole-body vibration combined with alendronate in ovariectomized rats: a random controlled osteoporosis prevention study. PLoS One. 2014;9:e96181. doi: 10.1371/journal.pone.0096181. This study demonstrates an additive effect in BMD enhancement when combining vibration therapy with pharmacological treatments, providing important insights into the potential for combination therapies to target bone disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roschger P, Paschalis EP, Fratzl P, Klaushofer K. Bone mineralization density distribution in health and disease. Bone. 2008;42:456–466. doi: 10.1016/j.bone.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 50.Hoy DG, Smith E, Cross M, et al. The global burden of musculoskeletal conditions for 2010: an overview of methods. Ann Rheum Dis. 2014;73:982–989. doi: 10.1136/annrheumdis-2013-204344. [DOI] [PubMed] [Google Scholar]
- 51.Komrakova M, Sehmisch S, Tezval M, et al. Identification of a vibration regime favorable for bone healing and muscle in estrogen-deficient rats. Calcif Tissue Int. 2013;92:509–520. doi: 10.1007/s00223-013-9706-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stuermer EK, Komrakova M, Sehmisch S, et al. Whole body vibration during fracture healing intensifies the effects of estradiol and raloxifene in estrogen-deficient rats. Bone. 2014;64:187–194. doi: 10.1016/j.bone.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 53.Rauch F, Sievanen H, Boonen S, et al. Reporting whole-body vibration intervention studies: recommendations of the International Society of Musculoskeletal and Neuronal Interactions. J Musculoskelet Neuronal Interact. 2010;10:193–198. [PubMed] [Google Scholar]
- 54.Rittweger J, Schiessl H, Felsenberg D. Oxygen uptake during whole-body vibration exercise: comparison with squatting as a slow voluntary movement. Eur J Appl Physiol. 2001;86:169–173. doi: 10.1007/s004210100511. [DOI] [PubMed] [Google Scholar]
- 55.Muir J, Kiel DP, Rubin CT. Safety and severity of accelerations delivered from whole body vibration exercise devices to standing adults. J Sci Med Sport. 2013;16:526–531. doi: 10.1016/j.jsams.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]