Abstract
Firm conclusions about whether mid-life or long-term statin use has an impact on cognitive decline and dementia remain elusive. Here, our objective was to systematically review, synthesize and critique the epidemiological literature that examines the relationship between statin use and cognition, so as to assess the current state of knowledge, identify gaps in our understanding, and make recommendations for future research. We summarize the findings of randomized controlled trials (RCTs) and observational studies, grouped according to study design. We discuss the methods for each, and consider likely sources of bias, such as reverse causation and confounding. Although observational studies that considered statin use at or near the time of dementia diagnosis suggest a protective effect of statins, these findings could be attributable to reverse causation. RCTs and well-conducted observational studies of baseline statin use and subsequent cognition over several years of follow-up do not support a causal preventative effect of late-life statin use on cognitive decline or dementia. Given that much of the human research on statins and cognition in the future will be observational, careful study design and analysis will be essential.
Introduction
The American College of Cardiology and American Heart Association guidelines on the management of cholesterol, published in 2013,1 substantially expanded the proportion of the US population that is eligible to receive statins: an estimated 56 million US adults—49% of those aged 40–75 years—are now eligible to receive statins, even though many do not have overt cardiovascular disease.2–4 Although the benefits of statins for primary and secondary prevention of cardiovascular outcomes have been demonstrated,5–7 their effects on cognition and the risk of dementia remain unclear. Case reports link cognitive impairment with statin use8,9 and, in the USA, the drugs now carry an FDA warning about statin-related reversible cognitive impairment or memory loss, 10 but these effects seem to be unrelated to dementia. Indeed, some evidence suggests that the pleiotropic effects of statins reduce the risk of dementia, for example, by decreasing levels of circulating cholesterol.
Hyperlipidaemia, particularly in mid-life, seems to be associated with an increased risk of dementia, 11–13 potentially by promoting damage to the brain vasculature.14 Consequently, treatment of hyperlipidaemia would be expected to reduce the risk of dementia. Statins also seem to promote cardiovascular and (by inference) cerebrovascular health through antioxidant and anti-inflammatory effects and improved endothelial function.15–17 However, they might also confer neuroprotection by other mechanisms. For example, statins—particularly lipophilic statins—might cross the blood–brain barrier and exert antioxidant and anti-inflammatory effects within the CNS, or modulate cholesterol metabolism in the brain. 16,18–23 Experiments in animal and cell models of Alzheimer disease (AD) also suggest that statins modulate amyloid-β; however, little evidence currently supports a similar effect in humans. 16,21,22,24–29 Finally, statins might modulate brain tau metabolism.22,27,30,31
Systematic reviews can synthesize data into a coherent evidential framework. However, existing reviews of statins and cognition have focused solely on clinical trials, have not systematically discussed study quality, or have discussed and meta-analysed observational studies as a group, which might be inappropriate when differences in study design or analyses yield noncomparable effect estimates.32–37 The purpose of this Review is to summarize findings from randomized controlled trials (RCTs) and observational cohort studies; these types of studies are the most useful for evaluating the putative causal effects of statin use on cognition. We group studies by design and statistical approach, and provide specific commentary on study methods and their likely influence on findings. We conclude with a summary of the state of the evidence and recommendations for future research.
Literature search and analysis
We did not register a review protocol; however, our process adhered to the AlzRisk review protocol,38 albeit with broader inclusion criteria. Briefly, references were identified through title and abstract screening and full-text review of citations identified by systematic searches of the MEDLINE and EMBASE databases (Supplementary Box 1 online) up to 15 June 2014, and by reviewing references included in identified eligible articles. No language restrictions were applied. One author (M.P.) was responsible for identifying eligible articles, extracting data, and conducting quality assessments in accordance with our study protocol; a reliability study conducted during protocol development indicated little, if any, benefit of adding a second reviewer.38 All co-authors reviewed the list of eligible articles to identify any missing studies on the basis of their expert knowledge.
We included all RCTs that reported on statin use in adults and any measure of cognitive status, with the exception of RCTs that exclusively included people with dementia or individuals who were administered statins as secondary prevention therapy (for example, after myocardial infarction or stroke). We also included any observational cohort study that listed statins as a primary exposure of interest if it considered the following: a cohort in which patients were known or assumed to be free of dementia at baseline (based on age or cognitive screening); populations that were not defined by clinical end points, with the exception of deliberate restriction to those with an indication for statin use for primary prevention of cardiovascular disease; and the association between statin use and either a change in neuropsychological test scores (without adjustment for baseline test scores 39) or incident diagnosis of dementia or AD. We retained multiple articles that reported on the same study population if their outcomes or analytical approaches were unique, or if they reported on overlapping but distinct samples; otherwise, we retained the report that had the longest follow-up period. We excluded conference abstracts and reports in supplements that were not peer-reviewed. These eligibility criteria focused our Review on studies that were the most likely to provide insight into the causal effects of statin use on cognition in the general population.
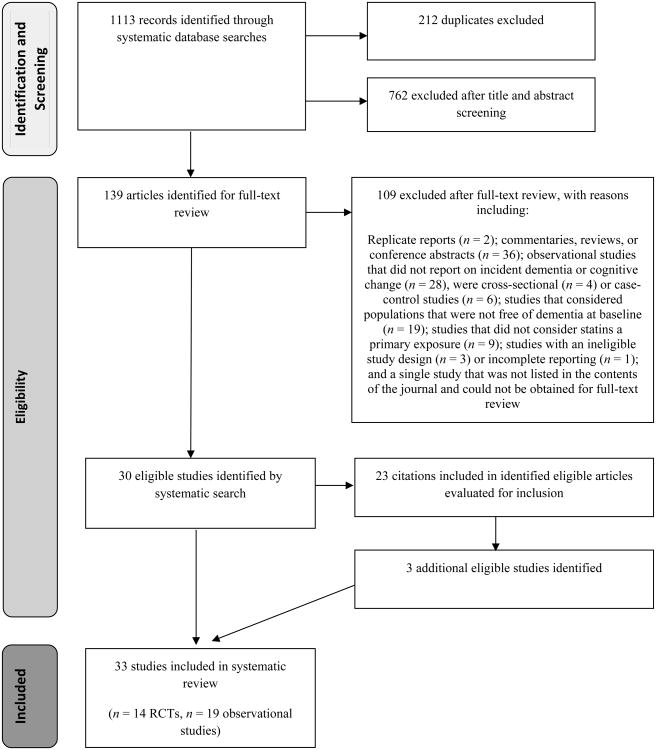
Ultimately, we identified 14 eligible articles40–53 that reported on 13 RCTs, and 19 eligible articles28,54–71 that reported on 17 observational studies (Figure 1). From one article,69 we excluded analyses of cognitive change that were adjusted for baseline cognitive performance, but included analyses of dementia.
Figure 1.
Flow diagram of the study selection process for the systematic review. Abbreviation: RCTs, randomized controlled trials
Details of data extraction from the included studies are provided in Supplementary Box 2 online. Each study was assessed for potential confounding, selection or misclassification bias, and reverse causation, as well as the applicability of the findings to other study populations with different characteristics. Studies were grouped by design and analytical approach for discussion. The Review was prepared with reference to published guidelines.72
Randomized controlled trials
Summary of published studies
Two large, double-blind, placebo-controlled RCTs of statins, the Heart Protection Study (HPS)51 and the Prospective Study of Pravastatin in the Elderly at Risk (PROSPER) trial,46,52 considered cognitive end points (Supplementary Table 1 online). In the HPS, 20,563 participants aged 40–80 years without dementia but with a high risk of vascular disease were randomly allocated to receive either 40 mg simvastatin daily or a placebo, and were followed up for an average of 5 years. Study personnel administered the Telephone Interview for Cognitive Status (TICS) to active study participants at the final follow-up interview. Neither the prevalence of cognitive impairment (indicated by a TICS score <22 or by reported dementia) nor the mean TICS score was associated with treatment assignment.51 In the PROSPER trial, 5,804 participants aged 70–82 years who had or were at risk of vascular disease were randomly allocated to receive 40 mg pravastatin daily or a placebo, and were followed up for an average of 3 years. Statin treatment was not associated with a change in cognitive test scores.46,52 Several small RCTs have reported on cognitive outcomes after 1–6 months of statin use,40–45,47–50,53 with mixed results (Supplementary Table 1 online).
