Abstract
Background:
Malaria is one of the major obstacles to the socioeconomic development of several developing countries. Adequate treatment of the disease is becoming increasingly difficult due to the worsening problems of drug resistance in many parts of the world. Therefore, increased efforts in antimalarial drug discovery are urgently needed.
Objectives:
This study was designed to evaluate the antimalarial activity of the leaf latex of Aloe citrina Carter and Brandham and its major constituent.
Materials and Methods:
The leaf latex of A. citrina was dissolved in methanol and subjected to preparative thin layer chromatography. Structure of the isolated compound was determined on the basis of its electrospray-ionization tandem mass spectrometry, 1H, 13C NMR and DEPT spectral data. The latex and its isolated compound were tested for their in vivo antimalarial activity using a 4-day suppressive test against chloroquine sensitive ANKA strain of Plasmodium berghei in mice.
Results:
Homonataloin A/B was isolated as a major component of the latex. Both the latex and isolated compound exhibited significant (P < 0.001) antimalarial activity at a dose of 400 mg/kg with parasite suppression of 60.59% and 67.52%, respectively. No significant adverse signs of toxicity were observed in mice treated with the leaf latex up to the highest dose (5000 mg/kg).
Conclusion:
The results of this study indicate that the antimalarial activity of the plant is attributed in part or in full to the presence of homonataloin A/B in the latex. It also validates the traditional use of the plant as an antimalarial agent.
KEY WORDS: 4-day suppressive test, acute toxicity, Aloe citrina, antimalarial activity, homonataloin A/B
INTRODUCTION
The magnitude of malaria in terms of morbidity and mortality in humans makes it a major public health problem in tropical and subtropical countries. More than half a million people die every year as a result of malaria. Africa faces the greatest impact of the disease.[1,2] The main reason for the dramatic increase in deaths from malaria in Africa is attributed to the alarming spread of drug resistance and limited number of effective antimalarial drugs.[3] In Ethiopia, malaria is one of the most important public health problems occurring in more than 75% of the landmass. Approximately, 52 million people (68%) of the total population live in areas at risk of malaria, primarily at altitudes below 2000 m.[4] This makes malaria the number one health problem in Ethiopia, and WHO has estimated 4,068,764 malaria cases and 1581 deaths in Ethiopia in 2010.[5]
The ever-increasing resistance of malarial parasites to the commonly available antimalarial drugs has necessitated the search for new drugs. Traditionally used medicinal plants have played an important role in malaria treatment in most African countries including Ethiopia.[6] Aloe citrina Carter and Brandham, locally known as “Hargissa,” is one of the indigenous Aloe species of Ethiopia. It grows in an open deciduous Bushland, with pubescent and papillate flowers, prominently spotted leaves and the relatively long pedicels.[7] People who live in and around Sof Omar caves in Bale Zone use leaves of the plant for the treatment of wounds, malaria, eye infections, and abdominal pain.[8] In this context, our present work has focused on the isolation and structural characterization of an antimalarial compound from the leaf latex of A. citrina.
MATERIALS AND METHODS
Plant material
The leaf latex of A. citrina was collected in December 2011 from Bale region, Sof Omar caves 110 km east of Bale Robe. The identity of the plant material was confirmed by Prof. Sebsebe Demissew, the National Herbarium, Department of Biology, Addis Ababa University (AAU) where voucher specimen (collection number: BG001) was deposited.
Spectroscopic analysis
NMR spectra were recorded on Bruker Avance DMX400 FT-NMR spectrometer operating at 400 MHz for 1H and 100 MHz for 13C at room temperature using MeOH-d4. NMR signals were referred to internal standard, tetramethylsilane. Chemical shifts are reported in d units. Mass spectra (MS) were recorded on ultimate 3000 LC-MS. The measurement was carried out by an electrospray ionization (ESI) method with negative mode. The source voltage and temperature were fixed at 3 kV and 250°C.
