Abstract
Objective
To determine the relationship between peak isometric muscle force and temporal characteristics of gait in individuals with sporadic inclusion body myositis (s-IBM).
Patients and Methods
An observational study of 42 individuals with s-IBM (12 female; age: 61.6 ±7.3 years [mean ±standard deviation]; disease duration 8.9 ±4.3 years) was conducted at a Federal hospital. Peak isometric force measurements for lower extremity (LE) muscle groups were obtained using quantitative muscle testing. Temporal characteristics of gait during habitual and fast walking conditions were measured using a portable gait analysis system.
Results
All observed muscle force values were significantly lower than predicted values (p <.001). During habitual walking, subjects’ gait speed and cadence were < 83% of normative literature values. During the fast walking, total gait cycle time was 133% of normal, while gait speed and cadence were 58% and 78%, respectively, of normative literature values. Scaled LE peak muscle forces showed significant moderate correlations with the temporal gait variables. Weaker subjects demonstrated greater limitations in gait speed and cadence compared to stronger subjects (p <.05). Peak isometric force of the knee flexors and ankle plantar flexors, but not knee extensors, were significantly correlated with most temporal features of habitual gait.
Conclusions
Muscle weakness associated with s-IBM disease activity may contribute to diminished gait kinematics. Temporal features of gait are not substantially influenced by knee extensor weakness alone, as the knee flexors and ankle plantar flexors play a compensatory role in maintaining the walking ability of individuals with s-IBM.
Sporadic inclusion body myositis (s-IBM) is recognized as the most commonly diagnosed idiopathic inflammatory myopathy (IIM) in adults over the age of 50 years. The incidence of s-IBM is thought to range from 1 per 100,000 to 5-10 per million in the United States, Europe, and Australia (1-5). It is thought to be the most common inflammatory myopathy among elders (6), and the condition is marked by an insidious onset and progressive clinical course (7-9). Although confirmed cases of s-IBM still may be considered relatively rare as a cause of muscle weakness in older individuals, misdiagnosis with other forms of myopathy is common, leading to under-estimation of the true number of cases in the population (1, 10).
Many investigators have reported clinically meaningful relationships between muscle strength and gait characteristics in both healthy adults and people with various types of lower extremity (LE) disablement (11-16). Nonetheless, there are very few published reports pertaining to the presence and etiology of specific gait deviations in individuals with s-IBM. Basquiera and associates (17) observed a heterogeneous presentation of LE weakness and concomitant gait abnormalities in their case report series involving 4 people diagnosed with IBM. Cortese and colleagues (18) reported that manual muscle test scores declined by 5.2% and quantitative dynamometry values for the knee extensors declined by 27.9% in their sample of individuals with s-IBM. Moreover, they stated that over 60% of their participants failed to maintain their independent ambulation status. Bernhardt and colleagues (19) identified a hyperextended knee during stance phase of gait in a proportion of their sample of individuals with s-IBM, while others displayed the typical knee flexion in loading response and neutral knee positioning during mid to late stance. All participants had normal peak knee flexion in early swing, with a few demonstrating excessive peak knee flexion during this portion of the gait cycle. The observed kinematic abnormalities were qualitatively associated with isometric strength values.
Limitations of existing studies affect our collective understanding of potential relationships between muscle function and gait kinematics in individuals with s-IBM. While clinical reports such as those by Cortese and colleagues (18) serve to inform both practitioners and investigators of the consequences of muscle weakness and other impairments that are part of the clinical presentation of s-IBM, they also highlight the need for clinical studies with adequate strength, falls, and gait outcomes. Basquiera and associates (17) report on much needed cross-sectional and longitudinal data concerning the natural history and physical performance of people with s-IBM. The combined strength and ambulation status findings presented by the investigators illustrate the utility of quantitative strength measures and a careful account of independence during upright mobility. Nevertheless, rehabilitation specialists depend on kinematic gait parameters to inform clinical decisions associated with interventions and adaptive equipment. The promising initial findings of Bernhardt and colleagues (19) provide objective data and greatly enhance our understanding of gait in people with s-IBM. Despite the rigor of this group's motion capture methods used to characterize gait kinematics and kinetics, they were constrained by a modest sample size. In addition, temporal features of gait and statistical analyses related to the potential etiology of observed deviations in gait kinematics in s-IBM were not reported.
The chief purpose of this study was to measure temporal features of gait in individuals with s-IBM. The secondary purpose of this study was to determine the association between temporal measures of gait and quantitative measures of muscle performance in our sample. We hypothesized that the LE muscle maximum voluntary isometric contraction (MVIC) values would significantly differ from literature-reported normative reference data, and that LE MVIC levels would determine meaningful differences in temporal gait characteristics.
Patients and Methods
Subjects
This study was conducted as part of a National Institute of Neurological Disorders and Stroke (NINDS; NCT00030212) clinical trial to characterize immune system irregularities in people with s-IBM and the resultant disablement associated with this form of muscle disease. Subjects were recruited from a NINDS Neuromuscular Diseases Section database. All subjects were referred to the Rehabilitation Medicine Department for the assessment of functional performance. Inclusion criteria were adults over the age of 40 years with a confirmed diagnosis of s-IBM. The pathological criteria for a definite diagnosis of s-IBM included open biopsy findings per previously published criteria (2). Exclusion criteria included cardiovascular disease, renal disease, and muscle disease that were advanced enough to preclude regular travel to the hospital for regular testing involved with the parent study, as well as joint instability that would contraindicate muscle testing. This study was approved by the NINDS Institutional Review Board.