Comment
Collectively, RCTs that evaluated cognitive outcomes after less than 6 months of statin use did not provide strong evidence for short-term effects of statins on cognition, and did not provide any information about the effects of long-term statin use on cognition or the risk of dementia. Thus, we focus our discussion on the HPS and the PROSPER trial.
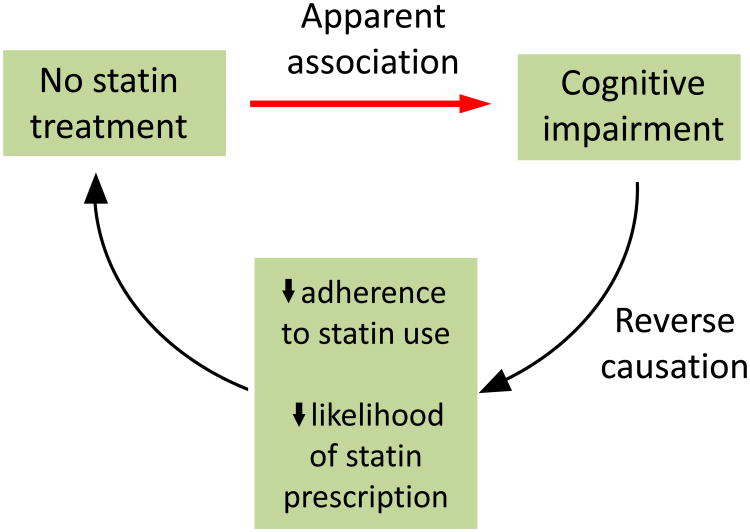
The key advantage of large, carefully conducted RCTs is the putative absence of confounding (because successful randomization balances characteristics related to both statin use and statin-independent cognitive change across treatment groups) and reverse causation (the situation in which cognition influences statin treatment rather than the reverse; Figure 2). However, other sources of bias remain. Non-adherence—assuming that it is unrelated to cognitive status—would cause an intention-to-treat analysis to underestimate the true aetiological effect of statin use. Although full adherence was not achieved in either the PROSPER trial or the HPS, the small degree of non-adherence that was observed is unlikely to explain the null results unless the size of the true aetiological effect is extremely small.
Figure 2.
Reverse causation in analyses that consider statin use and dementia risk. The underlying pathophysiology of dementia produces symptoms of cognitive impairment—including difficulties with memory, other cognitive processes and daily activities—that, once severe enough, lead to a dementia diagnosis. Development of cognitive impairment in study participants who do not use statins suggests that statins protect against cognitive decline, owing to an apparent association between no statin use and cognitive impairment. However, the difficulties caused by cognitive impairment are likely to reduce patient adherence to statin use (for example, if patients forget to take their medication or collect their prescription) and reduce the likelihood that a physician will prescribe statins (for example, if the physician is focused on treating the cognitive impairment or more-acute comorbidities, which might be exacerbated by cognitive impairment and increased inability to care for oneself). Thus, cognitive impairment might influence statin use, leading to reverse causation.
Likewise, little evidence exists for a strong selection bias that resulted from non-death attrition; loss to follow-up was minimal and did not differ substantially between treatment groups. Theoretically, differential attrition owing to death (for example, 13% mortality among simvastatin-allocated participants versus 15% mortality among placebo-allocated participants in the HPS) might mask a true aetiological protective effect of statins on cognition owing to survival bias (a form of selection bias);73 in other words, statins might not seem beneficial because they prevent death in people with poor cognition.5 In this case, the observed association would not necessarily answer the clinical question “does statin use reduce risk of dementia in any individual patient?” but might answer the question “will we observe a difference in the number of dementia cases if we increase use of statins in the population?” However, in this scenario, selective survival would only mask a protective aetiological effect of statin use on cognition if that effect were very small. Supplementary Box 3 online demonstrates that the magnitude of this potential bias is minimal under reasonable assumptions about the relationship between cognitive impairment and survival, and considering the small differences in mortality reported between the treatment groups in the HPS.
The relatively short follow-up times and old age of participants are limitations of both the HPS and the PROSPER trial. Detectable changes in brain structure and function are often present from years to decades before the diagnosis of dementia,74–76 and associations of other risk factors (for example, blood pressure or cholesterol levels) with cognitive impairment or dementia seem to relate to the time at which these risk factors are measured. Evidence suggests that mid-life or long-term exposure to such risk factors has a greater influence on the risk of dementia than does late-life or short-term exposure.11,77,78 These observations support the existence of a relevant aetiological window in mid-life or many years before symptoms of cognitive decline develop. Therefore, if statin use prevents or delays disease progression only at early stages of the pathological process or has small cumulative effects over many years, these RCTs would not be expected to demonstrate a benefit. Conversely, we would have expected to observe a benefit despite short follow-up times if statins have a marked and immediate benefit, for example, if they prevent or mitigate concomitant dementia-related cerebrovascular disease, thereby affecting progression or symptoms of preclinical dementia.79 Relative to the PROSPER trial, the HPS is further limited, as cognition was measured with a single test just once at the end of the study. However, we do not believe that this limitation is likely to account for the null results.
We conclude that the existing RCTs that assessed the effects of statin use on cognition do not indicate that statin use should be started in late life to prevent cognitive decline or dementia over the subsequent 3–5 years.
Observational studies
Baseline statin use and cognitive decline
Summary of published studies
Several observational studies (conducted almost exclusively in adults aged >65 years) have considered the association between statin use at the baseline study visit and subsequent cognitive decline or incident dementia (Supplementary Table 2 online).
Although statin use at baseline was strongly associated with a lower prevalence of dementia at baseline among participants in the Cache County Study, it was not associated with incident dementia or AD in the 3 years that followed.65 Similarly, among participants in the Religious Orders Study, no association was observed between statin use and the level or rate of change of global or domain-specific cognitive function or dementia over follow-up periods of up to 12 years.28 Likewise, no association was observed between baseline statin use and incident dementia in the Three City study, in which the follow-up period was 7 years.69
Conversely, in the Indianapolis sample of the Indianapolis–Ibadan Dementia Project, use of statins at baseline was associated with a smaller decline in scores on the Community Screening Instrument for Dementia over 3 years than was non-use of statins at baseline.61 However, when baseline statin users were further stratified according to statin use at the end of the 3-year follow-up period, only those who discontinued statin use during the 3-year interval exhibited significantly slower cognitive decline than participants who never used statins. Consistent statin users also seemed to exhibit slower cognitive decline than never users, but this association was not as strong as that for discontinuers, and was not statistically significant.