Experimental animals
Male and female Swiss albino mice, weighing 22–30 g and age of 6–8 weeks, were used in the study. The mice were obtained from the animal house of the Department of Biology, College of Natural Sciences, AAU. The mice were housed in an air-conditioned room and were allowed to acclimatize for 1-week before the commencement of the study and were fed with standard commercial pellet food and tap water ad libitum. All procedures complied with the guide for the care and use of laboratory animals[9] and approved by the Institutional Review Board of the School of Pharmacy, AAU.
Parasite
Plasmodium berghei ANKA strain (chloroquine sensitive) was obtained from Department of Biology, College of Natural Sciences, AAU. It was subsequently maintained in the laboratory by serial blood passage from mouse to mouse on weekly bases.
Extraction of the latex
Latex was collected from the leaves of A. citrina by cutting and arranging the leaves concentrically around a depression in the soil, which was covered with a plastic sheet. It was then left in open air for 3 days to allow evaporation of water, which yielded a dark brown powder.
Isolation of a compound
The latex was dissolved in methanol and directly applied to preparative thin layer chromatography plates over silica gel using chloroform and methanol mixture (4:1) as a solvent system. Chromatograms were visualized under ultraviolet light at 254 and 366 nm.
Acute oral toxicity test
Fifteen female Swiss albino mice were randomly divided into three groups of 5 mice/cage. Before the administration of a single dose of the latex, the mice were fasted for 24 h, and then the first group was given distilled water, the second group was given the leaf latex of A. citrina at a dose of 2000 mg/kg dissolved in distilled water orally. The third group was given the leaf latex at a dose of 5000 mg/kg dissolved in distilled water. The mice were observed continuously for 1 h after administration of the latex; intermittently for 4 h, over a period of 24 h and for 14 days. The mice were observed for gross behavioral changes such as loss of appetite, hair erection, lacrimation, tremors, convulsions, salivation, diarrhea, mortality and other signs of toxicity.[10]
In vivo antimalarial assay
The antimalarial activity against P. berghei infection was evaluated using a 4-day suppressive standard test as described by Peter and Anatoli.[11] Blood was taken from a donor mouse with rising parasitemia level of approximately 40% parasitized erythrocytes. The blood was then diluted with the normal saline medium and hence that each 0.2 ml contained approximately 107 infected red blood cells. Each Swiss albino mouse (male) weighed 22–30 g was infected with 0.2 ml (107 parasitized erythrocytes) P. berghei, which was expected to produce a steadily rising infection in mice.
The infected mice were weighed and divided into five groups of 5 mice/group. Three test groups and two control groups (one for the vehicle that serves as a negative control and the other for the standard drug, chloroquine, which serves as a positive control) were used for each sample. The latex and isolated compound at doses of 100, 200 and 400 mg/kg of body weight per day, and the vehicle (0.5 ml distilled water) and the standard drug chloroquine at a dose of 25 mg/kg/day were prepared.
The above treatment was continued for 4 consecutive days with an interval of 24 h starting from 3 h after the infection carried out on day 0. Twenty-four hours after the last treatment (5th day), thin smears of blood films were obtained from the peripheral blood of tail of each mouse. The smear was placed on microscopic slides, fixed with methanol and stained with 10% Giemsa at pH 7.2. The numbers of parasitized erythrocytes in each of the four fields of the microscope were counted 3 times and the average was calculated to give the parasitemia of the individual animal.[11,12]

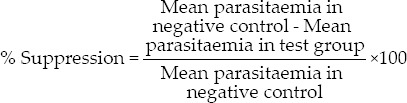
Statistical analysis
Results of this study were expressed as mean ± standard error of mean (M ± SEM). Data were analyzed using windows SPSS version 20 (IBM Company). Comparison of parasitaemia among groups and statistical significance was determined using one-way ANOVA and Student's t-test at a 95% confidence interval (α =0.05). The results were considered significant when P < 0.05.