Procedure
After signed informed consent was obtained, measurement of MVIC and gait kinematics occurred on separate days over a 2-day period.
MVIC assessment
Quantitative muscle testing (QMT, Biomech Designs, Ltd., Alberta, Canada) was used to measure MVIC. Precision of the force transducer (Interface, Scottsdale, AZ) was .5 N to1000 N with 150% overload protection (20). The QMT System software was used to negate passive force on the force transducer caused by the weight and position of the tested extremity prior to data collection. Calibration of the dynamometer was performed in accordance with manufacturer guidelines, and MVIC measures were recorded in kg. Two 5 s intrasession trials for a given muscle group were separated by a rest period of 20-30 s.
Mean MVIC values, expressed as an index of force scaled to body weight, represented the construct of muscle strength for the purposes of this study. The bilateral muscle groups assessed included the hip extensors, hip flexors, knee extensors, knee flexors, and ankle plantar flexors. The summed LE MVIC values were calculated. Subjects who exceeded the median summed LE MVIC were assigned to the “high force” subgroup, and subjects who exerted less than the median value for summed LE MVIC were assigned to the “low force” subgroup.
MVIC has been found to be a sensitive and reliable method of strength testing in subjects with neuromuscular disease (21, 22). Observed MVIC values were compared to predicted levels based on the method proposed by Stoll and associates (23). Their multiple regression models featured MVIC as the predicted variable with predictor variables based on the specific muscle group, right or left-sided extremity, sex, and age.
Gait kinematics
The Stride Analyzer (Model SA-Iv; B & L Engineering, Santa Fe Springs, CA) provided a measure of habitual and fast gait speed over a straight 10 m walkway. This device utilizes a light-controlled trigger in conjunction with shoe insoles that measure limb contact and swing temporal patterns and a portable data recorder. Participants were instructed to perform trials 1 and 2 at habitual walking speed and trials 3 and 4 at their fastest walking speed. The mean values for temporal gait characteristics were selected for use in the data analysis. Subjects used their gait aids and orthotics as necessary, and the examiner accompanied each participant during the trials to provide additional safety. Test-retest reliability for gait speed measured by the Stride Analyzer was reported as good to excellent (ICC2,1 = .88 to .98) in patients with neurological disorders (24, 25).
Gait parameters expressed in units of time included speed (s), cadence (steps/min), and the gait cycle (s). Additionally, gait speed was scaled to body height and expressed in statures/s. Gait parameters expressed as a percentage of the gait cycle included double limb support time, single limb support time, swing phase time, and stance phase time. Selected gait parameters were compared to reported normative values from various published studies. Many studies reported the influence of age and sex on gait characteristics (26-31). A priori criteria, based primarily on sex and then decade of age, were used to select normative data for comparisons. Additional considerations included the number of the participants and the gait analysis method featured in the studies. While it would have been optimal to use one study for all normative data comparisons, no single investigation featured all of the gait parameters assessed in this study.
Data from Murray and colleagues (26, 32) as well as from Bohannon and colleagues (33) were used for both habitual and fast speed normative values. Both of these authors reported gait speed as well as height measurements so gait speed was either expressed in statures/s or calculated from the raw data. Their speed data was reported by differing age ranges, thus our 50-59-year-old participants were compared to data from the Murray and associates (26, 32) studies while our 60-69-year-old and 70-79-year-old participants were compared to data from the Bohannon and colleagues (33) study. For habitual and fast cadence and total gait cycle time, reported values from Murray and associates (26, 32) and Oberg and associates (27) were used because both groups reported these values by sex and by age. Data that were not classified by sex or age were not utilized because of the significant influence of gender and age on gait kinematics in community-dwelling adults.
Data analysis
Statistical analyses were performed with SPSS for Windows (Version 10.0.5, SPSS Inc., Chicago, IL). Descriptive statistics were calculated for subject characteristics, MVIC, and temporal gait variables. Symmetry of summed LE MVIC and gait variables was estimated by ratios of right and left values. The relationship between MVIC and temporal gait variables was determined with the Spearman correlation coefficient (ρ). Multiple regression analysis was used to examine the association between gait speed and LE MVIC, with potential covariates such as body mass index (BMI), age, disease duration, age of disease onset, prednisone usage, and presence of orthopedic conditions. Point biserial correlation was used to determine the relationship between temporal gait variables and sex. Statistical significance of differences in temporal gait characteristics between high force/low force subgroups were analyzed using the Mann–Whitney U test; differences in strength and gait speed based on gait aid use were also examined. Principal axis factoring with varimax rotation and Kaiser-Meyer-Olkin sampling statistics was explored to determine the relative contribution of critical muscle groups to ambulation performance as described by Cohen (34). Alpha level was set at .05 for all statistical analyses, and normality of data and variance distribution of the data was examined. Non-parametric statistics were used for data exhibiting significant departures from normality regarding data and variance distribution based on the Shapiro-Wilk test, Levene's test, and a visual inspection of box plots.
Results
Participant characteristics
Our sample included 42 participants (12 females) with mean age of 61.6 ±7.3 years (range: 50-78 years) and a mean BMI of 27.3 ±4.6 (Table 1). All participants were diagnosed with s-IBM as confirmed by muscle biopsy. The mean age at disease onset for the sample was 53.8 ±9.0 years (38-72 years) and mean disease duration was 8.9 ±4.3 years (4-21 years). No strength or gait speed differences were attributed to sex or age (p > .05). The high force subgroup had a significantly lower BMI compared to the low-force subgroup (p < .05). However, only LE MVIC was significantly associated with gait speed within each subgroup and among the total sample. Nineteen individuals used a gait aid (cane: 12; rolling walker: 3; standard walker: 2; Loftstrand crutch: 1; axillary crutch: 1). Significant differences were observed in summed LE MVIC (p <.002) and habitual gait speed (p <.027) based on gait aid use. All force and gait parameters were approximately symmetrical between sides. Thus, data from the right LE was used for subsequent analyses.
