Abstract
Background:
Treatment of memory impairment associated with dementia such as Alzheimer's disease is still inadequate and requires development of new drugs.
Objective:
The objective was to evaluate the memory enhancing effect of Celastrus paniculatus seed oil.
Materials and Methods:
C. paniculatus seed oil was mixed with equal amount of pure ghee and administered orally to mice in the dose of 200 mg/kg/day. Piracetam was used as a standard nootropic. Elevated plus maze and passive avoidance tests were used as a models to test spatial and fear memory respectively. Scopolamine (3 mg/kg, i.p.), was used as an amnestic agent.
Results:
Mice receiving C. paniculatus showed significant memory enhancement as compared to scopolamine group. The effect of C. paniculatus and combination of C. paniculatus with piracetam was comparable to that with piracetam alone.
Conclusion:
The present study demonstrates that C. paniculatus seed oil has memory enhancing effect and hence can be developed as a potential drug in the treatment of dementia.
KEY WORDS: Ayurveda, Celastrus paniculatus, elevated plus maze, passive avoidance test, piracetam
INTRODUCTION
Dementia is a mental disorder characterized by loss of intellectual ability which is sufficiently severe to interfere with one's occupational and social activity. Prevalence of dementia increases with age. The incidence of dementia is increasing worldwide.[1] Treatment of memory impairment associated with cognitive disorders such as Alzheimer's disease (AD) is still inadequate and requires development of new drugs. Nootropic agents like cholinesterase inhibitors are most commonly used drugs for treatment of AD. However, the resulting adverse effects associated with these agents like nausea, diarrhea, vomiting, and hepatotoxicity have limited their use.[2]
Ayurveda the traditional system of medicine recommends the use of medicinal plants for treatment of “Apagatsmṛti” that is, loss of memory. In Ayurveda, there are three aspects of mental ability dhīḥ (process of acquisition/learning), dhṛti (process of retention), smṛti (process of recall). Any disturbance in these aspects results in loss of mental ability. Indian medicinal plants categorized as medhyarasāyana improve memory and cognitive deficits associated with chronic illness and aging. One of these is Jyotiṣmatī (Celastrus paniculatus) which is well known for its ability to improve memory.[3] Studies using Jyotiṣmatī oil have shown improved memory processes in rats.[4] Despite its mention in Ayurveda textbooks as memory enhancing agent, very few studies have been conducted to evaluate the nootropic activity of C. paniculatus on spatial memory. [4,5] Moreover, no study has been conducted to evaluate the nootropic activity of C. paniculatus seed oil in scopolamine induced amnesia in a model of fear memory. Hence, the present study was designed to test the effects of C. paniculatus seed oil on scopolamine induced amnesia using elevated plus maze (model of spatial memory) and passive avoidance test (model of fear memory). We have also studied the effect of co-administration of Jyotiṣmatī oil with piracetam in these model.
MATERIALS AND METHODS
Permission of the Institutional Animal Ethics Committee was obtained before initiating the study. The study was conducted in accordance with the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India guidelines.
Animals
Thirty six mice were randomized into different treatment groups as shown in Table 1. Animals used in the study were bred in the Central Animal House of the Institute (registration number 60/1999) registered with the CPCSEA. Animals were housed in polypropylene cages containing husk to keep them dry throughout the experiments. Identification of mice was done with cage number and individual marking on tail. The animals were housed under standard laboratory conditions such as room temperature at 23°C, humidity at 30–70%, and with 12 h light and dark cycle. They were fed with rat chow procured from Nav Maharashtra Chakan Oil Mills Ltd., Maharashtra and Aquaguard pure water in polyethylene bottle given ad libitum.
Table 1.
Experimental groups
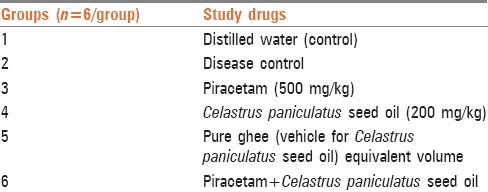
Drugs
Celastrus paniculatus was obtained from a botanical source from Dava Bazar, Mumbai. The sample was then authenticated with Agharkar Research Institute, Pune. C. paniculatus seed oil (Jo oil) was obtained by simple crushing in cold press as described in Ayurveda literature. The oil so obtained was stored at room temperature throughout the experiment. C. paniculatus seed oil (200 mg/kg/day)[5] was mixed with equal amount of pure ghee and administered orally.[3] Piracetam, a nootropic was administered orally at a dose of 500 mg/kg/day.[6] Commercially available tablets of piracetam (400 mg) of Intas Pharmaceuticals (Dehradun) was used. A suspension of piracetam was made in distilled water which served as vehicle. Scopolamine was purchased from Sigma-Aldrich and Administered in a dose of 3 mg/kg given i.p.[7]
Study procedure
Animals received the respective drugs/vehicle administered orally, once a day for a period of 14 days as depicted in Table 1. On 15th day, scopolamine/DW was injected intraperitoneally 30 min prior to training session of elevated plus maze test and passive avoidance test. Tests were done 24 h later to evaluate the effect of drugs on memory.