In a study that analysed data from The Health Improvement Network (THIN), a large database of general practice electronic medical records (EMRs), participants who initiated statin use were compared with age-matched and sex-matched control participants who were not receiving statins.66 Statin initiators were less likely than nonusers to receive a clinical diagnosis of dementia over a median follow-up period of 4.4 years. Similar analyses in the Decision Support System database of the US Veterans Affairs medical system suggested that simvastatin users, but not lovastatin or atorvastatin users, had a reduced risk of incident dementia of the Alzheimer type (as defined by the International Classification of Diseases, 9th Revision codes) compared with users of other cardiovascular medications.70
Comment
Bias in observational studies can be minimized or avoided by using appropriate study designs and analyses. For example, methods that have high validity for assessing participants' statin use (such as conducting a drug inventory, or considering prescription records and/or self-reported use80) reduce bias caused by misclassification of statin use. Similarly, cohort studies that systematically and prospectively evaluate participants for dementia are unlikely to be biased by differential misclassification of dementia (whereby the error in classification of dementia differs according to statin use) or substantial nondifferential misclassification (that is, random error). By contrast, use of EMRs to classify the dementia status of participants is not ideal:81–84 random error would simply make a true effect more difficult to detect, but EMR-derived errors in classification of dementia status could differ by statin use. If people with access to good health care are more likely to receive both treatment with statins and a diagnosis of dementia in the course of their normal clinical care, an erroneous adverse association could be obtained. Conversely, if people with comorbidities that lead to statin use are less likely to receive a diagnosis of dementia (because, for example, clinicians focus instead on the comorbidities), we might expect an erroneous protective association.
Observational studies that define statin use at baseline and monitor participants for several years are relatively resistant to reverse causation, provided that the duration of follow-up is sufficient. In the set of studies presented here, the maximum follow-up periods range from 3 to 12 years (Supplementary Table 2 online). If people whose cognition is intact are more likely to be prescribed or to continue taking statins, reverse causation would produce a misleading protective association (Figure 2). Several of the studies discussed here that have relatively short follow-up periods report a protective association,61,66,70 which could, in theory, be attributable to reverse causation. However, these studies have limitations besides the short follow-up periods—such as the use of EMRs to classify dementia, as discussed above, and confounding by ‘healthy user’ characteristics or small numbers of cases, as discussed below—that might account for the observed associations. We should also note that in the Indianapolis cohort,61 those who discontinued statin use during the 3-year follow-up had better cognitive trajectories, a finding opposite to that expected if a decline in cognition led to cessation of statin use in this sample. Cessation of statin use among health-conscious patients in response to the FDA warning10 could also produce erroneous associations, but this possibility is not relevant to this set of studies, as the warning was introduced after their completion.
Confounding is another concern, and the direction of total confounding bias is difficult to predict. Confounding by indication (for example, when vascular disease leads to both statin use and dementia) is likely to lead to spurious adverse associations, whereas confounding by ‘healthy user’ characteristics (for example, when preventive care leads to statin use and no dementia)85 would be expected to lead to spurious protective associations. Attempts that have been made to control for confounding by indication have had little impact on results,28,65,66 thereby arguing against severe uncontrolled confounding by indication, although the possibility cannot be discounted. All studies in which analyses were adjusted for education (one correlate of ‘healthy users’) reported null results, whereas two studies66,70 in which analyses were not adjusted for education reported a protective association. These findings would be expected if highly educated participants more readily obtained indicated statin treatments and were less likely to exhibit marked cognitive impairment.
As with the RCTs, bias from selective attrition could influence the findings of observational studies. In the Cache County Study, non-death attrition did not differ according to baseline statin use,65 suggesting minimal bias from selective loss to follow-up. However, as in the RCTs, deaths might mask a small aetiological benefit of statins, as delayed mortality provides a greater opportunity for dementia to develop.
Two additional characteristics of these studies are worth noting. First, the results from the Indianapolis cohort were based on only 32 participants with dementia, only three of whom were statin users.61 Similarly, only eight of the 182 participants with dementia in the Cache County Study were baseline statin users.65 Results based on such small numbers are more likely to be chance findings86 or to have incomplete control of confounding.87 Second, the participants in most studies were older adults (≥65 years at baseline; Supplementary Table 2 online), and in THIN66—the only study to include adults in mid-life—the follow-up period was too short to capture the risk of late-onset dementia in the younger participants. Therefore, these studies cannot provide information on the effects of mid-life or long-term statin use.
Three of the six observational studies discussed above reported associations that were consistent with a cognitive benefit of statin use;61,66,70 however, two of these three studies66,70 used EMRs to define dementia status and did not adjust for education or other socio-demographic factors, and the third61 was based on very small numbers of participants with dementia. The results of the remaining three studies concur with those of RCTs, and provide no evidence that statin use in late life prevents dementia over the few years following statin initiation.
Time-updated statin use and dementia
Summary of published studies
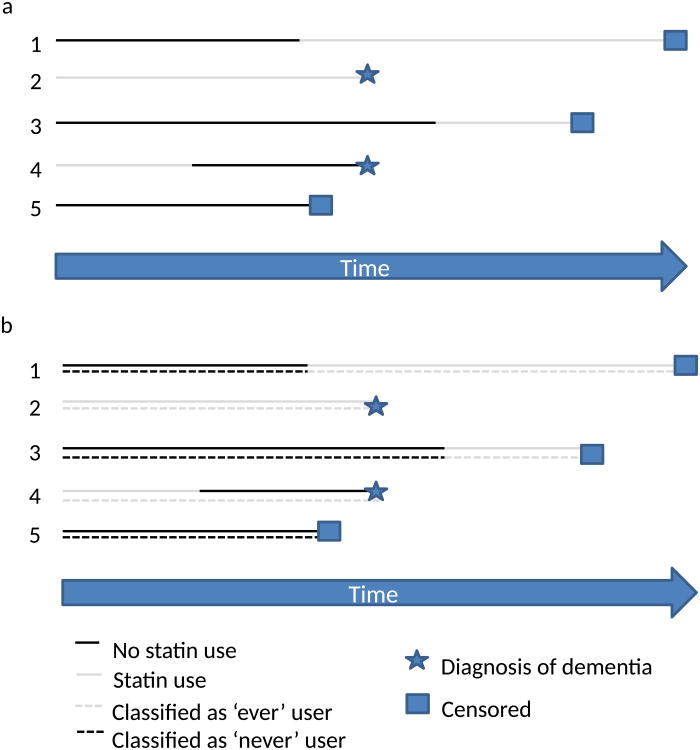
Several other observational studies (Supplementary Table 3 online) have considered the association between time-updated statin use and incident dementia, typically by reporting on results from a proportional hazards regression model in which statin use status during the follow-up period is entered into the model as a time-dependent covariate (Figure 3). Specifically, many of these analyses consider statin use as a time-updated version of ‘ever’ versus ‘never’ statin use; participants who were not using statins at baseline and do not start using statins during follow-up can be classified as ‘never’ users throughout, whereas participants who were using statins at baseline are classified as ‘ever’ users throughout (even if they subsequently stop using statins). Participants who initiate statin use during the study are classified as ‘never’ users until their time of statin initiation, when their status switches to ‘ever’ user. For example, a participant who begins taking statins in year 3 of follow-up could be considered a ‘never’ user for years 1 and 2, and an ‘ever’ user for year 3 and beyond. Studies that use this approach compare the risk of dementia among ‘ever’ users with that among ‘never’ users at the time of diagnostic assessment, an approach that is essentially cross-sectional in nature. (Figure 3b) Studies that incorporate a 1-year or 2-year lag compare the risk of dementia among ‘never’ and ‘ever’ users according to their statin use status at 1 year or 2 years, respectively, before diagnostic assessment.
Figure 3.
Interpretation of survival models using time-updated current or never–ever statin use with no lag. A comparison is made each time a participant is diagnosed with dementia. Comparisons at each time point are cross-sectional: statin use at other time points is not considered. a | Time-updated current statin use. The model compares the probability of a dementia diagnosis in participants currently using statins versus those not currently using statins. In this example, when patients 2 and 4 are diagnosed, the probability that any participant has a dementia diagnosis is 0.5, regardless of their current statin use, suggesting no benefit of statin use. b | Time-updated never–ever statin use. The model compares the probability of a dementia diagnosis in ever versus never statin users. In this example, when participants 2 and 4 are diagnosed, the probability of a dementia diagnosis in an ever user is 0.67, compared with 0 for never users, suggesting that statin use increases the risk of dementia.