RESULTS AND DISCUSSION
Acute toxicity
No significant signs of adverse toxicity or mortality were observed in mice after oral administration of the latex of A. citrina, up to a dose of 5000 mg/kg, indicating that the oral LD50 value of the latex is >5000 mg/kg. However, minor signs of toxicity such as temporary hair erection and diarrhea were noted in limited number of experimental animals. Plants or plant products with LD50 values higher than 2000–3000 mg/kg are generally considered to be free of any toxicity.[13]
Structural elucidation
Compound 1 was obtained from the latex as a yellow amorphous solid with an Rf value of 0.66 (chloroform/methanol, 4:1). A molecular formula of C22H24O9 was established by ESI tandem mass spectrometry (m/z 431 [M-H−), 1H and 13C NMR spectral data. However, the numbers of protons and carbons signals in the 1H and 13C NMR spectra [Table 1] of compound 1 do not match with the protons and carbons proposed in the molecular formula above. A close analysis of the spectra indicated that most of the signals occurred in pairs, indicating that compound 1 is a mixture of two related substances. Thus, compound 1 was unequivocally identified as the known anthrone, homonataloin A/B, by comparing its 1H and 13C NMR data with those data previously reported for the same compound [Figure 1].[14]
Table 1.
1H and 13C NMR spectral data of compound 1
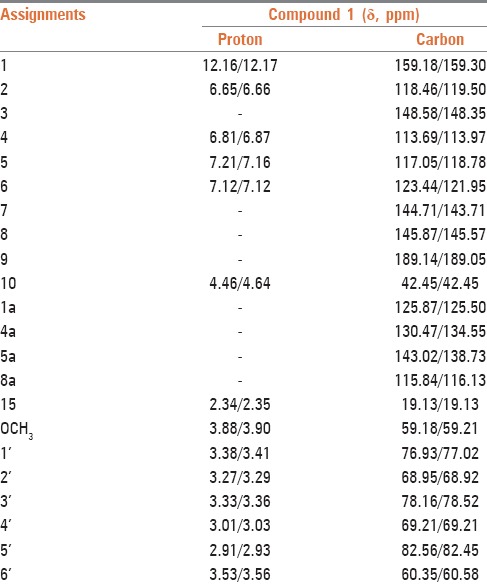
Figure 1.
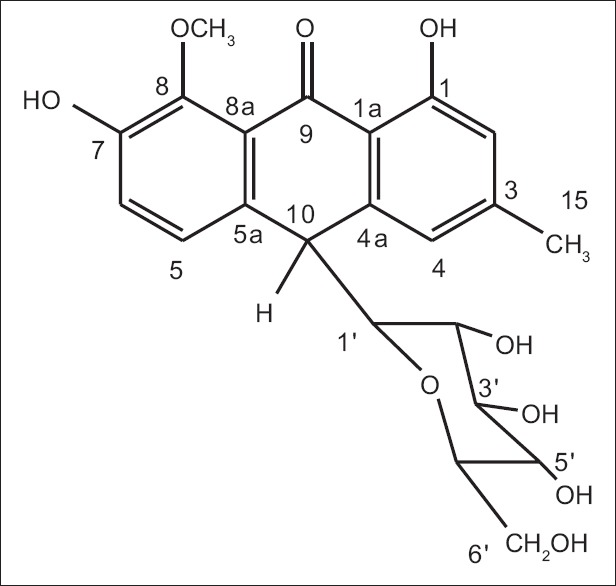
Chemical structures of homonataloin A/B
In vivo antimalarial activity
In vivo antimalarial activity of the leaf latex of A. citrina against chloroquine sensitive P. berghei was assessed using a 4-day suppression test. As shown in Table 2, the latex showed chemosuppression at doses of 100, 200 and 400 mg/kg/day. After 4 days of treatment at different doses, the mean parasitemia of the treated groups lowered from 22.81 ± 0.60% to 11.80 ± 0.36%, which was significant (P < 0.001) when compared to the negative control. Maximum parasite suppression (60.59%) was achieved by the latex at a dose of 400 mg/kg, while chloroquine at a dose of 25 mg/kg totally cleared the parasite on day 4, which was significant (P < 0.001) when compared to those groups treated with the latex. In general, the results of the in vivo evaluation indicated that the latex exhibits blood schizonticidal activity, which was also noted in other Aloe spp. such as A. debrana, which caused parasitemia suppression of 73.95% at a dose of 600 mg/kg[15] and A. otallensis which induced 60.70% suppression at a dose of 300 mg/kg. [16]
Table 2.