Elevated plus maze test
The elevated plus maze consists of two opposite open arms (16 cm × 5 cm) crossed with two closed arms of same dimension (16 cm × 5 cm × 12 cm). The arms are connected with a central square (5 cm × 5 cm) to give the apparatus the appearance of a plus sign. It is elevated 30 cm from the ground. Basal readings were recorded. Mouse was placed on one of the open arms facing opposite to the closed arm. Transfer latency (TL) time in seconds was noted for the first entry of the animal in a closed arm. TL is defined as the time elapsed between the point at which animal placed on the open arm and the point at which the animal enters the enclosed arm with all four legs inside the enclosed arm.
It is an exteroceptive behavioral model (wherein the stimulus exists outside the body). In elevated plus maze acquisition (learning) can be considered as TL on 1st day trials and the retention/consolidation (memory) is examined 24 h later.
Passive avoidance test
The two-chamber box consists of one light chamber connected to a dark chamber having an electrified grid where animal receives electric shock. Basal readings were recorded. The mouse was placed in the light chamber and the time spent by the animal in the light chamber was observed. Due to natural preference for the dark chamber animal spends more time in the dark chamber. The time taken in seconds for first entry of the animal in the dark chamber was recorded with the help of stopwatch. When the animal makes first entry into the dark chamber, a shock of 2 mA is transferred to the foot. The model is based upon conditional avoidance response wherein acquisition (learning) can be considered as TL on 1st day trials where animal receives foot shock and returns to the light chamber and the retention/consolidation (memory) is examined 24 h later.
Transfer latency is the time elapsed between the animal being placed in the light chamber and the time at which the animal enters the dark chamber with all four legs inside the dark chamber. TL was noted on 30 min after injection of scopolamine on day 1 and 24 h later on day 2.
Statistical analysis
The data were expressed in the form of mean ± standard deviation TLs were compared using ANOVA with post-hoc Tukey's test to evaluate the difference between groups. For analyzing effects within group, paired t-test was used. The level of significance was set at P < 0.05. GraphPad InStat software version 3.06 (GraphPad Software, Inc., California, US) was used for statistical analysis.
RESULTS
Elevated plus maze test
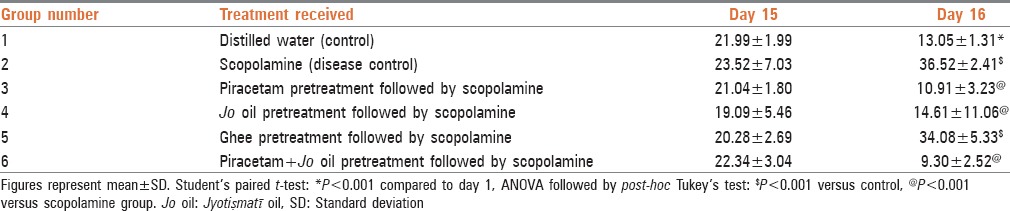
As presented in Table 2, TL for all the groups noted on day 15 was comparable to each other. Scopolamine group (Group 2) showed significantly high TL on day 16 when compared to the mice in the control group. The mice which received ghee (vehicle for Jo oil) also showed an increase in TL compared to control and values were comparable to the disease control. The mice pretreated with piracetam (Group 3) prior to scopolamine had statistically significant decrease (P < 0.001) in TL as compared to scopolamine group. Mice receiving Jo oil showed significant fall in TL as compared to scopolamine group (P < 0.001). The concomitant administration of piracetam with Jo oil also exhibited further decrease in TL (P < 0.001) compared to scopolamine group. Though the values of TL in the mice given concomitant administration of piracetam with Jo oil were lesser than the individual treatment groups, statistically all the three treatment groups were comparable with one another [Table 2]
Table 2.