Table 1.
Demographic data for all subjects and a priori designated subgroups. All values given as mean ± standard deviation (range), except where indicated. P-value references comparison between low force and high force subgroups (independent-samples t-test).
| Total Sample (n = 42) | Low Force Subgroup (n = 21) | High Force Subgroup (n = 21) | |
|---|---|---|---|
| Age (yrs) | 61.8 ±7.3 (50 – 78) | 62.4 ±8.0 (50 – 78) | 63.0 ±6.7 (52 – 77) |
| Weight (kg) | 87.7 ±19.7 (49.0 – 134.6) | 93.9 ±20.7 (59.0 – 134.6) | 78.1 ±14.9 (49.0 – 101.1)† |
| Height (cm) | 178.5 ±9.7 (160.0 – 195.6) | 181.4 ±10.8 (162.5 – 195.6) | 174.7 ±8.1 (160.0 – 187.0)* |
| Body mass index | 27.3 ±4.6 (18.9 – 40.2) | 28.4±5.4 (18.9 – 40.2) | 25.4 ±3.6 (19.1 – 31.6)† |
| Summed LE MVIC§ | 1.38 ±0.39 (0.72 – 2.38) | 1.05 ±0.18 (0.72 – 1.30) | 1.67 ±0.29 (1.31 – 2.38)* |
| Prednisone∥ | 6 (n/a) | 4 (n/a) | 2 (n/a) |
| LE orthopedic conditions‡∥ | 16 (n/a) | 9 (n/a) | 7 (n/a) |
statistically significant, p < .01
statistically significant, p < .05
scaled to body weight (kg/kg)
LE orthopedic conditions within the sample: history of gout (6), osteoarthritis (6), previous fracture (3), total hip arthroplasty (1), total knee arthroplasty (1); note: frequency sum exceeds 16 due to co-existing conditions
data expressed as a frequency count
Abbreviations:
cm: centimeters
kg: kilograms
LE: lower extremity
MVIC: maximum voluntary isometric contraction
SD: standard deviation
yrs: years
MVIC characteristics
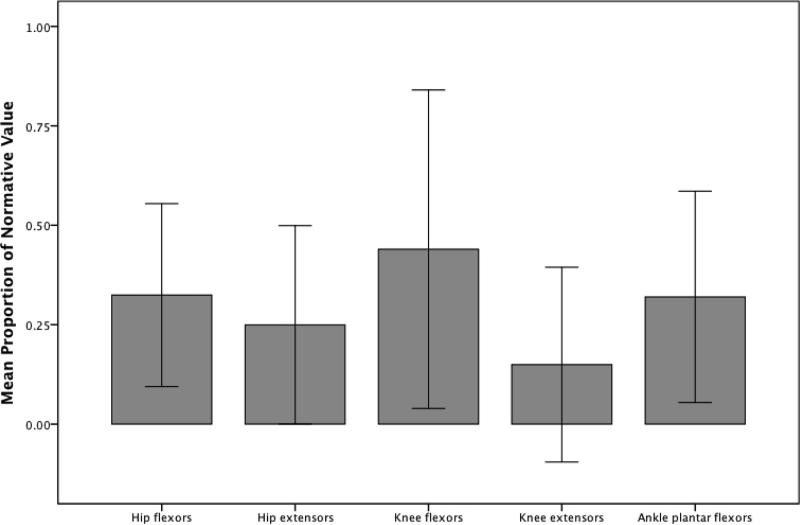
All observed MVIC values were significantly lower than predicted values (p < .001; Figure 1). The knee extensors and hip extensors demonstrated the greatest relative weakness compared to predicted values, with 14.9% and 23.8% of predicted, respectively. The hip flexors were 32.0% of predicted, and the ankle plantar flexors were 31.8% of predicted values. Knee flexors in individuals with s-IBM showed the greatest preservation of MVIC compared to predicted levels, with values of 41.9% of predicted. Aggregate values of all 5 muscle groups compared to the predicted normal reference values were 27%.
Figure 1.
Peak isometric force for lower extremity muscle groups in our sample of individuals with inclusion body myositis (n = 42) expressed as a proportion of literature-based normative values (1.00). Error bars represent ±2 standard deviations. All differences between the cohort and normative values are statistically significant (p < .001; Mann–Whitney U test).