Four studies that used this design suggested a strong beneficial effect of statin use on the risk of dementia. Among participants of the Rotterdam Study, time-updated ever statin use was associated with a reduced risk of AD dementia.67 Analyses of ever–never statin use with a 2-year lag and current statin use at 1 year before the date of diagnosis produced comparable results. Additional analyses failed to identify differences in this association according to statin lipophilicity (one determinant of whether the drug can cross the blood–brain barrier), dosage or duration of use, or to apolipoprotein E (APOE) allele status.
In the Sacramento Area Latino Study on Aging (SALSA), time-updated ever use of statins with a 1-year lag was associated with a reduced risk of dementia or “cognitive impairment without dementia” (a research diagnosis akin to mild cognitive impairment [MCI]).64 Analyses from the Gingko Evaluation of Memory Study (GEMS)60 showed that time-updated ever statin use was associated with a reduced risk of dementia in participants without MCI at baseline. Further analyses suggested that this result was primarily due to an association between current statin use at the time of dementia ascertainment and a reduced risk of dementia, as no association with time-updated former use was observed. Similar, but not significant, associations were observed after participants with cardiovascular disease at baseline were excluded, or when AD dementia or dementia with a vascular component was considered; associations were strongest with lipophilic statins and when analyses were restricted to participants who initiated statin use during the follow-up period. Associations observed among participants with MCI at baseline were also protective, though not significant. In addition, the Baltimore Memory Study (BMS) showed that time-updated ever statin use was associated with a reduced risk of dementia and AD dementia, but not with a reduced risk of MCI.57
Two articles that we identified evaluated data from the Adult Changes in Thought (ACT) study, but drew slightly different conclusions. In the initial work, no association was observed between time-updated ever statin use and the risk of dementia or AD dementia; analyses that considered dose, duration of use or time-updated ever statin use with a 1-year lag were similarly null.54 However, in subsequent work that analysed an expanded study population, time-updated ever statin use was associated with a reduced risk of AD in participants younger than 80 years, but not in those aged ≥80 years.55 APOE*ε4 allele carrier status did not modify the association.
Data from the Cardiovascular Health Study (CHS) did not suggest a protective effect of statins.58 Three analyses were reported: time-updated current statin use, time-updated ever statin use with a 1-year lag, and time-updated categories of statin use (current, former, never) with a 1-year lag. Interestingly, analyses of time-updated current statin use that did not incorporate a lag demonstrated protective associations between current statin use and both AD and all-cause dementia. However, neither ever (versus never) nor current (versus never) statin use with a 1-year lag was associated with an increased risk of dementia or dementia subtypes, and time-updated former use of statins (versus never use) was associated with an increased risk of all-cause and AD dementia. The study conclusions were unchanged when the analysis was restricted to people with indications for statin use, or when duration of statin use and statin lipophilicity were considered.
Comment
The interpretation of studies that consider statin use as a time-dependent covariate (with no lag) in a proportional hazards regression model is nearly equivalent to the interpretation of a cross-sectional study (Figure 3). Statin use in incident (rather than prevalent) cases of dementia is compared with statin use in those without dementia at a given point in time. The introduction of a 1-year or 2-year lag to this time-updated approach necessitates an interpretation similar to that of a prospective cohort study with a follow-up period of just 1 or 2 years. Thus, analyses of time-updated statin use and dementia have the same limitations as cross-sectional studies or prospective studies with short follow-up periods.
Analyses of time-updated statin use and dementia are susceptible to reverse causation, in that their findings can easily reflect an effect of declining cognition on statin use (Figure 2).88 This phenomenon is illustrated by the GEMS61 and the CHS:58 the GEMS showed a reduced risk of dementia in current, but not former, statin users, whereas the CHS, which incorporated a 1-year lag in the primary analysis, showed an increased risk of AD dementia in former, but not current, statin users. The apparent lack of a protection against dementia among former users could be explained by discontinuation of statin use as a result of cognitive decline. Similarly, the apparent protective effect of current statin use might reflect the fact that intact cognition affects continued statin use. Lagged exposures can be used to avoid issues of reverse causation. However, if 1-year or 2-year lags were enough to avoid reverse causation, we might expect different results with and without incorporation of lags, but this pattern was not seen consistently. Although the CHS58 reported an inverse association when no lag was incorporated and null results when a 1-year lag was incorporated, both the ACT54 and the Rotterdam67 studies produced similar results regardless of whether a 1-year lag was incorporated. As with observational studies that consider baseline statin use, other sources of bias remain a concern in studies of time-updated statin use; however, given that reverse causation probably accounts for the majority of findings in this group of studies, we will not discuss these biases further.
In summary, although multiple studies that considered the association between time-updated statin use and dementia showed a reduced risk of dementia with statin use, concerns about reverse causation preclude conclusions of a strong beneficial effect.
Alternative study designs
Summary of published studies
Several observational studies have incorporated alternative study designs or analyses to investigate the association between statin use and risk of dementia (Supplementary Table 4 online). Additional analyses in the GEMS60 used a linear mixed effects regression model to assess the impact of statin use on scores in cognitive tests; this model included both a time-updated statin use variable and the interaction of this variable with time. In participants without MCI at baseline, this approach demonstrated an association between time-updated statin use and cognitive change, as determined by performance on a battery of cognitive tests.
Several other studies have looked for an association between statin use during follow-up, which was summarized at the end of the follow-up period, and cognitive decline or dementia during this period (Supplementary Table 4 online). In the CHS, for example, participants were classified as consistent statin users (>4 years of continuous use), intermittent users (2–4 years of continuous use or 3–5 years of intermittent use), or untreated (<2 years of use).59 The untreated group was further classified, according to the 1993 National Cholesterol Education Program guidelines, into three groups: treatment not recommended, diet treatment recommended, and drug treatment recommended. In adjusted analyses, consistent statin users exhibited significantly slower cognitive decline over up to 7 years (as evaluated with the modified Mini-Mental State Examination [MMSE]) compared with untreated users for whom treatment was not recommended, and marginally slower decline compared with untreated users for whom drug treatment was recommended. Other comparisons were not reported.
Another study used data from the Alzheimer's Disease Anti-inflammatory Prevention Trial (ADAPT) to classify participants into four groups according to statin use at the end of ∼4 years of follow-up: no notable use of lipid-lowering medication, consistent statin use, use of lipid-lowering medication other than statins, and inconsistent statin use.56 The authors concluded that the incidence of AD dementia was significantly reduced by long-term statin use, although several exposure groups included fewer than five patients with AD.
A similar study considered the Uniform Data Set maintained by the National Alzheimer's Coordinating Centre.62 In this study, statin use throughout an average follow-up period of 3 years—when compared with no statin use during follow-up—was associated with slower cognitive decline assessed by using the Clinical Dementia Rating Scale Sum of Boxes, and was marginally associated with slower cognitive decline assessed by using the MMSE. Statin use was not associated with a change in cognitive ability assessed by domain-specific cognitive tests. Results were null among participants who had MCI at baseline.
In a follow-up of the 1932 Scottish Mental Health Survey of the Lothian Birth Cohort 1921, participants who were using statins at age 80 years attained, on average, higher age-normalized scores in the Moray House Test of intelligence at age 80 years than at age 11 years. The reverse was true for participants who were not using statins at age 80 years.68 Notably, a report that analysed primary care EMR data from the QResearch database (England and Wales) found no elevated risk of dementia among new users of statins (who entered the cohort on receipt of their first statin prescription) during follow-up compared with participants who did not use statins during the follow-up period, although the results suggested that the risk of dementia was reduced with simvastatin or atorvastatin use in women.63 Conversely, new statin use during follow-up was associated with a reduced risk of incident dementia in analyses that used EMR data from the Longitudinal Health Insurance Database 2000 (Taiwan).71 Associations were stronger with high-potency statins and longer durations of use, but did not differ according to lipophilicity of statins or age of participants. The authors did, however, suggest sex-related differences in the associations. Finally, additional analyses in the Baltimore Longitudinal Study of Aging58 showed that ever statin use defined at the end of the follow-up period was associated with reduced risks of dementia, AD and MCI.
