Abstract
Mammalian spermatozoa must undergo complex physiological and morphological alterations within the female reproductive tract before they become fertilization-competent. Two important alterations are capacitation and the acrosome reaction (AR), by which spermatozoa become capable of penetrating the zona pellucida (ZP) of the oocyte. Although various biochemical stimulants have been reported to induce the AR, the true physiological inducer in vivo remains to be identified. Previously, it was reported that most fertilizing spermatozoa undergo the AR before contacting the ZP, and that only a small fraction of in vitro capacitated spermatozoa can penetrate the ZP. Therefore, it is important to identify which capacitating spermatozoa undergo the AR in response to potential AR inducers such as progesterone. Here we show that spermatozoa undergo a dynamic rearrangement of the acrosome during in vitro capacitation. This involves the rapid movement of an artificially introduced soluble component of the acrosome, enhanced green fluorescent protein (EGFP), from the acrosomal cap region to the equatorial segment of the sperm head (EQ). Spermatozoa exhibiting the EQ pattern were more sensitive to progesterone than those without it. We suggest that spermatozoa that are ready to undergo acrosomal exocytosis can be detected by real-time EGFP imaging. This offers a promising new method for identifying where spermatozoa undergo the AR in the female reproductive tract in vivo.
Keywords: Sperm, gamete biology, acrosomal exocytosis, fertilization
Introduction
Mammalian spermatozoa are not able to fertilize oocytes until they have resided in the female reproductive tract for a certain time (Austin, 1951; Chang, 1951). The changes that occur in spermatozoa during this period are collectively called capacitation. Capacitated spermatozoa must then undergo the acrosome reaction (AR), another step before they can pass through the oocyte extracellular matrix called the zona pellucida (ZP) and fuse with the oolemma. It has long been thought that the AR is induced by contact of the fertilizing spermatozoon with the zona pellucida (ZP) (Wassarman and Litscher, 2001), although others reported in hamster and rabbit that spermatozoa could undergo exocytosis within the cumulus (Yanagimachi, 1966; Kuzan et al., 1984). However, recent studies using transgenic mice expressing enhanced green fluorescent protein (EGFP) inside the acrosome as a method to visualize acrosomal exocytosis revealed that: 1) simple sperm binding to the ZP is not sufficient to induce the AR (Baibakov et al., 2007); 2) spermatozoa that have completed the AR are able to bind to the ZP and penetrate it (Jin et al., 2011); and 3) acrosome-reacted spermatozoa collected from the perivitelline space of an oocyte are still able to fertilize other cumulus-enclosed oocytes (Kuzan et al., 1984; Inoue et al., 2011). These observations suggest that fertilizing spermatozoa do not necessarily undergo the AR upon interaction with the ZP proteins in their original three-dimensional structures.
Two obvious questions that have remained unanswered are where and when fertilizing spermatozoa begin their AR in vivo (Bedford, 2011; Yanagimachi, 2011; Buffone et al., 2014). Although these questions are important, examinations of the AR within oviducts have been difficult. Whereas spermatozoa with intact acrosomes are able to reach the ampullary region of the oviduct following copulation (Chang and Suarez, 2012), acrosome-reacted spermatozoa are present within the oviductal ampulla in vivo before and near the time of fertilization (Yanagimachi, 1966; Yanagimachi and Mahi, 1976). Another unresolved question concerns the real inducer(s) of the AR in fertilizing spermatozoa in vivo. Although the cumulus oophorus surrounding oocytes has been implicated as the site of the AR in some mammalian species such as human (Stock et al., 1989) and shrew (Kaneko et al., 2001) there is no unequivocal evidence to support this view in mouse. In a previous work from our group using in vitro fertilization (IVF), we observed that the AR, as shown by a loss of EGFP fluorescence in the acrosome, rarely begins while spermatozoa are traveling through the cumulus (Hirohashi et al., 2011). An inherent problem of using spermatozoa treated in capacitation medium is that only a small fraction of cells are functionally capacitated and others fail to progress through the steps of sperm-egg interaction, suggesting that the changes occurring in the majority of the spermatozoa do not necessary represent what actually happens during fertilization. This feature may also explain why only a small fraction of the sperm undergoes acrosomal exocytosis when stimulated by progesterone. Therefore, we attempted to identify those spermatozoa whose acrosomes are ready to undergo AR in response to progesterone, one of the major secretory products from the cumulus cells and a potential physiological inducer of the AR (Parinaud et al., 1992; Roldan et al., 1994). It is well established that during capacitation, changes in the acrosome architecture such as acrosomal swelling, invaginations of the outer acrosomal membrane, and membrane docking are essential for exocytosis to occur (Zanetti and Mayorga, 2009); however, these changes are difficult to observe using standard microcopy methods. Our hypothesis is that these changes that the acrosome undergoes during capacitation can be visualized using transgenic EGFP sperm, and therefore we might be able to determine spermatozoa whose acrosomes are ready to trigger the AR in response to progesterone. In this report, we studied the relationship between pre-AR changes in spermatozoa expressing EGFP in their acrosomes and their response to progesterone.