Temporal characteristics of gait
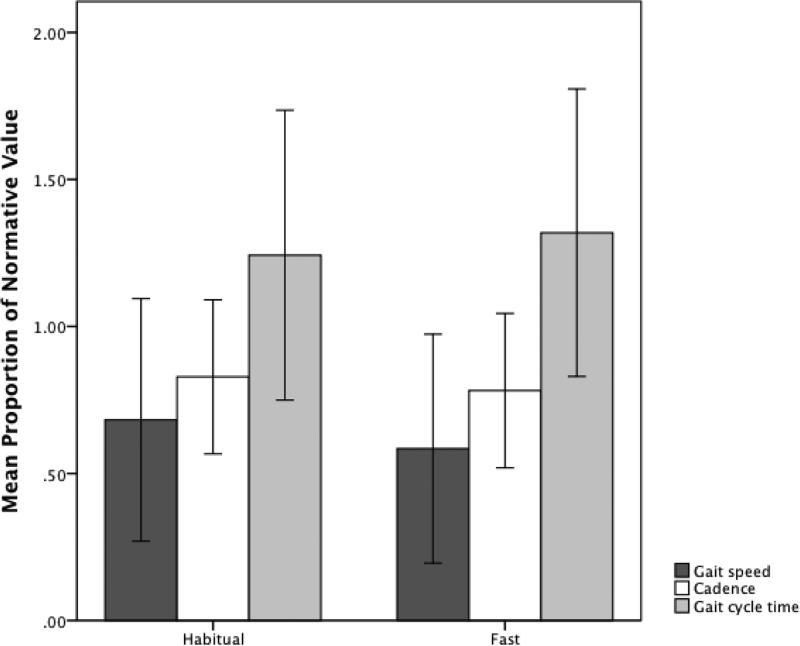
Participants exhibited comparatively longer gait cycle time, slower gait speed, and decreased cadence than the reference literature values during both habitual and fast gait conditions (Figure 2). None of the temporal gait variables were significantly associated with age, sex, disease duration, or age of disease onset. Gait cycle time appeared to be longer, and gait speed slower, in comparison to the normative values across both gait conditions. However, discrepancies between temporal gait characteristics for individuals with s-IBM and normative data increased during the fast walking condition (Figure 2). Additionally, the percentage of gait cycle time spent in double limb support, stance phase, and swing phase did not appreciably differ between habitual and fast walking conditions.
Figure 2.
Temporal characteristics of gait in our sample of individuals with inclusion body myositis (n = 42) during gait at habitual walking speed and fast walking speed, expressed as a proportion of published literature values (1.00). Error bars represent ±2 standard deviations.
Relationship between temporal gait variables and MVIC
The scaled LE MVIC values showed a significant, but moderate association, with the temporal gait variables (Table 3). Ankle plantar flexors and knee flexors were the muscle groups with the strongest association with walking performance. For the habitual walking speed condition, ankle plantar flexion MVIC was significantly associated with gait speed, cadence, and gait cycle time (p < .01). During habitual walking, the knee flexion MVIC was significantly associated with gait speed, cadence, gait cycle time, and stance time (p < .05). In general, ankle plantar flexion and knee flexion were the MVICs exhibiting the strongest and most consistent associations with the gait variables (Table 3). The association of knee extension with gait speed, cadence, and gait cycle time became significant in the fast gait speed condition (p < .05). The magnitude of the association between strength and walking performance was mildly improved by using aggregate LE forces. The summed LE MVIC was significantly correlated with gait speed, cadence, and gait cycle time in both walking conditions (p < .05).
Table 3.
Association between isometric peak force (scaled to body weight) and temporal measurements of gait during habitual speed and fast speed conditions in our sample of individuals with inclusion body myositis (n = 42). Values represent Spearman correlation coefficients.
| Speed (statures) | Cadence (steps/min) | Gait cycle time(s) | Double limb support time (%GC) | Stance time (%GC) | Swing time (%GC) | |
|---|---|---|---|---|---|---|
| Habitual gait speed | ||||||
| Hip flexion | .319† | .330† | −.339† | −.258 | .308† | .186 |
| Hip extension | .157 | .103 | −.099 | −.129 | .243 | −.007 |
| Knee flexion | .333† | .398* | −.396* | −.461* | .306 | .466* |
| Knee extension | .317† | .265 | −.258 | .096 | −.069 | −.059 |
| Ankle plantar flexion | .515* | .455* | −.453* | −.259 | .168 | .273 |
| LE summed force | .458* | .418* | −.414* | −.259 | .317† | .180 |
| Fast gait speed | ||||||
| Hip flexion | .315† | .317† | −.315† | −.231 | .240 | .151 |
| Hip extension | .289 | .321† | .321† | −.116 | .234 | .077 |
| Knee flexion | .333† | .433* | −.435* | −.497* | .320† | .448* |
| Knee extension | .423* | .386† | −.387† | .147 | −.098 | −.104 |
| Ankle plantar flexion | .503* | .485* | −.493* | −.259 | .073 | .171 |
| LE summed force | .537* | .558* | −.561* | −.248 | .218 | .156 |
statistically significant, p < .01
statistically significant, p < .05
Abbreviations
%GC: percentage of the gait cycle
LE: lower extremity
min: minute
s: seconds
Principal axis factoring revealed potentially relevant groupings of muscles in participants who performed below median walking speed in the habitual and fast gait conditions (Table 4). A 2-factor solution was identified for both gait speed conditions. Factor 1 consisted of proximal LE muscle groups, whereas Factor 2 consisted solely of the ankle plantar flexors (varimax-rotated factor loading fl: .860) in the habitual gait condition. Knee extensor MVIC did not load onto either factor in the habitual gait speed condition. However, in the 2-factor solution identified for the fast gait speed condition, Factor 1 again consisted of primarily the proximal LE muscle groups and Factor 2 consisted only of knee extensor MVIC (FL: .758). The identified 2-factor solutions accounted for 67% of the sample variance in both gait speed conditions (Kaiser-Meyer-Olkin sampling statistic, p < .05).
Table 4.