Comment
Statin use is not a point exposure, so consideration of an individual's history of exposure is desirable. However, existing studies that take this history into account have substantial limitations.
Analyses of cognitive change that use a time-updated exposure (for example, the GEMS analyses60) are almost impossible to interpret, and do not clearly answer a clinically relevant question. Analyses that use summaries of statin use defined at the end of the follow-up period are more interpretable, but might not reflect the causal effects of statins on cognition. Existing studies that consider such summaries of statin use have employed variable follow-up periods; participants with shorter follow-up periods are less likely to exhibit variable statin use, although it is unclear whether this factor affects the observed findings. Analyses that incorporate summaries of exposure and concurrent cognition might be biased by reverse causation, because changes in cognitive status can influence statin use.
Confounding is an additional concern. Although several studies included only new users of statins, which might aid identification of and adjustment for relevant confounders, every study that adjusted for confounding seemed to adjust only for baseline characteristics. This approach might be insufficient when using summaries of exposure data collected throughout the follow-up period. In this context, use of time-updated covariates in standard regression models probably also results in bias, given the high probability of time-dependent confounding, which occurs when potential confounders are also mediators of the impact of statins on cognition (Figure 4).89 Appropriate methods are available to account for this time-dependent confounding.89–91 Finally, the studies presented here cannot provide information on the effect of mid-life or long-term statin use, given that participants were primarily aged >65 years during follow-up, and the follow-up periods were relatively short (Supplementary Table 4 online).
Figure 4.
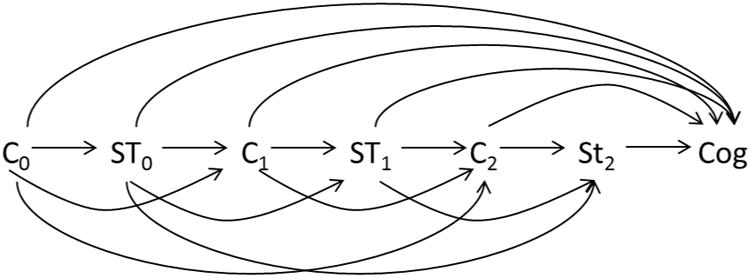
Time-dependent confounding. Directed acyclic graph that illustrates the potential for time-dependent confounding when studying the influence of statin history on cognition. Arrows between variables denote a causal effect of one variable on the next. Time-dependent confounding occurs when a variable that confounds the effect of interest (in this case, the effect of statins on cognition) also acts as a mediator of that effect. In this example, cholesterol status acts as a confounder, because it is associated with both future cognitive status and future statin use (as it is an indication for statin use). However, it also mediates the effect of statin use on cognition because statin use affects future cholesterol levels, which then affect future cognitive status. Standard regression methods to adjust for confounding (for example, adjusting for time-varying cholesterol status) will produce biased results, so other methods (for example, inverse probability weighting in a marginal structural model) are required.
Sti denotes statin use at time i, Ci denotes cholesterol status or any other measured, time-varying confounder of statins and cognition (e.g. access to preventive medical care), and Cog is cognitive status at the end of the study.
Legitimate concerns about bias and interpretability in this body of studies preclude the conclusion that statins have a causal protective effect on cognition, despite the relatively consistent reports of protective associations.
Overview
Collectively, RCTs and observational studies of the association between statin use at baseline and subsequent cognition do not support the initiation of statin use in late life to prevent cognitive decline and dementia within the subsequent few years. Protective associations demonstrated by observational studies that considered time-updated statin use could be explained by reverse causation. Other existing observational studies, particularly those that defined statin use at the end of the follow-up period, did not sufficiently address potential sources of bias, so cannot be used to comment on the causal relationship between statins and late-life cognitive change or dementia. Whether long-term statin use or the timing of statin use (for example, initiation in mid-life versus late life) affects subsequent cognitive status is unclear. Similarly, questions remain about the impact of the statin dose and treatment duration. Furthermore, the type of statin used might be important, as the hypothesized cognitive benefits might be conferred by only a subset of statins (for example, lipophilic statins); differential associations would provide insight into the mechanisms of any beneficial effects. Such questions can only be answered by use of observational research, as the required RCTs are not feasible given cost, ethical concerns, and participant burden.
Reverse causation, misclassification, confounding by indication or ‘healthy user’ characteristics, and selection bias can be sufficiently addressed through careful study design and data collection, and appropriate analysis: well-conducted observational studies can generate results that are consistent with high-quality RCTs. Reverse causation can be completely eliminated by the enrolment of participants without overt or preclinical dementia, either by recruiting participants during mid-life, or by using emerging preclinical markers to exclude participants with preclinical dementia. Although existing studies that considered time-updated statin use have significant limitations, fruitful use of time-varying data is possible and of interest. Such efforts must address the issue of time-dependent confounding (Figure 4) by using appropriate analytical approaches.89,91 The incorporation of exposure lags of at least 3 years is also justified. Adjustment for correlates of adherence to medical recommendations and use of preventative care should allow appropriate control of confounding, but requires thoughtful data collection. Nevertheless, residual confounding could remain. We recommend that future observational research report analyses are restricted to participants with an indication for treatment with statins, so as to aid interpretability.
Finally, future reviews of the relationship between statins and cognition must carefully consider study design and the potential for bias when summarizing the literature. As discussed in this Review, variations in study design and analysis lead to effect estimates that are fundamentally different and should not be pooled for meta-analyses or discussion. Furthermore, meta-analyses must be conducted with caution, as greater numbers (of studies or participants) do not alleviate issues of bias.
Conclusions
Initiation of statin use in late life does not seem to prevent cognitive decline and dementia over the subsequent few years. However, this conclusion in no way undermines existing recommendations regarding statin use for primary and secondary prevention of cardiovascular disease, and does not preclude a beneficial effect of mid-life or long-term statin use on cognition. Given the ethical and feasibility considerations associated with RCTs, carefully designed observational studies that use appropriate analytical methods are our best hope for answering important questions about the relationships between mid-life or long-term statin use and cognitive ability
Supplementary Material
Key points.
Initiation of statin use in late life does not seem to prevent cognitive decline and dementia over the subsequent few years
The current literature does not address the questions of whether mid-life or long-term statin use have beneficial effects on subsequent cognition
Many studies that assessed time-updated statin use suggest a protective effect of statin use on cognitive decline, but these findings are probably attributable to reverse causation
Ethical and feasibility considerations limit randomized controlled trials; carefully designed observational studies that use appropriate analytical methods are our best hope for determining the effect of statin use on cognition
Future observational work must incorporate study designs and analytical techniques that minimize the potential for bias, especially that which is due to reverse causation and confounding
Acknowledgments
M.C.P. receives a grant from the National Institute on Aging (T32 AG027668). Funding was provided to M.C.P., J.W. and D.B. from the Alzheimer's Drug Discovery Foundation (ADDF). The ADDF catalyses and funds drug discovery and drug development for Alzheimer disease (AD) and related disorders. To learn more about the ADDF, visit the website at www.alzdiscovery.org. The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. This work has not previously been presented in any form. However, the literature review and quality assessment were completed in parallel with work completed for the AlzRisk website (www.alzrisk.org), which attempts to catalogue epidemiological reports on risk factors for AD and to provide a continually updated, publically available assessment of the state of the literature. The AlzRisk database entry on statins, which is currently limited to studies reporting on AD as an end point, will be updated as new studies are published. We would like to thank John Jackson for his work in developing AlzRisk search strategies, which informed our search strategy, and his insight into the accuracy of electronic medical records in ascertaining dementia status.