Materials and Methods
Materials
Human tubal fluid (HTF) medium and Hepes-buffered HTF designed for human IVF and intracytoplasmic sperm injection (ICSI) were purchased from InVitroCare (Frederick, MD, USA). Bovine serum albumin (BSA) and progesterone were obtained from Sigma-Aldrich (St Louis, MO, USA). Progesterone was dissolved in dimethyl sulfoxide (DMSO) for experimental use.
Transgenic Mouse Spermatozoa
Spermatozoa of double-gene knock-in male mice [BDF1-Tg (CAG-mtDsRed2, Acr-EGFP) RBGS0020sb] have acrosomes expressing EGFP fluorescence and midpiece mitochondria displaying Ds-Red2 fluorescence (Hasuwa et al., 2010). The EGFP was designed to be expressed as a soluble protein under the acrosin promoter with a proacrosin signal sequence. These male mice were crossed with imprinting control region (ICR) strain females and their gametes were used for our observations. All experiments were performed with the approval of the Animal Care and Use Committee of Ochanomizu University and IBYME. In addition, investigations using experimental animals were conducted in accordance with the NIH specific guidelines (Guide for the Care and Use of Laboratory Animals, 1996).
Sperm Capacitation
Spermatozoa recovered from the cauda epididymidis were induced to capacitate by suspending them in a 100 μL droplet of HTF–BSA medium at ~105 cells/mL and incubating them for 1–4 h at 37°C under a humidified atmosphere of 5% CO2 and 95% air. In some experiment, spermatozoa were incubated with 2 mg/mL propidium iodide to determine the viability of the cells, and observe using fluorescence microscopy.
Analysis of the AR Using EGFP-expressing Spermatozoa
To determine the percentage of spermatozoa with EGFP in their acrosomes, 10 μL aliquots of the sperm suspensions were placed on poly-l-lysine-coated glass slides and covered with coverslips. To detect the presence of EGFP in the acrosomes, the cells were quantified using a Nikon TE2000 inverted fluorescence microscope with fluorescence optics (excitation 480 nm, emission 515 nm) as previously described (Buffone et al., 2009a, b). In other experiments, acrosome reaction was quantified as previously described using flow cytometry using a FACSCanto II flow cytometer (BD) [21]. The viable sperm were selected by staining with propidium iodide (final concentration, 10 μg/ml), and their acrosomal integrity was determined by the presence of acrosomal EGFP as previously reported (Muro et al., 2012).
Real-time Observations of Acrosomal EGFP
Spermatozoa were collected as described above. Coverslips were washed overnight with 85% ethanol, rinsed with water and coated with laminin by adding a drop of 20 mg/mL laminin (Sigma-Aldrich) and allowing them to air-dry. The laminin-covered coverslips were placed into a Leiden chamber (Medical Systems, Greenvale, NY). Spermatozoa were affixed to these coverslips for fluorescence imaging of acrosomal EGFP. The coverslips were then washed twice with 400 μL of HTF to remove nonattached spermatozoa. The chamber was placed onto the temperature-controlled stage of an inverted epifluorescence Nikon TE2000 microscope at 37 °C equipped with a 100 × 0.5–1.3 NA S Fluor oil objective and a Princeton Instruments MicroMAX CCD camera (Roper Scientific, Trenton, NJ, USA). Basal sperm fluorescence levels were recorded, and—without interruption of imaging—a small volume of DMSO containing progesterone (Sigma-Aldrich) was added to the coverslip with spermatozoa and incubated for 30 sec at 37 °C for a final concentration of 0-20 μM progesterone. For analysis, we selected spermatozoa that were oriented such that the side of the head could be visualized and the apical, dorsal, and posterior regions of the sperm head could be identified readily. The fluorescence signal was measured every 0.5 sec for 10 min. The fluorescence intensity was quantified using ImageJ software 1.47 V (National Institute of Health, USA). The intensity of fluorescence was calculated in the regions of interest localized in the sperm head. The background intensity was subtracted.
In some experiments, a confocal microscope (Nikon Eclipse C1) was used to determine the localization of EGFP in spermatozoa during the capacitating incubation.