Varimax rotated principal axis factor loadings for scaled peak isometric forces of selected lower extremity muscles, considering subjects performing below median walking speeds in the habitual and fast gait conditions. Results suggest unique contribution of the ankle plantar flexor peak isometric force to habitual gait performance and knee extensor peak isometric force to fast gait performance in this subgroup of subjects with inclusion body myositis.
| Habitual gait speed less than median value | ||
|---|---|---|
| Factor 1 | Factor 2 | |
| Hip flexors | .957 | .086 |
| Hip extensors | .494 | .204 |
| Knee flexors | .672 | .100 |
| Knee extensors | −.041 | −.343 |
| Ankle plantar flexors | .222 | .860 |
| Fast gait speed less than median value | ||
|---|---|---|
| Factor 1 | Factor 2 | |
| Hip flexors | .951 | −.153 |
| Hip extensors | .566 | −.003 |
| Knee flexors | .632 | −.159 |
| Knee extensors | .036 | .782 |
| Ankle plantar flexors | .244 | −.355 |
High force and low force subgroup analysis
Significant differences in walking performance were detected between the high force and low force subgroups (Table 2). Participants in the low force subgroup demonstrated greater limitations in gait speed and cadence (p < .01) during the habitual and fast gait conditions compared to the high force subgroup. Gait cycle time was significantly longer for the low force subgroup in the fast gait condition compared to the high force subgroup (p = .01). Also, double limb support time was significantly longer for the low force subgroup in both habitual and fast gait conditions (p < .01).
Table 2.
Temporal features of gait for individuals with inclusion body myositis who scored above the median value for summed lower extremity isometric peak force (high force subgroup) and below the median value for summed lower extremity peak force (low force subgroup). P-value references comparison between low force and high force subgroups (Mann–Whitney U test).
| Low Force Subgroup (n=21) | High Force Subgroup (n=21) | |||
|---|---|---|---|---|
| Median (IQR) | % of normal | Median (IQR) | % of normal | |
| Habitual gait speed | ||||
| Speed (statures) | .52 (.36 – .60) | 59 ±20 | .65 (.57 – .73)* | 76 ±17 |
| Cadence (steps/min) | 96.8 (80.8 – 100.9) | 79 ±12 | 104.4 (98.1 – 112.5)* | 86 ±13 |
| Gait cycle time (s) | 1.24 (1.19 – 1.49) | 129 ±21 | 1.15 (1.06 – 1.22)† | 120 ±26 |
| Double limb support time (%GC) | 35.5 (32.2 – 38.7) | n/a | 31.8 (29.0 – 33.5)* | n/a |
| Stance time (%GC) | 32.6 (31.1 – 34.2) | n/a | 34.1 (33.5 – 36.3)* | n/a |
| Swing time (%GC) | 32.2 (30.0 – 33.7) | n/a | 34.4 (32.7 – 35.5)† | n/a |
| Fast gait speed | ||||
| Speed (statures) | .57 (.38 – .71) | 48 ±18 | .81 (.65 – .97)* | 66 ±16 |
| Cadence (steps/min) | 107.2 (85.5 – 112.3) | 73 ±13 | 120.3 (113.3 – 131.7)* | 83 ±11 |
| Gait cycle time (s) | 1.12 (1.07 – 1.41) | 142 ±35 | 1.00 (.91 – 1.06)* | 123 ±19 |
| Double limb support time (%GC) | 33.7 (31.2 – 41.0) | n/a | 30.3 (28.0 – 33.4)† | n/a |
| Stance time (%GC) | 33.6 (30.4 – 35.3) | n/a | 34.8 (32.8 – 36.4) | n/a |
| Swing time (%GC) | 31.6 (29.9 – 34.5) | n/a | 35.3 (31.6 – 35.7) | n/a |
statistically significant, p < .01
statistically significant, p < .05
Abbreviations
%GC: percentage of the gait cycle
IQR: interquartile range
min: minutes
s: seconds
Discussion
We have documented temporal characteristics of gait, LE MVIC, and the association between gait and LE strength in individuals with s-IBM. Compared to age-referenced normative values, our study participants appeared to have slower gait speed, cadence, and longer gait cycle time. Walking in older individuals is commonly characterized by decreased speed and stride length, a maintained or increased cadence, and increased double limb support and gait cycle time, compared to younger adults (27, 28, 32, 33, 35-37). In this study, temporal kinematic data from individuals with s-IBM generally resembled these age-related differences. However, participants in this study demonstrated slower temporal characteristics than their age- and sex-matched reference samples in all of the selected categories.
Habitual cadence came closest of the temporal gait measurements obtained in this study to normative values, and may represent a strategy employed to control speed. Habitual cadence was significantly different between low force and high force subgroups, and the high force subgroup used a faster cadence and a reduced double limb support time. We noted that all individuals were able to increase their walking speed in the fast gait condition, but they could not significantly decrease their double limb support. Since a reduction in double limb support is coupled with increases in stride length, we speculate that our participants increased gait speed between habitual and fast walking conditions by increasing cadence without increasing stride length.
Analyzing gait characteristics based on summed LE MVIC revealed significant and clinically important limitations in walking performance in the low force subgroup in comparison to the high force subgroup. Thus, we believe LE muscle weakness of s-IBM mediates the observed changes in temporal gait characteristics, rather than typical age-related sarcopenia and balance changes. In support of this assertion, participants in the high force subgroup demonstrated a habitual speed that was almost 25% faster than the low force subgroup and yet only approached 75% of normal. Interestingly, despite the reported asymmetry of muscle strength in s-IBM, all unilateral gait parameters and summed LE forces for our subjects were symmetric.