Biographies
Melinda C. Power, earned her doctorate at the Harvard School of Public Health, USA, and is now a postdoctoral fellow at the Johns Hopkins Bloomberg School of Public Health. Her research focuses the contribution of vascular and environmental factors to the risk of cognitive decline and dementia.
Jennifer Weuve is Assistant Professor of Medicine at Rush University's Institute for Healthy Aging, USA, and codirector of the AlzRisk project, a web-based compendium of epidemiological studies of nongenetic risk factors for Alzheimer disease. She investigates questions on the determinants of age-related cognitive decline and dementia, as well as the human health effects of exposures to environmental toxicants. Increasingly, her research has explored the intersection between the two subjects: the possibility that these exposures could impair the ageing brain. To accomplish this, she brings her training in epidemiological methods together with collaborations across many disciplines.
A. Richey Sharrett is a cardiovascular epidemiologist. He was involved in the inception of the Atheroclerosis Risk in Communities (ARIC) study in 1987 when employed by the Epidemiology Program of the National Heart Lung and Blood Institute, Bethesda, USA, and continues to work in the cohort today. Now a professor in the Department of Epidemiology at the Johns Hopkins Bloomberg School of Public Health, he focuses on the study of factors associated with atherosclerosis, microvascular disease and on vascular contributions to cognitive impairments.
Deborah Blacker is a geriatric psychiatrist and epidemiologist who focuses on the epidemiology, genetics, and early recognition of Alzheimer disease. She is Professor of Psychiatry at Harvard Medical School and in Epidemiology at Harvard School of Public Health (HSPH), USA. At Massachusetts General Hospital, she serves as Associate Chief of Psychiatry for Research, directs the Gerontology Research Unit, leads the Education and Outreach Core, and co-leads the Clinical Core for the Mass Alzheimer's Disease Research Center. At HSPH, she directs the Psychiatric Epidemiology concentration, supervises graduate students, teaches a course on assessment methods, and collaborates on methodological research.
Rebecca F. Gottesman is an Associate Professor of Neurology and Epidemiology at Johns Hopkins University School of Medicine, and a core faculty member in the Welch Center for Prevention, Epidemiology, and Clinical Research, USA. As a stroke neurologist with a PhD in Clinical Investigation, she works primarily on the epidemiology of stroke and vascular cognitive impairment. She is a coinvestigator with the Atherosclerosis Risk in Communities (ARIC) study, studying vascular contributions to cognitive impairment and leukoaraiosis, and is the principal investigator of an ARIC ancillary study in which she is evaluating the relationship between vascular risk factors and amyloid deposition.
Footnotes
The authors declare no competing interests.
Author contributions: M.C.P. conducted the literature search, determined study eligibility, extracted the data, conducted the quality assessment, and drafted the manuscript. All authors made substantial contributions to the analysis and interpretation of the data and critically revised the manuscript for important intellectual content. All authors gave final approval for publication.
Supplementary information is linked to the online version of the paper at www.nature.com/nrneurol.
References
- 1.Stone NJ, et al. 2013 ACC/AHA Guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl. 2):S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 2.Pencina MJ, et al. Application of new cholesterol guidelines to a population-based sample. New Engl J Med. 2014;370:1422–1431. doi: 10.1056/NEJMoa1315665. [DOI] [PubMed] [Google Scholar]
- 3.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 4.Grundy SM, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol. 2004;44:720–732. doi: 10.1016/j.jacc.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Naci H, et al. Comparative benefits of statins in the primary and secondary prevention of major coronary events and all-cause mortality: a network meta-analysis of placebo-controlled and active-comparator trials. Eur J Prev Cardiol. 2013;20:641–657. doi: 10.1177/2047487313480435. [DOI] [PubMed] [Google Scholar]
- 6.Udell JA, Ray JG. Primary and secondary prevention of heart failure with statins. Expert Rev Cardiovasc Ther. 2006;4:917–926. doi: 10.1586/14779072.4.6.917. [DOI] [PubMed] [Google Scholar]
- 7.Taylor F, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews. (1) doi: 10.1002/14651858.CD004816.pub4. Art. No.: CD004816. http://dx.doi.org/10.1002/14651858.CD004816.pub4. [DOI] [PMC free article] [PubMed]
- 8.Rojas-Fernandez CH, Cameron JC. Is statin-associated cognitive impairment clinically relevant? A narrative review and clinical recommendations. Ann Pharmacother. 2012;46:549–557. doi: 10.1345/aph.1Q620. [DOI] [PubMed] [Google Scholar]
- 9.Evans MA, Golomb BA. Statin-associated adverse cognitive effects: survey results from 171 patients. Pharmacotherapy. 2009;29:800–811. doi: 10.1592/phco.29.7.800. [DOI] [PubMed] [Google Scholar]
- 10.U.S. Food and Drug Administration; 2012. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. [online] http://www.fda.gov/drugs/drugsafety/ucm293101.htm. [Google Scholar]
- 11.Anstey KJ, Lipnicki DM, Low LF. Cholesterol as a risk factor for dementia and cognitive decline: a systematic review of prospective studies with meta-analysis. Am J Geriatr Psychiatry. 2008;16:343–354. doi: 10.1097/JGP.0b013e31816b72d4. [DOI] [PubMed] [Google Scholar]
- 12.Stewart R, White LR, Xue QL, Launer LJ. Twenty-six-year change in total cholesterol levels and incident dementia: the Honolulu–Asia Aging Study. Arch Neurol. 2007;64:103–107. doi: 10.1001/archneur.64.1.103. [DOI] [PubMed] [Google Scholar]
- 13.Panza F, et al. Lipid metabolism in cognitive decline and dementia. Brain Res Rev. 2006;51:275–292. doi: 10.1016/j.brainresrev.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Gorelick PB, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov. 2005;4:977–987. doi: 10.1038/nrd1901. [DOI] [PubMed] [Google Scholar]
- 16.Miida T, Takahashi A, Ikeuchi T. Prevention of stroke and dementia by statin therapy: experimental and clinical evidence of their pleiotropic effects. Pharmacol Ther. 2007;113:378–393. doi: 10.1016/j.pharmthera.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Bifulco M, Malfitano AM, Marasco G. Potential therapeutic role of statins in neurological disorders. Expert Rev Neurother. 2008;8:827–837. doi: 10.1586/14737175.8.5.827. [DOI] [PubMed] [Google Scholar]
- 18.Sierra S, et al. Statins as neuroprotectants: a comparative in vitro study of lipophilicity, blood–brain-barrier penetration, lowering of brain cholesterol, and decrease of neuron cell death. J Alzheimers Dis. 2011;23:307–318. doi: 10.3233/JAD-2010-101179. [DOI] [PubMed] [Google Scholar]
- 19.Carlsson CM, et al. Effects of atorvastatin on cerebral blood flow in middle-aged adults at risk for Alzheimer's disease: a pilot study. Curr Alzheimer Res. 2012;9:990–997. doi: 10.2174/156720512803251075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Locatelli S, et al. Reduction of plasma 24S-hydroxycholesterol (cerebrosterol) levels using high-dosage simvastatin in patients with hypercholesterolemia: evidence that simvastatin affects cholesterol metabolism in the human brain. Arch Neurol. 2002;59:213–216. doi: 10.1001/archneur.59.2.213. [DOI] [PubMed] [Google Scholar]