Observation of the sperm acrosomal status in the oviduct
(C57BL/6J×BALB/c)F1 females were superovulated by intraperitoneal injection of 5U of pregnant mare's serum gonadotropin followed 48 h later by 5U of human chorionic gonadotropin (hCG). Superovulated females were caged together with CAG-mtDsRed2, Acr-EGFP males 12 h after hCG injection. At 1 and 4 h after coitus, oviducts and uterus were dissected out. Oviducts were gently manipulated and straightened out by cutting the mesosalpinx. They were mounted on slides, covered with coverslips and examined by confocal microscopy (Nikon Eclipse C1) to determine the presence of sperm containing the acrosomal EGFP marker.
Statistical analysis
Data are expressed as mean ± SEM of at least three experiments for all determinations. Statistical analyses were performed using ANOVA and a Tukey's multiple comparisons test using the GraphPad Prism 6 software (La Jolla, CA USA). Statistical significance is indicated in the figure legends.
Results
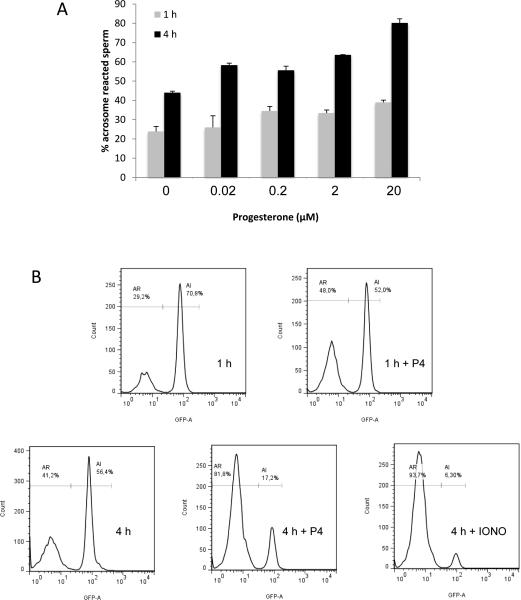
Progesterone has long been a candidate for inducing the AR (Parinaud et al., 1992; Roldan et al., 1994). However, the reasons have remained elusive as to why only a small proportion of capacitated spermatozoa can undergo the AR when exposed to progesterone at physiological concentrations. Although most IVF protocols use 1-2 h incubation of epididymal spermatozoa in a chemically-defined medium for sperm capacitation, we wondered if longer incubation can result in better outcomes in the progesterone-induced AR. A recent report showed that mouse spermatozoa remain in the lower part of the oviduct for about 3 h before ascending to the ampulla (Miki and Clapham, 2013). We, therefore, studied how spermatozoa would respond to progesterone after various times of in vitro capacitation (1–4 h) with different concentrations of progesterone (0–20 μM) (Figure 1). The results showed a dose-dependent increase in the progesterone-induced AR and the rate of such increase was accelerated when initiated following 4 h-incubation period compared to a 1 h-incubation (Figure 1). Thus, the ability of spermatozoa to undergo the AR in response to progesterone was enhanced after extended incubation under capacitating conditions.
Figure 1.
Spermatozoa are more responsive to progesterone after 4 h incubation in capacitating conditions (compared with 1 h). (A) After 1 h or 4 h incubation under capacitating conditions, the spermatozoa were exposed to increasing concentrations of progesterone (0.01–20 μM) and the percentages of sperm with or without EGFP in the acrosome were observed and counted using a fluorescence microscope. As a control, the vehicle (DMSO) was added to the samples at both time points (0 μM of Progesterone). The data represent the mean ± standard error of the mean (SEM; n = 4 experiments). *represents significant difference compared with 1h (P< 0.05). (B) Representative experiments showing acrosomal exocytosis after progesterone treatment at 1 h or 4 h incubation under capacitating conditions. Live spermatozoa as judged by propidium iodide staining were used independently to determine the percentage of sperm showing the presence (AI) or absence (AR) of the GFP fluorescence using flow cytometry. After 1 h incubation in a capacitating medium, sperm were treated with or without 20 μM progesterone (upper right and left panel, respectively). After 4 h incubation in a capacitating medium, spermatozoa were treated with or without 20 μM progesterone or 1 μM ionomycin (middle, left and right panel, respectively).