Antimalarial activity of the latex and homonataloin A/B isolated from the leaves of Aloe citrina in mice infected with Plasmodium berghei
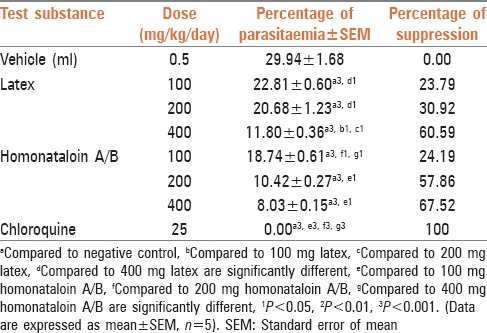
According to Deharo et al.,[17]in vivo antiplasmodial activity can be classified as moderate, good, and very good if an extract displayed percent parasite suppression ≥50% at a dose of 500, 250 and 100 mg/kg body weight/day, respectively. Based on this classification the leaf latex of A. citrina can be considered as a good antiplasmodial agent. The activity of the latex may be due to the presence of anthrones, which are characteristic constituents of the genus Aloe.[18] Thus, homonataloin A/B, which was isolated from the leaf latex of A. citrina was tested for its chemosuppression effects at doses of 100, 200 and 400 mg/kg/day. After a 4 days treatment with different doses of this compound, the mean parasitemia was lowered from 18.74 ± 0.61% to 8.03 ± 0.03% with maximum parasite suppression (67.52%) at a dose of 400 mg/kg/day [Table 2]. All groups of mice treated with homonataloin A/B showed significant reduction of parasitemia (P < 0.001) when compared to the negative control. These results indicated that the isolated compound possesses blood schizonticidal activity on early infection of mice with P. berghei.
Anthraquinones and anthrones are known to exhibit antiplasmodial activity.[19,20] For example, several anthraquinones isolated from the root extract of Rennellia elliptica Korth were found to inhibit strongly in vitro growth of a chloroquine sensitive strain of P. falciparum (3D7).[21] Similarly, aloe saponarin I isolated from A. saponaria has been reported to possess a good antiplasmodial activity against the P. falciparum Dd2.[19] 10-(Chrysophanol-7′-yl)-10-(β)-hydroxychrysophanol-9-anthrone and chryslandicin, isolated from the root of Kinphofia foliosa have also been found to display a strong in vitro antimalarial activity against the chloroquine-sensitive 3D7 strain of P. falciparum.[22]
One of the effects of malaria is a reduction of body weight. In the present investigation, weight difference of the infected mice before and after administration of the latex of A. citrina was recorded. As shown in Table 3, mice which received the vehicle (negative control) and treated with the latex with the exception of the group treated with 100 mg/kg of the compound, showed weight loss on the 5th day of infection. The latter showed statistically significant (P < 0.05) improvement of weight when compared to the weight before treatment. However, mice treated with 25 mg/kg chloroquine showed significant (P < 0.01) weight gain and higher weight increment than those received 100 mg/kg of the latex. Animals treated with the latex decreased their weight with increasing dose implying the possible appetite suppressive effects of the latex. Similar results have been reported in the literature,[23,24] whereby the percent weight loss preventive effect of the butanol fraction of Asparagus africanus declined with increasing dose.
Table 3.