- 21.Lutjohann D, von Bergmann K. 24S-Hydroxycholesterol: a marker of brain cholesterol metabolism. Pharmacopsychiatry. 2003;36(Suppl. 2):S102–S106. doi: 10.1055/s-2003-43053. [DOI] [PubMed] [Google Scholar]
- 22.Serrano-Pozo A, et al. Effects of simvastatin on cholesterol metabolism and Alzheimer disease biomarkers. Alzheimer Dis Assoc Disord. 2010;24:220–226. doi: 10.1097/WAD.0b013e3181d61fea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vega GL, et al. Reduction in levels of 24S-hydroxycholesterol by statin treatment in patients with Alzheimer disease. Arch Neurol. 2003;60:510–515. doi: 10.1001/archneur.60.4.510. [DOI] [PubMed] [Google Scholar]
- 24.Höglund K, Blennow K. Effect of HMG-CoA reductase inhibitors on β-amyloid peptide levels: implications for Alzheimer's disease. CNS Drugs. 2007;21:449–462. doi: 10.2165/00023210-200721060-00002. [DOI] [PubMed] [Google Scholar]
- 25.Fassbender K, et al. Effects of statins on human cerebral cholesterol metabolism and secretion of Alzheimer amyloid peptide. Neurology. 2002;59:1257–1258. doi: 10.1212/wnl.59.8.1257. [DOI] [PubMed] [Google Scholar]
- 26.Höglund K, et al. Statin treatment and a disease-specific pattern of β-amyloid peptides in Alzheimer's disease. Exp Brain Res. 2005;164:205–214. doi: 10.1007/s00221-005-2243-8. [DOI] [PubMed] [Google Scholar]
- 27.Riekse RG, et al. Effect of statins on Alzheimer's disease biomarkers in cerebrospinal fluid. J Alzheimers Dis. 2006;10:399–406. doi: 10.3233/jad-2006-10408. [DOI] [PubMed] [Google Scholar]
- 28.Arvanitakis Z, et al. Statins, incident Alzheimer disease, change in cognitive function, and neuropathology. Neurology. 2008;70:1795–1802. doi: 10.1212/01.wnl.0000288181.00826.63. [DOI] [PubMed] [Google Scholar]
- 29.Refolo LM, et al. Hypercholesterolemia accelerates the Alzheimer's amyloid pathology in a transgenic mouse model. Neurobiol Dis. 2000;7:321–331. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- 30.Pollen DA, et al. Prevention of Alzheimer's disease in high risk groups: statin therapy in subjects with PSEN1 mutations or heterozygosity for apolipoprotein Eε4. Alzheimers Res Ther. 2010;2:31. doi: 10.1186/alzrt55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li G, et al. Statin therapy is associated with reduced neuropathologic changes of Alzheimer disease. Neurology. 2007;69:878–885. doi: 10.1212/01.wnl.0000277657.95487.1c. [DOI] [PubMed] [Google Scholar]
- 32.McGuinness B, Craig D, Bullock R, Passmore P. Statins for the prevention of dementia. Cochrane Database of Systematic Reviews. (2) doi: 10.1002/14651858.CD003160.pub2. Art. No.: CD003160 http://dx.doi.org/10.1002/14651858.CD003160.pub2. [DOI] [PubMed]
- 33.Swiger KJ, Manalac RJ, Blumenthal RS, Blaha MJ, Martin SS. Statins and cognition: a systematic review and meta-analysis of short- and long-term cognitive effects. Mayo Clin Proc. 2013;88:1213–1221. doi: 10.1016/j.mayocp.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 34.Muangpaisan W, Brayne C Alzheimer's Society Vascular Dementia Systematic Review Group. Systematic review of statins for the prevention of vascular dementia or dementia. Geriatr Gerontol Int. 2010;10:199–208. doi: 10.1111/j.1447-0594.2009.00579.x. [DOI] [PubMed] [Google Scholar]
- 35.Zhou B, Teramukai S, Fukushima M. Prevention and treatment of dementia or Alzheimer's disease by statins: a meta-analysis. Dement Geriatr Cogn Disord. 2007;23:194–201. doi: 10.1159/000099037. [DOI] [PubMed] [Google Scholar]
- 36.Wong WB, Lin VW, Boudreau D, Devine EB. Statins in the prevention of dementia and Alzheimer's disease: a meta-analysis of observational studies and an assessment of confounding. Pharmacoepidemiol Drug Saf. 2013;22:345–358. doi: 10.1002/pds.3381. [DOI] [PubMed] [Google Scholar]
- 37.Richardson K, et al. Statins and cognitive function: a systematic review. Ann Intern Med. 2013;159:688–697. doi: 10.7326/0003-4819-159-10-201311190-00007. [DOI] [PubMed] [Google Scholar]
- 38.AlzRisk methods. Alzforum. 2015 [online] http://www.alzrisk.org/methods.aspx.
- 39.Glymour MM, Weuve J, Berkman LF, Kawachi I, Robins JM. When is baseline adjustment useful in analyses of change? An example with education and cognitive change. Am J Epidemiol. 2005;162:267–278. doi: 10.1093/aje/kwi187. [DOI] [PubMed] [Google Scholar]
- 40.Carlsson CM, et al. Effects of simvastatin on cerebrospinal fluid biomarkers and cognition in middle-aged adults at risk for Alzheimer's disease. J Alzheimers Dis. 2008;13:187–197. doi: 10.3233/jad-2008-13209. [DOI] [PubMed] [Google Scholar]
- 41.Gibellato MG, Moore JL, Selby K, Bower EA. Effects of lovastatin and pravastatin on cognitive function in military aircrew. Aviat Space Environ Med. 2001;72:805–812. [PubMed] [Google Scholar]
- 42.Kostis JB, Rosen RC, Wilson AC. Central nervous system effects of HMG CoA reductase inhibitors: lovastatin and pravastatin on sleep and cognitive performance in patients with hypercholesterolemia. J Clin Pharmacol. 1994;34:989–996. doi: 10.1002/j.1552-4604.1994.tb01971.x. [DOI] [PubMed] [Google Scholar]
- 43.Muldoon MF, et al. Effects of lovastatin on cognitive function and psychological well-being. Am J Med. 2000;108:538–546. doi: 10.1016/s0002-9343(00)00353-3. [DOI] [PubMed] [Google Scholar]
- 44.Muldoon MF, Ryan CM, Sereika SM, Flory JD, Manuck SB. Randomized trial of the effects of simvastatin on cognitive functioning in hypercholesterolemic adults. Am J Med. 2004;117:823–829. doi: 10.1016/j.amjmed.2004.07.041. [DOI] [PubMed] [Google Scholar]
- 45.Summers MJ, Oliver KR, Coombes JS, Fassett RG. Effect of atorvastatin on cognitive function in patients from the Lipid Lowering and Onset of Renal Disease (LORD) trial. Pharmacotherapy. 2007;27:183–190. doi: 10.1592/phco.27.2.183. [DOI] [PubMed] [Google Scholar]
- 46.Trompet S, et al. Pravastatin and cognitive function in the elderly. Results of the PROSPER study. J Neurol. 2010;257:85–90. doi: 10.1007/s00415-009-5271-7. [DOI] [PubMed] [Google Scholar]
- 47.Harrison RW, Ashton CH. Do cholesterol-lowering agents affect brain activity? A comparison of simvastatin, pravastatin, and placebo in healthy volunteers. Br J Clin Pharmacol. 1994;37:231–236. doi: 10.1111/j.1365-2125.1994.tb04268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Santanello NC, et al. Effect of pharmacologic lipid lowering on health-related quality of life in older persons: results from the Cholesterol Reduction in Seniors Program (CRISP) Pilot Study. J Am Geriatr Soc. 1997;45:8–14. doi: 10.1111/j.1532-5415.1997.tb00971.x. [DOI] [PubMed] [Google Scholar]
- 49.Gengo F, et al. Effects of treatment with lovastatin and pravastatin on daytime cognitive performance. Clin Cardiol. 1995;18:209–214. doi: 10.1002/clc.4960180406. [DOI] [PubMed] [Google Scholar]
- 50.Roth T, et al. Comparative effects of pravastatin and lovastatin on nighttime sleep and daytime performance. Clin Cardiol. 1992;15:426–432. doi: 10.1002/clc.4960150607. [DOI] [PubMed] [Google Scholar]
- 51.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20, 536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 52.Shepherd J, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–1630. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 53.Cutler N, et al. Effects of treatment with simvastatin and pravastatin on cognitive function in patients with hypercholesterolaemia. Br J Clin Pharmacol. 1995;39:333–336. doi: 10.1111/j.1365-2125.1995.tb04458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li G, et al. Statin therapy and risk of dementia in the elderly: a community-based prospective cohort study. Neurology. 2004;63:1624–1628. doi: 10.1212/01.wnl.0000142963.90204.58. [DOI] [PubMed] [Google Scholar]