Temporal features of gait in individuals with s-IBM were significantly related to distal LE muscle weakness in this study. Ankle plantar flexor MVIC had the strongest association with walking speed in the habitual and fast gait conditions. The results of the factor analysis also suggested the ankle plantar flexor MVIC as uniquely important in individuals with slow habitual gait speed. Our observations parallel the findings of other investigators who found ankle plantar flexor strength to be a strong predictor of gait speed in older persons (11, 29, 31, 38). Indeed, the ankle plantar flexor muscle group demonstrated the highest level of force (Figure 1) of all muscle groups measured in this study and may be the key to both stability and propulsion. Normally, hip extensors work with ankle plantar flexors to provide upright stability and help maintain knee posture during early stance and forward momentum during late stance of the gait cycle (37). In this study, however, hip extensor MVIC showed low and non-significant correlations with many temporal gait features. By contrast, knee flexor MVIC demonstrated significant moderate correlations with many temporal gait features. These findings suggest the hip extensors work to small advantage for forward momentum while the knee flexors and plantar flexors may co-contract to maintain a prolonged knee extension moment for stability in stance, in the presence of knee extensor weakness. The ankle plantar flexors, the strongest muscle group in our sample, may then continue to function through terminal stance for forward propulsion.
One recent study of gait and LE muscle force in individuals with s-IBM provides context for the findings in our study. Bernhardt and colleagues (19) reported on 9 subjects with s-IBM and quadriceps weakness. Using motion capture and force plates, these authors described the range of walking patterns that were present in their sample. Knee extensor weakness was thought to be a primary predictor of gait deviations at the knee, because subjects with lower knee extensor strength were qualitatively observed to demonstrate knee hyperextension during midstance more frequently than subjects with higher knee extensor strength. In our study, however, knee extensor MVIC was not significantly associated with temporal features of gait at habitual speeds. Factor analysis revealed knee extensor muscle weakness to be uniquely relevant in participants attempting fast gait speeds who were below the median value for our sample. Although knee extensor MVIC may be responsible for kinematic and kinetic abnormalities of knee mechanics during fast gait (19), our findings suggest temporal features of gait are not substantially influenced by knee extensor weakness alone. Bernhardt and colleagues (19) posited that the performance of other muscles crossing the hip, knee, and ankle may allow compensation for knee extensor weakness and produce a range of walking abilities in individuals with s-IBM. Our findings corroborate this assertion, because knee flexor and ankle plantar flexor MVIC was significantly associated with most temporal features of gait. In this study, temporal features of gait were significantly different at habitual and fast walking speeds between subgroups of individuals with s-IBM based on summed LE MVIC. Consequently, this aggregate force profile may be more pertinent to the explanation of gait performance and fall risk in individuals with s-IBM than a more isolated approach that emphasizes kinematics and kinetics at a single joint.
This study adds to our collective understanding of the nuanced interplay between strength and ambulatory function in people with s-IBM; future research should continue to build on its findings by addressing certain methodological limitations. Measurements of spatial features (e.g., step length), joint-specific kinematics, and kinetics of gait were not collected in this study, so conclusions regarding these characteristics cannot be drawn based on our data. Also, the use of differing sources of normative reference data for gait parameters may introduce potential confounding factors in the interpretation of the descriptive comparisons. However, it is important to note that these reference data were not subject to any inferential statistical analysis. MVIC measurements are common in the s-IBM literature, so they were utilized in this study. Nevertheless, MVIC measurements may not generalize well to the functional requirements of gait, which are typically repetitive submaximal contractions. Thus, future studies may feature an examination of muscle power and fatigability measurements. Our observations concerning the temporal features of gait and LE MVIC may have been affected by the use of assistive devices by 19 individuals. The magnitude of weakness in some of the participants, along with the significant fall history within both groups, required the use of these devices as a matter of clinical necessity in order to safely measure gait performance. Additionally, the principal axis factoring used in the analysis was exploratory and descriptive in nature, and has limitations regarding its generalization beyond our study sample. Finally, the authors examined muscle groups that have a primary action in sagittal plane during gait. The finding that force decrement of multiple muscles across the lower extremities has a substantial influence on walking patterns in our sample suggests a similar need for future work to measure force output of muscles with primary action in the frontal or transverse plane to better understand pathological gait in people with s-IBM.
Significance and Innovation.
Despite our participant assignment to low force and high force subgroups, the entire sample was notable for their severe lower extremity strength deficits (27.4% of predicted values). Nevertheless, significant differences in peak muscle force distinguished meaningful differences in temporal gait characteristics among our participants with s-IBM and frank muscle weakness.
Our observations suggest that individuals with s-IBM were able to increase their walking speed by increasing cadence, but not stride length.
Previous investigators have cited an important relationship between knee extensor strength and gait characteristics in individuals with s-IBM. However, our findings indicate that ankle plantar flexor and knee flexor muscle groups are more strongly associated with temporal gait characteristics in the presence of significant knee extensor weakness (14.9% of predicted values).
Acknowledgments
This study was funded by the National Institute of Neurological Disorders and Stroke (Protocol: 02-N-0121) Intramural Research Program and supported by the Rehabilitation Medicine Department, NIH.
Footnotes
Any opinions or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the U.S. Department of Veterans Affairs or the U.S. Department of Health and Human Services.
The authors declare no financial or commercial conflicts of interests related to the material presented in this manuscript.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published.
Study conception. Harris-Love and Dalakas.
Study design. Harris-Love, Benson, Baker, Gracey, Davenport, and Shrader.