Body weight of Plasmodium berghei infected mice after administration of the latex and homonataloin A/B isolated from the leaves of Aloe citrina
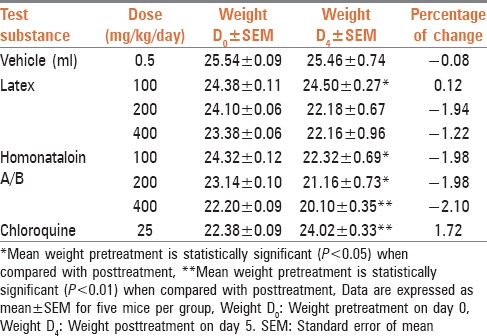
Table 3 also depicts that mice treated with different doses of homonataloin A/B lost weight on the 5th day of infection. The loss of weight was found to be dose-dependent with the maximum weight loss observed on animals treated with 400 mg/kg of the pure compound. In all compound and vehicle-treated groups the loss of weight was significant (P < 0.05). The loss of weight by homonataloin A/B treated mice could be attributed to the appetite suppressing effect of the compound as toxicity may be ruled out based on the results obtained from acute toxicity tests.
Homonataloin A/B contains a chelating moiety (C‑1 hydroxy-C‑9 ketone combination), which is thought to be responsible for the antiplasmodial activity of potent compounds such as rufigallol.[25] Therefore, the mechanism by which homonataloin A/B exerts its antimalarial action could be similar to that of rufigallol, which has been shown to involve chelation of iron, an essential element for cellular metabolism and DNA synthesis of the parasite.
CONCLUSION
Results of this study confirm the genuine antiplasmodial activity of the latex of A. citrina, which has been shown to contain the anthrone, homonataloin A/B as a major constituent. The investigation also proved that the antimalarial activity of A. citrina could be attributed in part or in full to the presence of homonataloin A/B in the latex.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Farooq U, Mahajan RC. Drug resistance in malaria. J Vector Borne Dis. 2004;41:45–53. [PubMed] [Google Scholar]
- 2.Pierre S, Toua VT, Fohouo TF, Nloga NA, Jean M. Medicinal plants used in traditional treatment of malaria in Cameroon. J Ecol Nat Environ. 2011;3:104–17. [Google Scholar]
- 3.Chenniappan K, Kadarkarai M. In vitro antimalarial activity of traditionally used Western Ghats plants from India and their interactions with chloroquine against chloroquine-resistant Plasmodium falciparum. Parasitol Res. 2010;107:1351–64. doi: 10.1007/s00436-010-2005-9. [DOI] [PubMed] [Google Scholar]
- 4.Malaria Programme Performance Review, Ethiopia. Addis Ababa: Ministry of Health; 2012. Federal Democratic Republic of Ethiopia. [Google Scholar]
- 5.WHO Global Malaria Programme – World Malaria Report. Geneva: WHO Press; 2011. World Health Organization. [Google Scholar]
- 6.Mesfin A, Giday M, Animut A, Teklehaymanot T. Ethnobotanical study of antimalarial plants in Shinile District, Somali Region, Ethiopia, and in vivo evaluation of selected ones against Plasmodium berghei. J Ethnopharmacol. 2012;139:221–7. doi: 10.1016/j.jep.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Demissew S, Nordal I. 2nd ed. Addis Ababa: Shama Books; 2010. Aloes and Lilies of Ethiopia and Eritrea; pp. 42–57. [Google Scholar]
- 8.Girma B. M. Sc. Thesis. Addis Ababa University; 2013. Anthrones Isolated from the Leaf Latex of Aloe citrina Carter and Brandham: Their Antimicrobial and Antimalarial Activities. [Google Scholar]
- 9.Guide for the Care and Use of Laboratory Animals. 7th ed. Washington, DC: National Academy Press; 1996. Institute of Laboratory Animal Resources. [Google Scholar]