- 55.Li G, et al. Age-varying association between statin use and incident Alzheimer's disease. J Am Geriatr Soc. 2010;58:1311–1317. doi: 10.1111/j.1532-5415.2010.02906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sparks DL, et al. Reduced risk of incident AD with elective statin use in a clinical trial cohort. Curr Alzheimer Res. 2008;5:416–421. doi: 10.2174/156720508785132316. [DOI] [PubMed] [Google Scholar]
- 57.Beydoun MA, et al. Statins and serum cholesterol's associations with incident dementia and mild cognitive impairment. J Epidemiol Community Health. 2011;65:949–957. doi: 10.1136/jech.2009.100826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rea TD, et al. Statin use and the risk of incident dementia: the Cardiovascular Health Study. Arch Neurol. 2005;62:1047–1051. doi: 10.1001/archneur.62.7.1047. [DOI] [PubMed] [Google Scholar]
- 59.Bernick C, et al. Statins and cognitive function in the elderly: the Cardiovascular Health Study. Neurology. 2005;65:1388–1394. doi: 10.1212/01.wnl.0000182897.18229.ec. [DOI] [PubMed] [Google Scholar]
- 60.Bettermann K, et al. Statins, risk of dementia, and cognitive function: secondary analysis of the ginkgo evaluation of memory study. J Stroke Cerebrovasc Dis. 2012;21:436–444. doi: 10.1016/j.jstrokecerebrovasdis.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Szwast SJ, et al. Association of statin use with cognitive decline in elderly African Americans. Neurology. 2007;69:1873–1880. doi: 10.1212/01.wnl.0000279333.77404.d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Steenland K, Zhao L, Goldstein FC, Levey AI. Statins and cognitive decline in older adults with normal cognition or mild cognitive impairment. J Am Geriatr Soc. 2013;61:1449–1455. doi: 10.1111/jgs.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ. 2010;340:c2197. doi: 10.1136/bmj.c2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cramer C, Haan MN, Galea S, Langa KM, Kalbfleisch JD. Use of statins and incidence of dementia and cognitive impairment without dementia in a cohort study. Neurology. 2008;71:344–350. doi: 10.1212/01.wnl.0000319647.15752.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zandi PP, et al. Do statins reduce risk of incident dementia and Alzheimer disease? The Cache County Study. Arch Gen Psychiatry. 2005;62:217–224. doi: 10.1001/archpsyc.62.2.217. [DOI] [PubMed] [Google Scholar]
- 66.Smeeth L, Douglas I, Hall AJ, Hubbard R, Evans S. Effect of statins on a wide range of health outcomes: a cohort study validated by comparison with randomized trials. Br J Clin Pharmacol. 2009;67:99–109. doi: 10.1111/j.1365-2125.2008.03308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haag MD, Hofman A, Koudstaal PJ, Stricker BH, Breteler MM. Statins are associated with a reduced risk of Alzheimer disease regardless of lipophilicity. The Rotterdam Study. J Neurol Neurosurg Psychiatry. 2009;80:13–17. doi: 10.1136/jnnp.2008.150433. [DOI] [PubMed] [Google Scholar]
- 68.Starr JM, et al. Life long changes in cognitive ability are associated with prescribed medications in old age. Int J Geriatr Psychiatry. 2004;19:327–332. doi: 10.1002/gps.1093. [DOI] [PubMed] [Google Scholar]
- 69.Ancelin ML, et al. Lipid lowering agents, cognitive decline, and dementia: the three-city study. J Alzheimers Dis. 2012;30:629–637. doi: 10.3233/JAD-2012-120064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wolozin B, et al. Simvastatin is associated with a reduced incidence of dementia and Parkinson's disease. BMC Med. 2007;5:20. doi: 10.1186/1741-7015-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chou CY, Chou YC, Chou YJ, Yang YF, Huang N. Statin use and incident dementia: a nationwide cohort study of Taiwan. Int J Cardiol. 2014;173:305–310. doi: 10.1016/j.ijcard.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 72.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hernán MA, Hernández-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15:615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 74.Fox NC, Warrington EK, Rossor MN. Serial magnetic resonance imaging of cerebral atrophy in preclinical Alzheimer's disease. Lancet. 1999;353:2125. doi: 10.1016/S0140-6736(99)00496-1. [DOI] [PubMed] [Google Scholar]
- 75.Jack CR, Jr, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sperling RA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Power MC, Tchetgen EJ, Sparrow D, Schwartz J, Weisskopf MG. Blood pressure and cognition: factors that may account for their inconsistent association. Epidemiology. 2013;24:886–893. doi: 10.1097/EDE.0b013e3182a7121c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 79.Paglieri C, et al. Hypertension and cognitive function. Clin Exp Hypertens. 2008;30:701–710. doi: 10.1080/10641960802563584. [DOI] [PubMed] [Google Scholar]
- 80.Dufouil C, et al. APOE genotype, cholesterol level, lipid-lowering treatment, and dementia: the Three-City Study. Neurology. 2005;64:1531–1538. doi: 10.1212/01.WNL.0000160114.42643.31. [DOI] [PubMed] [Google Scholar]
- 81.Rait G, et al. Survival of people with clinical diagnosis of dementia in primary care: cohort study. BMJ. 2010;341:c3584. doi: 10.1136/bmj.c3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van den Dungen P, et al. The accuracy of family physicians' dementia diagnoses at different stages of dementia: a systematic review. Int J Geriatr Psychiatry. 2012;27:342–354. doi: 10.1002/gps.2726. [DOI] [PubMed] [Google Scholar]
- 83.Taylor DH, Jr, Østbye T, Langa KM, Weir D, Plassman BL. The accuracy of Medicare claims as an epidemiological tool: the case of dementia revisited. J Alzheimers Dis. 2009;17:807–815. doi: 10.3233/JAD-2009-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Butler D, Kowall NW, Lawler E, Michael Gaziano J, Driver JA. Underuse of diagnostic codes for specific dementias in the Veterans Affairs New England healthcare system. J Am Geriatr Soc. 2012;60:910–915. doi: 10.1111/j.1532-5415.2012.03933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brookhart MA, et al. Adherence to lipid-lowering therapy and the use of preventive health services: an investigation of the healthy user effect. Am J Epidemiol. 2007;166:348–354. doi: 10.1093/aje/kwm070. [DOI] [PubMed] [Google Scholar]
- 86.Button KS, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14:365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- 87.Greenland S, Morgenstern H. Confounding in health research. Annu Rev Public Health. 2001;22:189–212. doi: 10.1146/annurev.publhealth.22.1.189. [DOI] [PubMed] [Google Scholar]
- 88.Benner JS, et al. Long-term persistence in use of statin therapy in elderly patients. JAMA. 2002;288:455–461. doi: 10.1001/jama.288.4.455. [DOI] [PubMed] [Google Scholar]
- 89.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 90.Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11:561–570. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 91.Robins JM. In: Statistical Models in Epidemiology: The Environment and Clinical Trials. Berry D, Halloran ME, editors. Springer-Verlag; 1999. pp. 95–134. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.