Acquisition of data. Harris-Love, Benson, Dalakas, Rakocevic, and McElroy.
Analysis and interpretation of data. Davenport, Harris-Love, and Shrader.
References
- 1.Badrising UA, Maat-Schieman M, van Duinen SG, Breedveld F, van Doorn P, van Engelen B, et al. Epidemiology of inclusion body myositis in the Netherlands: a nationwide study. Neurology. 2000;55(9):1385–7. doi: 10.1212/wnl.55.9.1385. [DOI] [PubMed] [Google Scholar]
- 2.Dalakas MC. Polymyositis, dermatomyositis and inclusion-body myositis. N Engl J Med. 1991;325(21):1487–98. doi: 10.1056/NEJM199111213252107. [DOI] [PubMed] [Google Scholar]
- 3.Dalakas MC. Sporadic inclusion body myositis - diagnosis, pathogenesis and therapeutic strategies. Nat Clin Pract Neurol. 2006;2(8):437–47. doi: 10.1038/ncpneuro0261. [DOI] [PubMed] [Google Scholar]
- 4.Dalakas MC. Interplay between inflammation and degeneration: using inclusion body myositis to study “neuroinflammation”. Ann Neurol. 2008;64(1):1–3. doi: 10.1002/ana.21452. [DOI] [PubMed] [Google Scholar]
- 5.Phillips BA, Zilko PJ, Mastaglia FL. Prevalence of sporadic inclusion body myositis in Western Australia. Muscle Nerve. 2000;23(6):970–2. doi: 10.1002/(sici)1097-4598(200006)23:6<970::aid-mus20>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 6.Maat-Schieman ML, Macfarlane JD, Bots GT, Wintzen AR. Inclusion body myositis: its relative frequency in elderly people. Clin Neurol Neurosurg. 1992;94(Suppl):S118–20. doi: 10.1016/0303-8467(92)90043-3. [DOI] [PubMed] [Google Scholar]
- 7.Spector SA, Lemmer JT, Koffman BM, Fleisher TA, Feuerstein IM, Hurley BF, et al. Safety and efficacy of strength training in patients with sporadic inclusion body myositis. Muscle Nerve. 1997;20(10):1242–8. doi: 10.1002/(sici)1097-4598(199710)20:10<1242::aid-mus6>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 8.Phillips BA, Cala LA, Thickbroom GW, Melsom A, Zilko PJ, Mastaglia FL. Patterns of muscle involvement in inclusion body myositis: clinical and magnetic resonance imaging study. Muscle Nerve. 2001;24(11):1526–34. doi: 10.1002/mus.1178. [DOI] [PubMed] [Google Scholar]
- 9.Amato AA, Gronseth GS, Jackson CE, Wolfe GI, Katz JS, Bryan WW, et al. Inclusion body myositis: clinical and pathological boundaries. Ann Neurol. 1996;40(4):581–6. doi: 10.1002/ana.410400407. [DOI] [PubMed] [Google Scholar]
- 10.Hopkinson ND, Hunt C, Powell RJ, Lowe J. Inclusion body myositis: an underdiagnosed condition? Ann Rheum Dis. 1993;52(2):147–51. doi: 10.1136/ard.52.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perry J, Mulroy SJ, Renwick SE. The relationship of lower extremity strength and gait parameters in patients with post-polio syndrome. Arch Phys Med Rehabil. 1993;74(2):165–9. [PubMed] [Google Scholar]
- 12.Powers CM, Boyd LA, Fontaine CA, Perry J. The influence of lower-extremity muscle force on gait characteristics in individuals with below-knee amputations secondary to vascular disease. Phys Ther. 1996;76(4):369–77. doi: 10.1093/ptj/76.4.369. discussion 78-85. [DOI] [PubMed] [Google Scholar]
- 13.Willen C, Sunnerhagen KS, Ekman C, Grimby G. How is walking speed related to muscle strength? A study of healthy persons and persons with late effects of polio. Arch Phys Med Rehabil. 2004;85(12):1923–8. doi: 10.1016/j.apmr.2003.11.040. [DOI] [PubMed] [Google Scholar]
- 14.Slavin MD, Jette DU, Andres PL, Munsat TL. Lower extremity muscle force measures and functional ambulation in patients with amyotrophic lateral sclerosis. Arch Phys Med Rehabil. 1998;79(8):950–4. doi: 10.1016/s0003-9993(98)90093-4. [DOI] [PubMed] [Google Scholar]
- 15.Jette DU, Slavin MD, Andres PL, Munsat TL. The relationship of lower-limb muscle force to walking ability in patients with amyotrophic lateral sclerosis. Phys Ther. 1999;79(7):672–81. doi: 10.1093/ptj/79.7.672. [DOI] [PubMed] [Google Scholar]
- 16.Ferrucci L, Guralnik JM, Buchner D, Kasper J, Lamb SE, Simonsick EM, et al. Departures from linearity in the relationship between measures of muscular strength and physical performance of the lower extremities: the Women's Health and Aging Study. J Gerontol A Biol Sci Med Sci. 1997;52(5):M275–85. doi: 10.1093/gerona/52a.5.m275. [DOI] [PubMed] [Google Scholar]
- 17.Basquiera AL, Caeiro F, Palacio S, Theaux R, Casale A, Lucero C, et al. Inclusion body myositis. Report of 4 cases. Medicina (B Aires) 2002;62(1):37–40. [PubMed] [Google Scholar]