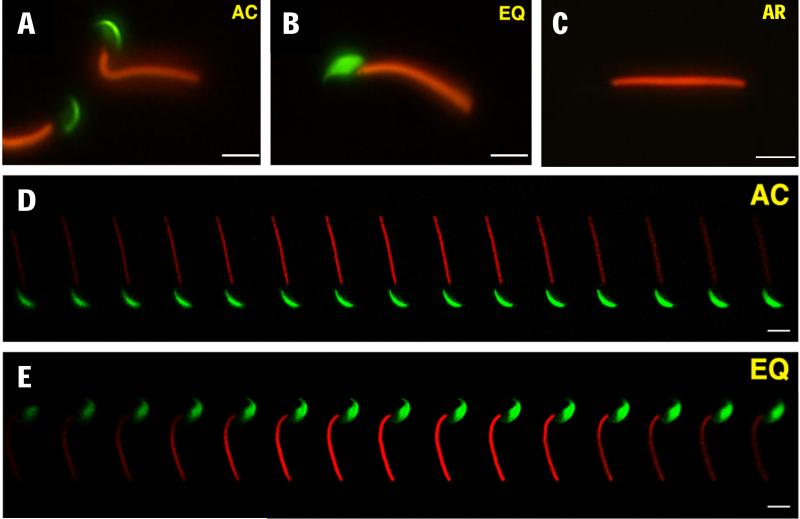
When these spermatozoa from transgenic mice (Ho et al., 2009) were incubated for 4 h and observed by epifluorescence microscopy, we noted that a significant number of acrosome-intact spermatozoa exhibited an altered EGFP distribution, i.e., the EGFP fluorescence originally restricted to the acrosomal cap region (Fig. 2A) was extended to the equatorial segment of the acrosome (Fig. 2B), hereinafter referred to as “EQ sperm” that are distinguishable from non-capacitated, acrosome-intact spermatozoa with a distinct acrosomal cap staining pattern (AC sperm). The difference in EGFP distribution between AC and EQ spermatozoa was not caused by a difference in the focal plane of the microscope, because we observed the same difference when using a confocal microscope (Fig. 2D, E). The EQ sperm were alive and motile, as judged by propidium iodide vital staining and video motion analysis (Figure 3, which represents Supplementary Movie S1).
Figure 2.
Two different types of acrosome-intact spermatozoa: (A) acrosomal cap (AC) and (B) equatorial segment (EQ). (C) Acrosome-reacted sperm lacking EGFP. (D, E) Z-stack confocal images of AC (D) and EQ (E) spermatozoa, respectively. After 4 h of incubation, many spermatozoa showed expansion of the soluble acrosomal components—represented by EGFP presence in the equatorial region.
Figure 3.
Representative time-lapse images of an EQ spermatozoon exhibiting active motility and no membrane permeability to propidium iodide. Images are taken from the Supplemental Movies S1.
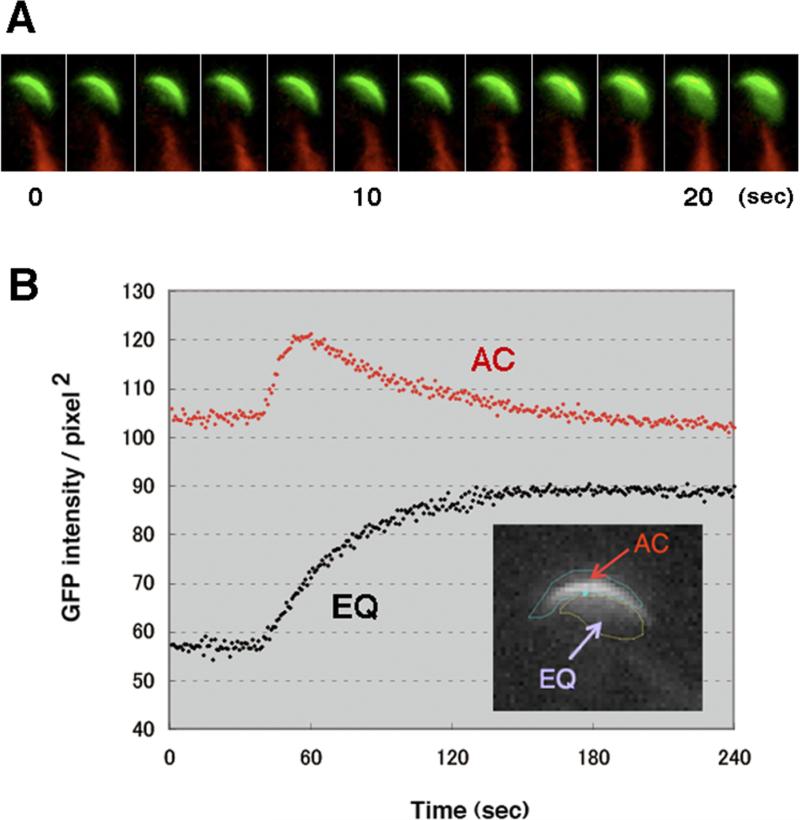
Population analysis of spermatozoa revealed that the transition from the AC to the EQ pattern occurred gradually during 4 h of incubation (Fig. 4A). In a control experiment where BSA was absent in medium (noncapacitating conditions), the transition was seldom seen, suggesting its association with sperm capacitation (Fig. 4B). Interestingly, single cell analysis revealed that the transition from the AC to the EQ pattern occurred very rapidly (~10 s) (Fig. 5, Supplementary Movie S2).
Figure 4.