Transfer latency in seconds in the model of elevated plus maze test
Passive avoidance test
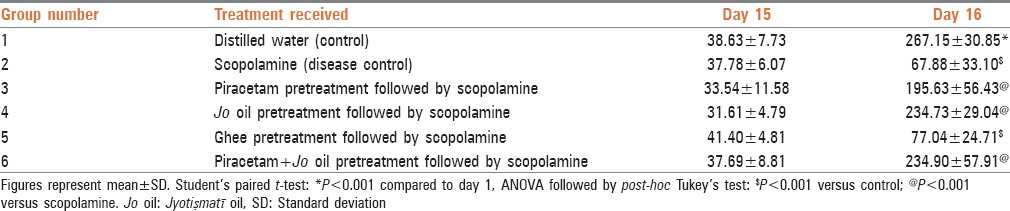
TLs for all the groups were comparable to one another on day 15. Scopolamine group showed a decrease in TL as compared to control on day 16 [Table 3]. Similarly, the mice which received ghee also showed significantly lesser TL compared to control group mice and values were comparable to the scopolamine group on day 16. The mice that received piracetam had statistically significant increase (P < 0.001) in TL as compared to scopolamine group. Mice receiving Jo oil too showed significant rise in TL (P < 0.001 vs. scopolamine group). The concomitant administration of piracetam with Jo oil exhibited increase in TL (P < 0.001) compared to control. The values of TL in these three treatment groups were comparable with one another on day 16, but they were less than that observed with control [Table 3].
Table 3.
Transfer latency in seconds in the model of passive avoidance test
DISCUSSION
In the present study, scopolamine was used to establish the model of impairment of learning and memory in mice with a dose of 3 mg/kg intraperitoneally.[7,8] Scopolamine is known to cause amnesia by interfering with acetylcholine (ACh) transmission in the central nervous system (CNS).[8] Jyotiṣmatī seed oil improved both spatial and fear memory as shown by decrease in TL in elevated plus maze test and increase in TL in passive avoidance test as compared to disease control. Combination of Jyotiṣmatī seed oil with piracetam did not show statistically significant difference when compared to those groups which received Jyotiṣmatī alone and piracetam alone. Piracetam, a known nootropic agent was selected as the positive control for this experiment. It has multiple mechanisms such as increasing high affinity choline uptake which facilitates ACh production and neuroprotection.[9]
As per Ayurveda, cow's ghee enhances learning, imparts intelligence, and strengthens memory.[10] Drugs such as Jyotiṣmatī and other Ayurvedic memory enhancers are usually given with ghee.[3] However, administration of ghee alone in this present study did not show any beneficial effect on the TLs in both the models. Hence, it appears from this model that ghee given with Jyotiṣmatī oil may be serving mainly as a vehicle to facilitate the transfer of Jyotiṣmatī oil across blood brain barrier.
In the present study, both Jyotiṣmatī seed oil and piracetam did not show a significant change in the TL as compared to control on the trial on the 1st day. This indicates that both the drugs did not have any effect on learning. Further it was demonstrated that Jyotiṣmatī seed oil enhances spatial and fear memory. The animals receiving Jyotiṣmatī oil and concomitant administration of piracetam with Jyotiṣmatī oil also showed a fall in TL on elevated plus maze. It has been reported that continued treatment with Jyotiṣmatī oil reversed the scopolamine induced deficits in maze performance, whereas the acute treatment was much less effective.[5] The present study also indicated good effect with 14 days. Another study has also shown that there needs to be at least 15 days of administration for maximal drug responses.[11] It has been postulated that Jyotiṣmatī oil induces adaptive cellular change within the CNS, thus needing long term administration of drug.[5]
The exact mechanism by which Jyotiṣmatī oil enhances learning and memory performance in behavioral tasks is not known. There are diverse reports in the literature regarding its probable mechanism of action. It has been demonstrated that Jyotiṣmatī oil enhances cognition due to increased ACh level in rat brain.[4] However, according to Gattu et al., it did not appear to inhibit brain cholinesterase activity, nor were there any symptoms of cholinergic receptor stimulation exhibited by the animals at any time during treatment.[5] Moreover, acute administration of Jyotiṣmatī oil failed to revert scopolamine's amnestic actions. Kumar and Gupta have reported the antioxidant activity of aqueous extract of Jyotiṣmatī which is responsible for reducing oxidative stress by increasing the endogenous antioxidant enzymes.