- 18.Cortese A, Machado P, Morrow J, Dewar L, Hiscock A, Miller A, et al. Longitudinal observational study of sporadic inclusion body myositis: implications for clinical trials. Neuromuscul Disord. 2013;23(5):404–12. doi: 10.1016/j.nmd.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Bernhardt KA, Oh TH, Kaufman KR. Gait patterns of patients with inclusion body myositis. Gait Posture. 2011;33(3):442–6. doi: 10.1016/j.gaitpost.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 20.Biomech Designs L. The QMT System: Quantitative Muscle Testing - Software Manual (Version 6.8) Biomech Designs, Ltd.; Edmonton, Alberta, Canada: 1990. [Google Scholar]
- 21.Personius KE, Pandya S, King WM, Tawil R, McDermott MP. Facioscapulohumeral dystrophy natural-history study - standardization of testing procedures and reliability of measurements. Phys Ther. 1994;74(3):253–63. doi: 10.1093/ptj/74.3.253. [DOI] [PubMed] [Google Scholar]
- 22.Stoll T, Bruhlmann P, Stucki G, Seifert B, Michel BA. Muscle strength assessment in polymyositis and dermatomyositis evaluation of the reliability and clinical use of a new, quantitative, easily applicable method. J Rheumatol. 1995;22(3):473–7. [PubMed] [Google Scholar]
- 23.Stoll T, Huber E, Seifert B, Michel BA, Stucki G. Maximal isometric muscle strength: normative values and gender-specific relation to age. Clin Rheumatol. 2000;19(2):105–13. doi: 10.1007/s100670050026. [DOI] [PubMed] [Google Scholar]
- 24.Morris ME, Matyas TA, Iansek R, Summers JJ. Temporal stability of gait in Parkinson's disease. Phys Ther. 1996;76(7):763–77. doi: 10.1093/ptj/76.7.763. discussion 78-80. [DOI] [PubMed] [Google Scholar]
- 25.Hill KD, Goldie PA, Baker PA, Greenwood KM. Retest reliability of the temporal and distance characteristics of hemiplegic gait using a footswitch system. Arch Phys Med Rehabil. 1994;75(5):577–83. [PubMed] [Google Scholar]
- 26.Murray MP, Kory RC, Sepic SB. Walking patterns of normal women. Arch Phys Med Rehabil. 1970;51(11):637–50. [PubMed] [Google Scholar]
- 27.Oberg T, Karsznia A, Oberg K. Basic gait parameters: reference data for normal subjects, 10-79 years of age. J Rehabil Res Dev. 1993;30(2):210–23. [PubMed] [Google Scholar]
- 28.Blanc Y, Balmer C, Landis T, Vingerhoets F. Temporal parameters and patterns of the foot roll over during walking: normative data for healthy adults. Gait Posture. 1999;10(2):97–108. doi: 10.1016/s0966-6362(99)00019-3. [DOI] [PubMed] [Google Scholar]
- 29.Bassey EJ, Macdonald IA, Patrick JM. Factors affecting the heart rate during self-paced walking. Eur J Appl Physiol Occup Physiol. 1982;48(1):105–15. doi: 10.1007/BF00421170. [DOI] [PubMed] [Google Scholar]
- 30.Himann JE, Cunningham DA, Rechnitzer PA, Paterson DH. Age-related changes in speed of walking. Medicine and science in sports and exercise. 1988;20(2):161–6. doi: 10.1249/00005768-198820020-00010. [DOI] [PubMed] [Google Scholar]
- 31.Bendall MJ, Bassey EJ, Pearson MB. Factors affecting walking speed of elderly people. Age Ageing. 1989;18(5):327–32. doi: 10.1093/ageing/18.5.327. [DOI] [PubMed] [Google Scholar]
- 32.Murray MP, Kory RC, Clarkson BH. Walking patterns in healthy old men. J Gerontol. 1969;24(2):169–78. doi: 10.1093/geronj/24.2.169. [DOI] [PubMed] [Google Scholar]
- 33.Bohannon RW. Comfortable and maximum walking speed of adults aged 20-79 years: reference values and determinants. Age Ageing. 1997;26(1):15–9. doi: 10.1093/ageing/26.1.15. [DOI] [PubMed] [Google Scholar]
- 34.Cohen J. Statistical Power Analyses for the Behavioral Sciences. Academic Press; New York City: 1988. [Google Scholar]
- 35.Elble RJ, Thomas SS, Higgins C, Colliver J. Stride-dependent changes in gait of older people. J Neurol. 1991;238(1):1–5. doi: 10.1007/BF00319700. [DOI] [PubMed] [Google Scholar]
- 36.Perron M, Malouin F, Moffet H, McFadyen BJ. Three-dimensional gait analysis in women with a total hip arthroplasty. Clin Biomech (Bristol, Avon) 2000;15(7):504–15. doi: 10.1016/s0268-0033(00)00002-4. [DOI] [PubMed] [Google Scholar]
- 37.Sadeghi H, Allard P, Barbier F, Sadeghi S, Hinse S, Perrault R, et al. Main functional roles of knee flexors/extensors in able-bodied gait using principal component analysis (I). Knee. 2002;9(1):47–53. doi: 10.1016/s0968-0160(01)00134-x. [DOI] [PubMed] [Google Scholar]
- 38.Mueller MJ, Minor SD, Sahrmann SA, Schaaf JA, Strube MJ. Differences in the gait characteristics of patients with diabetes and peripheral neuropathy compared with age-matched controls. Phys Ther. 1994;74(4):299–308. doi: 10.1093/ptj/74.4.299. discussion 9-13. [DOI] [PubMed] [Google Scholar]