The transition from the AC pattern to the EQ pattern depended on capacitating conditions. Time course analysis of the AC (black columns) and EQ (grey columns) patterns: (A) under capacitating conditions and (B) under non-capacitating conditions. Incubation of spermatozoa in medium lacking BSA did not permit the transition. Data represent the mean ± SEM (n = 4 experiments). *represents significant difference compared with AC pattern (P< 0.05).
Figure 5.
Live imaging analysis of the transition between the AC and the EQ patterns. (A) Representative time-lapse images of a spermatozoon undergoing this transition. (B) The fluorescence intensity in the equatorial region area became stably higher after the transition, whereas upon the AC to EQ transition, the fluorescence in the acrosomal cap region increased transiently and returned to the original level. The fluorescence intensities of two arbitrary delimited areas of interest were measured over time.
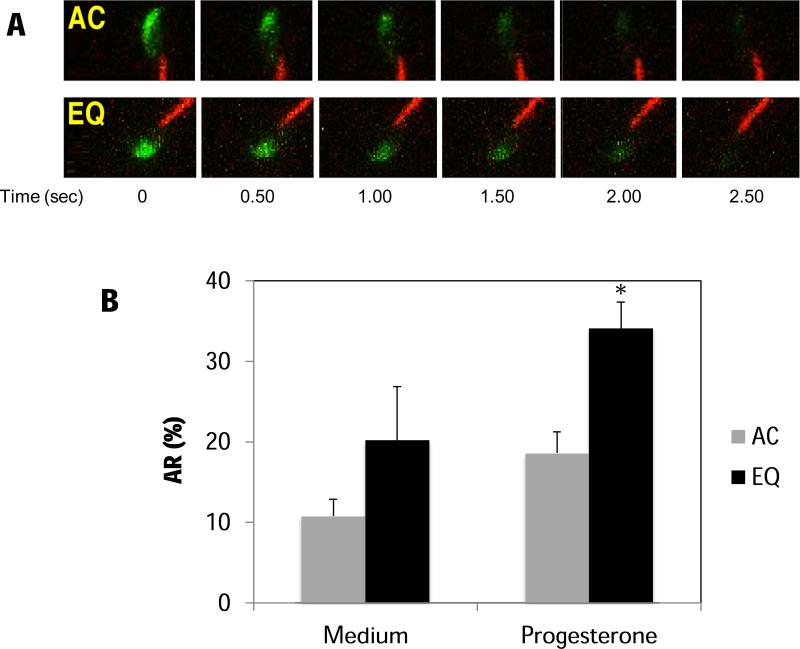
We speculated that the change in the EGFP distribution pattern might reflect an important physiological consequence during sperm capacitation prior to the AR, which render spermatozoa more susceptible to progesterone. When spermatozoa were treated with 20 μM progesterone after 4 h incubation, (Figure 6A, Supplementary Movie S3 and S4), both AC and EQ sperm underwent AR. However, the induced AR occurred to a greater extent in EQ spermatozoa than in AC spermatozoa (Fig. 6B).
Figure 6.
Spermatozoa with both patterns of EGFP distribution could undergo the AR after the addition of progesterone. (A) Time-lapse images of EGFP-expressing spermatozoa with the AC pattern (upper panels) or the EQ pattern (lower panels) undergoing acrosomal exocytosis after the addition of 20 μM of progesterone. (B) The percentages of ARs of both AC- and EQ-type spermatozoa in the presence (induced AR) or absence (spontaneous AR) of 20 μM of progesterone. As a control, the vehicle DMSO was added to the samples (Medium). Spermatozoa were attached to laminin-coated slides and recorded for 10 min. During this time, both patterns underwent spontaneous ARs at similar rates. However, in the presence of 20 μM progesterone, more EQ sperm tended to undergo exocytosis than the AC sperm. Data represent the mean ± SEM (n = 4 experiments). *represents significant difference compared with AC pattern (P< 0.05).
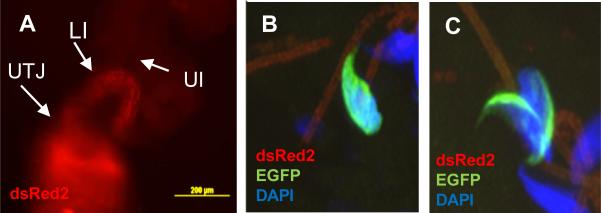
Next we addressed if sperm can evoke this change after being deposited in the female reproductive tract by copulation. At 4h post-mating, the wild-type females were sacrificed to isolate the reproductive organs (uterus and oviduct). Ejaculated spermatozoa in the isolated organs were observed using epifluorescence or confocal microscopy. As shown in figure 7A, spermatozoa migrated through the utero-tubal junction (UTJ) and took up residence in the oviduct. The sperm density in the UTJ or in the lower parts of the isthmus (close to the UTJ) was much higher than that in other parts of the oviduct, and markedly decreased in the upper part (toward the ampulla) of the fallopian tube. According to a previous report (Ho et al., 2009), spermatozoa arrived at the isthmus in 60-90 min after mating. After 4 h, the sperm density in each section of the oviduct was quite similar to the distribution observed after 1 h (data not shown). We were unable to find spermatozoa with the EQ pattern in the uterus or the UTJ (data not shown); however some EQ sperm were evident in upper segments of the isthmus only after 4 h post mating (Fig. 7B and C). However, an important limitation in quantifying accurately the percentage of EQ sperm in this region is that very few cells arrive at this segment of the oviduct, and some of them already lost their acrosomes.