- 10.Camargo ME, Berdeja B, Miranda G. Diuretic effect of the aqueous extract of Bidens odorata in the rat. J Ethnopharmacol. 2004;95:363–6. doi: 10.1016/j.jep.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Peter IT, Anatoli VK. 1st ed. Washington, DC: ASM Press; 1998. The current global malaria situation; Malaria Parasite Biology, Pathogenesis, and Protection; pp. 11–22. [Google Scholar]
- 12.Fidock DA, Rosenthal PJ, Croft SL, Brun R, Nwaka S. Antimalarial drug discovery: Efficacy models for compound screening. Nat Rev Drug Discov. 2004;3:509–20. doi: 10.1038/nrd1416. [DOI] [PubMed] [Google Scholar]
- 13.Arthi N, Murugan K. Antimalarial activity and phytochemical screening of ethanolic leaf extract of Phyllanthus niruri and Mimosa pudica. Int J Pharm Res Dev. 2011;3:198–205. [Google Scholar]
- 14.Conner JM, Gray AI, Reynold T, Waterman PG. Anthrone and chromone components of Aloe cremnophila and A. jacksonii leaf exudates. Phytochemistry. 1990;29:941–4. [Google Scholar]
- 15.Deressa T, Mekonnen Y, Animut A. In vivo anti-malarial activities of Clerodendrum myricoides, Dodonaea angustifolia and Aloe debrana against Plasmodium berghei. Ethiop J Health Dev. 2010;24:25–9. [Google Scholar]
- 16.Paulos B, Bisrat D, Gedif T, Asres K. Antimalarial and Antioxidant activities of the leaf exudates and a naphthalene derivative from Aloe otallensis Baker. Ethiop Pharm J. 2011;29:100–7. [Google Scholar]
- 17.Deharo E, Bourdy G, Quenevo C, Muñoz V, Ruiz G, Sauvain M. A search for natural bioactive compounds in Bolivia through a multidisciplinary approach Part V. Evaluation of the antimalarial activity of plants used by the Tacana Indians. J Ethnopharmacol. 2001;77:91–8. doi: 10.1016/s0378-8741(01)00270-7. [DOI] [PubMed] [Google Scholar]
- 18.Dagne E, Bisrat D, Viljoen A, Van Wyk B. Chemistry of Aloe species. Curr Org Chem. 2000;4:1055–78. [Google Scholar]
- 19.Nogueira CR, Lopes LM. Antiplasmodial natural products. Molecules. 2011;16:2146–90. [Google Scholar]
- 20.Endale M, Ekberg A, Alao JP, Akala HM, Ndakala A, Sunnerhagen P, et al. Anthraquinones of the roots of Pentas micrantha. Molecules. 2012;18:311–21. doi: 10.3390/molecules18010311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osman CP, Ismail NH, Ahmad R, Ahmat N, Awang K, Jaafar FM. Anthraquinones with antiplasmodial activity from the roots of Rennellia elliptica Korth. (Rubiaceae) Molecules. 2010;15:7218–26. doi: 10.3390/molecules15107218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wube AA, Bucar F, Asres K, Gibbons S, Rattray L, Croft SL. Antimalarial compounds from Kniphofia foliosa roots. Phytother Res. 2005;19:472–6. doi: 10.1002/ptr.1635. [DOI] [PubMed] [Google Scholar]
- 23.Dikasso D, Makonnen E, Debella A, Abebe D, Urga K, Makonnen W, et al. In vivo antimalarial activity of hydroalcoholic extracts from Asparagus africanus Lam. in mice infected with Plasmodium berghei. Ethiop J Health Dev. 2006;20:112–8. [Google Scholar]
- 24.Yared D, Mekonnen Y, Debella A. In vivo antimalarial activities of fractionated extracts of Asparagus africanus in mice infected with Plasmodium Berghei. Phol. 2012;3:88–94. [Google Scholar]
- 25.Winter RW, Cornell KA, Johnson LL, Isabelle LM, Hinrichs DJ, Riscoe MK. Hydroxy-anthraquinones as antimalarial agents. Bioorg Med Chem Lett. 1995;5:1927–32. [Google Scholar]