[12] Jyotiṣmatī has been also shown to possess antioxidant properties in in vitro studies.[13] Further experiments are needed to confirm these possibilities. Although exact mechanism of Jyotiṣmatī has not been studied till date, it is postulated that the nootropic effect may be due to multiple mechanisms and phytoconstitutents such as sesquiterpene[14] and β-sitosterol.[15] The combination of piracetam with Jyotiṣmatī oil did not produce any additive effect over their individual effects. Perhaps both the drugs may be working through same mechanism and thus not leading to better effects on combination than when used as single agents. Based on the present study, there appears no scope for the concomitant therapy of these two agents. Future studies with other models of amnesia should be conducted to confirm nootropic potential of C. paniculatus and its mechanism of action should be explored.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Prince M, Bryce R, Ferri C. London: Alzheimer's Disease International; 2011. World Alzheimer Report 2011: The Benefits of Early Diagnosis and Intervention. [Google Scholar]
- 2.Brunton LL, Chabner BA, Knollmann BC, editors. Goodman and Gilman's the Pharmacological Basis of Therapeutics. 12th ed. China: McGraw Hill; 2011. [Google Scholar]
- 3.Gogte VM. Ayurvedic pharmacology and therapeutic uses of medicinal plants (Dravyagunavidnyan) In: Ramkrishnan S, editor. 1st ed. Mumbai: Bharatiya Vidya Bhavan; 2000. p. 378. [Google Scholar]
- 4.Bhanumathy M, Harish MS, Shivaprasad HN, Sushma G. Nootropic activity of Celastrus paniculatus seed. Pharm Biol. 2010;48:324–7. doi: 10.3109/13880200903127391. [DOI] [PubMed] [Google Scholar]
- 5.Gattu M, Boss KL, Terry AV, Jr, Buccafusco JJ. Reversal of scopolamine-induced deficits in navigational memory performance by the seed oil of Celastrus paniculatus. Pharmacol Biochem Behav. 1997;57:793–9. doi: 10.1016/s0091-3057(96)00391-7. [DOI] [PubMed] [Google Scholar]
- 6.Zhang LH, Zhang SS. Relationship between facilitatory effect of piracetam on memory and glutamate receptors. Zhongguo Yao Li Xue Bao. 1991;12:145–7. [PubMed] [Google Scholar]
- 7.Vogel GH. Drug effects on learning and memory. In: Vogel G, editor. Drug Discovery and Evaluation of Pharmacological Assay. 2nd ed. New York: Springer-Verlag Berlin Heidelberg; 2002. [Google Scholar]
- 8.Itoh J, Nabeshima T, Kameyama T. Utility of an elevated plus-maze for the evaluation of memory in mice: Effects of nootropics, scopolamine and electroconvulsive shock. Psychopharmacology (Berl) 1990;101:27–33. doi: 10.1007/BF02253713. [DOI] [PubMed] [Google Scholar]
- 9.Balaraman R, Shingala J. Molecule of the millenium. Nootropics. Indian J Pharmacol. 2002;34:439–40. [Google Scholar]
- 10.Tripathi B, editor. Ch. 13. Varanasi: Chaukhamba Surbharati Prakashan; 2001. Charaka Samhita of Agnivesha, Sutrasthana; pp. 264–5. [Google Scholar]
- 11.Nalini K, Karanth KS, Rao A, Aroor AR. Effects of Celastrus paniculatus on passive avoidance performance and biogenic amine turnover in albino rats. J Ethnopharmacol. 1995;47:101–8. doi: 10.1016/0378-8741(95)01264-e. [DOI] [PubMed] [Google Scholar]
- 12.Kumar MH, Gupta YK. Antioxidant property of Celastrus paniculatus willd. A possible mechanism in enhancing cognition. Phytomedicine. 2002;9:302–11. doi: 10.1078/0944-7113-00136. [DOI] [PubMed] [Google Scholar]
- 13.Russo A, Izzo AA, Cardile V, Borrelli F, Vanella A. Indian medicinal plants as antiradicals and DNA cleavage protectors. Phytomedicine. 2001;8:125–32. doi: 10.1078/0944-7113-00021. [DOI] [PubMed] [Google Scholar]
- 14.Melikov EM, Serkerov SV, Movsumov GD, Mir-Babaev NF. The sesquiterpene lactone “azerin” has memory enhancing properties. Biull Eksp Biol Med. 1993;115:166–7. [PubMed] [Google Scholar]
- 15.Huanling Y, Yanxia B, Rong X, Weiwei M, Li X, Bingjie D, et al. Effects of β-sitosterol on spatial learning and memory of mice with hypercholesterolemic diet. J Cap Med Univ. 2008;29:724–7. [Google Scholar]