Figure 7.
Representative images of sperm inside the female reproductive tract after natural mating with transgenic males carrying Acr-EGFP and Ds-Red2. The photographs show spermatozoa migrating through the female reproductive tract detected by Ds-Red2 using an epi-fluorescence microscope (A). B-C: Representative confocal images of a cross section of the upper isthmus isolated 4 h after mating containing EQ (B) and AC (C) spermatozoon. UTJ: utero-tubal junction; LI: lower isthmus; UI: upper isthmus.
Discussion
In mammals, many millions of spermatozoa are ejaculated into the vagina or the uterus, yet only very few can reach the site of fertilization (the ampulla). An extreme case reported in mice was that the sperm-to-egg ratio was nearly 1:1 in the ampulla (Stewart-Savage and Bavister, 1988), suggesting that every single sperm arrived at the fertilization site have gained fertilization competence. However, the mechanism by which how such a few spermatozoa are selectively transported has been a longstanding mystery. In contrast, conventional IVF requires thousands of excess capacitated spermatozoa to ensure fertilization. In standard IVF protocols, epididymal sperm are subjected to a minimum of 1 h incubation in a chemically defined capacitating medium and thereafter only 10~20% of the population can undergo the AR by the inducers such as ZP3 or progesterone. These results can draw a tentative conclusion that in vitro capacitated spermatozoa exhibit the heterogeneous fertilizability due to chronological or susceptibility differences in acquiring competence for exocytosis. Meanwhile, we realized during our IVF attempts (Hirohashi et al., 2011; Jin et al., 2011) that mouse spermatozoa incubated for 4–5 h in a capacitating medium gave better fertilization outcomes than 1 h. This observation prompted us to examine the morphology of the acrosome in great detail during in vitro capacitation. Because the rate of spontaneous AR does not correlate with fertilization outcomes (data not shown) and fertilization readily occurs if the cumulus oophorus remains intact, fertilizing spermatozoa are thought to have intact acrosomes during capacitation and undergo the AR upon stimulation by some soluble or insoluble component(s) from the cumulus matrix.
Interestingly, we observed an increased rate of the AR after 4 h of preincubation in capacitating condition compared to 1–2 h preincubation. In the extended incubation condition, there was a dynamic and rapid redistribution of acrosomal EGFP. The EGFP protein expanded from the apical region of the head (acrosomal crescent) to the equatorial domain in a substantial proportion of spermatozoa. Although spermatozoa with either AC or EQ patterns were capable of triggering the AR when exposed to progesterone, the EQ spermatozoa underwent the AR more frequently. This change might be related to acrosomal swelling (Zanetti and Mayorga, 2009), a prerequisite to the acrosomal exocytosis that occur following sperm capacitation. According to Zanetti and Mayorga (Zanetti and Mayorga, 2009), human spermatozoa increase their acrosomal volume during capacitation, and this is of critical importance for membrane docking and hybrid vesicle formation during the AR. It remains unknown how acrosomal swelling occurs during sperm capacitation; however, alkalization of intra acrosomal milieu could be a possible mechanism (isotonic swelling). Initially, acrosomal pH is maintained at acidic levels thorough the homeostatic action of a vacuolar-type proton ATPase (V-ATPase), a sodium-proton exchanger (NHE) and possibly a Cl-/HCO3- exchanger (Nishigaki et al., 2014). Upon stimulation with a capacitating medium, acrosomal pH gradually increases over the incubation period of 120 min from pH 5.3 ± 0.1 to pH 6.2 ± 0.3 (Nakanishi et al., 2001). However, because a rate of pH increase is so gradual, alkalosis-induced swelling does not simply explain the observed dynamics of AC-to-EQ transition, rather the transition must be set by a commitment threshold.
To investigate the physiological role of the capacitation-associated EGFP distribution, we investigated the acrosomal status of spermatozoa in the uterus and different parts of the oviduct. We observed some EQ sperm in the upper segments of the oviduct. However, due to the limited sperm number arriving at this region, it was difficult to estimate the accurate AC/EQ proportion. In addition, we met difficulty in distinguishing the EQ from AC in some oviductal spermatozoa that preclude an equatorial view. When we compared the sperm density in each part of the oviduct, there was no significant difference between 1 h and 4 h post mating except for the upper segments of the oviduct. A marked difference was the absence of EQ sperm at any part of the oviduct at 1 h post mating. Therefore, EQ sperm could be at the primed state where the AR takes place by physiological stimulation in the vicinity of the ampulla.
Transition from a nonexcitable (AC) to an excitable (EQ) state might also be a rapid process leading to acquired exocytotic competence as well as an increased sensitivity to progesterone. In this paper, we provide for the first time a non-invasive method by which live cell imaging of capacitated spermatozoa with an exocytotic competence could be performed. These findings are important not only for dissecting the molecular events that occur prior to the AR, but also for identifying bona fide inducer(s) for the AR from the natural environment of female reproductive organs.
Supplementary Material
Supplementary video 1: A representative EQ spermatozoon exhibiting active motility and no membrane permeability to propidium iodide.
Supplementary video 2: Examples of the transition from the AC to the EQ patterns.
Supplementary video 3: A representative AC-type spermatozoon undergoing the AR in response to 20 μM of progesterone.
Supplementary video 4: A representative EQ spermatozoon undergoing the AR in response to 20 μM of progesterone.
Acknowledgments
We thank Drs. Ryuzo Yanagimachi and George L. Gerton for their insightful comments. We also thank Dr. Masaru Okabe for kindly providing the “green-red” sperm mice (CAG-mtDsRed2, Acr-EGFP). We thank N. Osafune, M. Takada, H. Ochi and K. Moriwaki for their assistance in the experiments.
Funding: This work was supported by National Institutes of Health (RO1TW008662); Agencia Nacional de Promoción Científica y Tecnológica (PICT 2010-2426 and 2012-1175) and the Japanese Society for Promotion of Science (JSPS) (FY2011).
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Austin CR. Observations on the penetration of the sperm in the mammalian egg. Australian Journal of Scientific Research. Ser. B: Biological Sciences. 1951;4:581–596. doi: 10.1071/bi9510581. [DOI] [PubMed] [Google Scholar]
- Baibakov B, Gauthier L, Talbot P, Rankin TL, Dean J. Sperm binding to the zona pellucida is not sufficient to induce acrosome exocytosis. Development (Cambridge, England) 2007;134:933–943. doi: 10.1242/dev.02752. [DOI] [PubMed] [Google Scholar]
- Bedford JM. Site of the mammalian sperm physiological acrosome reaction. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:4703–4704. doi: 10.1073/pnas.1102296108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffone MG, Rodriguez-Miranda E, Storey BT, Gerton G. Acrosomal exocytosis of mouse sperm progresses in a consistent direction in response to zona pellucida. Journal of Cellular Physiology. 2009a;220:611–620. doi: 10.1002/jcp.21781. [DOI] [PubMed] [Google Scholar]
- Buffone MG, Kim K- S, Doak BJ, Rodriguez-Miranda E, Gerton GL. Functional consequences of cleavage, dissociation and exocytotic release of ZP3R, a C4BP-related protein, from the mouse sperm acrosomal matrix. Journal of Cell Science. 2009b;122:3153–3160. doi: 10.1242/jcs.052977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffone MG, Hirohashi N, Gerton G. Unresolved questions concerning mammalian sperm acrosomal exocytosis. Biology of Reproduction. 2014;90:112. doi: 10.1095/biolreprod.114.117911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MC. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature. 1951;168:697–698. doi: 10.1038/168697b0. [DOI] [PubMed] [Google Scholar]
- Chang H, Suarez S. Unexpected flagellar movement patterns and epithelial binding behavior of mouse sperm in the oviduct. Biology of Reproduction. 2012;86(140):1–8. doi: 10.1095/biolreprod.111.096578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasuwa H, Muro Y, Ikawa M, Kato N, Tsujimoto Y, Okabe M. Transgenic mouse sperm that have green acrosome and red mitochondria allow visualization of sperm and their acrosome reaction in vivo. Experimental Animals / Japanese Association for Laboratory Animal Science. 2010;59:105–107. doi: 10.1538/expanim.59.105. [DOI] [PubMed] [Google Scholar]
- Hirohashi N, Gerton GL, Buffone M. Video imaging of the sperm acrosome reaction during in vitro fertilization. Communicative & Integrative Biology. 2011;4:471–476. doi: 10.4161/cib.4.4.15636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho K, Wolff CA, Suarez S. CatSper-null mutant spermatozoa are unable to ascend beyond the oviductal reservoir. Reproduction, Fertility, and Development. 2009;21:345–350. doi: 10.1071/rd08183. [DOI] [PubMed] [Google Scholar]
- Inoue N, Satouh Y, Ikawa M, Okabe M, Yanagimachi R. Acrosome-reacted mouse spermatozoa recovered from the perivitelline space can fertilize other eggs. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:20008–20011. doi: 10.1073/pnas.1116965108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Fujiwara E, Kakiuchi Y, Okabe M, Satouh Y, Baba SA, Chiba K, Hirohashi N. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:4892–4896. doi: 10.1073/pnas.1018202108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T, Iida H, Bedford JM, Mōri T. Spermatozoa of the shrew, Suncus murinus, undergo the acrosome reaction and then selectively kill cells in penetrating the cumulus oophorus. Biology of Reproduction. 2001;65:544–553. doi: 10.1095/biolreprod65.2.544. [DOI] [PubMed] [Google Scholar]
- Kuzan FB, Fleming AD, Seidel GE., Jr Successful fertilization in vitro of fresh intact oocytes by perivitelline (acrosome-reacted) spermatozoa of the rabbit. Fertility and Sterility. 1984;41:766–770. doi: 10.1016/s0015-0282(16)47847-7. [DOI] [PubMed] [Google Scholar]
- Miki K, Clapham D. Rheotaxis guides mammalian sperm. Current Biology: CB. 2013;23:443–452. doi: 10.1016/j.cub.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muro Y, Buffone MG, Okabe M, Gerton G. Function of the acrosomal matrix: zona pellucida 3 receptor (ZP3R/sp56) is not essential for mouse fertilization. Biology of Reproduction. 2012;86:1–6. doi: 10.1095/biolreprod.111.095877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi T, Ikawa M, Yamada S, Toshimori K, Okabe M. Alkalinization of acrosome measured by GFP as a pH indicator and its relation to sperm capacitation. Developmental Biology. 2001;237:222–231. doi: 10.1006/dbio.2001.0353. [DOI] [PubMed] [Google Scholar]
- Nishigaki T, José O, González-Cota AL, Romero F, Treviño CL and Darszon A. Intracellular pH in sperm physiology. Biochemical and Biophysical Research Communications. 2014;450:1149–1158. doi: 10.1016/j.bbrc.2014.05.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parinaud J, Labal B, Vieitez G. High progesterone concentrations induce acrosome reaction with a low cytotoxic effect. Fertility and Sterility. 1992;58:599–602. doi: 10.1016/s0015-0282(16)55270-4. [DOI] [PubMed] [Google Scholar]
- Roldan ER, Murase T, Shi Q. Exocytosis in spermatozoa in response to progesterone and zona pellucida. Science (New York, N.Y.) 1994;266:1578–1581. doi: 10.1126/science.7985030. [DOI] [PubMed] [Google Scholar]
- Stewart-Savage J, Bavister BD. Success of fertilization in golden hamsters is a function of the relative gamete ratio. Gamete Research. 1988;21:1–10. doi: 10.1002/mrd.1120210102. [DOI] [PubMed] [Google Scholar]
- Stock CE, Bates R, Lindsay KS, Edmonds DK, Fraser L. Human oocyte cumulus complexes stimulate the human acrosome reaction. Journal of Reproduction and Fertility. 1989;86:723–730. doi: 10.1530/jrf.0.0860723. [DOI] [PubMed] [Google Scholar]
- Wassarman PM, Litscher E. Towards the molecular basis of sperm and egg interaction during mammalian fertilization. Cells, Tissues, Organs. 2001;168:36–45. doi: 10.1159/000016804. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R. Time and process of sperm penetration into hamster ova in vivo and in vitro. Journal of Reproduction and Fertility. 1966;11:359–370. doi: 10.1530/jrf.0.0110359. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R. Mammalian sperm acrosome reaction: where does it begin before fertilization? Biology of Reproduction. 2011;85:4–5.. doi: 10.1095/biolreprod.111.092601. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R, Mahi C. The sperm acrosome reaction and fertilization in the guinea-pig: a study in vivo. Journal of Reproduction and Fertility. 1976;46:49–54. doi: 10.1530/jrf.0.0460049. [DOI] [PubMed] [Google Scholar]
- Zanetti N, Mayorga L. Acrosomal swelling and membrane docking are required for hybrid vesicle formation during the human sperm acrosome reaction. Biology of Reproduction. 2009;81:396–405. doi: 10.1095/biolreprod.109.076166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary video 1: A representative EQ spermatozoon exhibiting active motility and no membrane permeability to propidium iodide.
Supplementary video 2: Examples of the transition from the AC to the EQ patterns.
Supplementary video 3: A representative AC-type spermatozoon undergoing the AR in response to 20 μM of progesterone.
Supplementary video 4: A representative EQ spermatozoon undergoing the AR in response to 20 μM of progesterone.