Abstract
Sports and orthopaedic physical therapists have long used a multitude of techniques in order to address pain and dysfunction associated with myofascial trigger points. One technique in particular has recently received overwhelming attention: trigger point dry needling (DN). Despite its efficacy and low risk, questions remain as to its effectiveness, safety, and whether the technique is within the scope of practice of physical therapists. Therefore, the purpose of this clinical commentary is to summarize the current literature related to the associated mechanisms of action of DN, the safety of DN, as well as to discuss relevant scope of practice concerns.
Level of Evidence
5
Keywords: Dry needling, TDN, DN, Trigger point dry needling
INTRODUCTION
Dry needling (also known as intramuscular manual stimulation, or intramuscular needling) is a treatment technique that has been utilized by physiotherapists in Canada, Chile, Ireland, Spain, South Africa and the United Kingdom since the 1980's, and in the United States since 1984.1 While the technique is typically not taught in entry‐level education, there has been a dramatic increase in dry needling (DN) certification programs and continuing education courses in recent years. Additionally, the practice of DN has received significant attention at the federal level, as the Federation of State Boards of Physical Therapy (FSBPT) has released four editions of a resource paper between 2010 and 2013, all regarding physical therapist use of DN.1 The American Physical Therapy Association (APTA) and the American Academy of Orthopaedic Manual Physical Therapists (AAOMPT) have both created position statements supporting physical therapists’ use of the technique.2,3 With the increase in exposure to the practice of DN, therapists should question its efficacy, as well its associated risks. In order to appreciate both, it is vital to have a robust understanding of the various models for DN, as well as the proposed associated outcomes when treating pain of myofascial trigger point origin. Lastly, physical therapists must fully understand the scope of practice challenges that are associated with the performance of DN.
Myofascial Trigger Point Pain
Myofascial trigger point pain is defined as “pain arising from one or more myofascial trigger points (MTrPs), which are hyperirritable spots in skeletal muscle that are associated with hypersensitive palpable nodules in taut bands.”4 With MTrPs, the entire muscle is not hard, cramped, nor tender; the tenderness is strictly limited to the taut band.5 Typically, MTrPs are painful on compression and can give rise to referred pain and/or tenderness, as well as autonomic phenomena (localized sweating, vasoconstriction or vasodilation, and pilomotor activity).1,4,6 Additionally, they can be divided into active and latent types. These must be differentiated from tender points found in a muscle, which in contrast to MTrPs, only cause local pain upon compression.7 These differences underscore the need for the diagnosis of MTrP's to be classified not only as a motor or architectural abnormality, but as also including painful sensory dysfunction.
Several theories of precipitating and perpetuating factors responsible for creating MTrPs have been proposed (Table 1). The leading belief is that MTrPs are caused from an excessive release of acetylcholine (Ach) from motor endplates.8,9 The prolonged release of Ach results in chronic shortening and contractures of sarcomeres, coupled with decreased circulation leading to hypoxia and local ischemia.7,10 As a result, prostaglandins, bradykinins, cytokines, and histamine are released, which then sensitize the sensory afferent nerve fibers of the muscle, likely accounting for the specific point tenderness commonly seen with MTrPs.10-12 Furthermore, the bombardment of nociceptors by the endogenous chemicals often leads to central sensitization of the dorsal horn neurons.11,12
Table 1.
Precipitating & perpetuating factors of MTrPs
| Mechanism Category | Specific Mechanisms | Clinical Examples |
|---|---|---|
| Trauma83,84 | Macrotrauma | Contusions, sprains, strains |
| Microtrauma | Chronic repetitive overloading | |
| Mechanical86,86 | Internal factors | Poor posture, scoliosis |
| External factors | Poor ergonomics | |
| Degeneration7 | Articular degeneration | Osteoarthritis, with associated loss of myofascial flexibility |
| Nerve Root Compression53,87,88 | Nerve root sensitization | Sensitization of spinal segment and associated muscles |
| Emotional & Psychological11 | Anxiety, increased sympathetic output, sleep deprivation | Muscle tension, fatigue, decreased myofascial threshold |
| Endocrine & Metabolic7 | Thyroid & estrogen insufficiencies | Can give rise to, or perpetuate, myofascial pain |
| Nutritional7 | Vitamin & mineral insufficiencies | Can give rise to, or perpetuate, myofascial pain |
| Infection7 | Virus or parasite | Can perpetuate myofascial pain |
| Respiratory89,90 | Hypocapnia | Can lead to a decrease in oxygenation of the myofascial system due to the Bohr effect, thus causing myofascial inflammation |
Central sensitization is “an increase in the excitability of neurons within the central nervous system” that elicits pain hypersensitivity, so that normal inputs begin to produce abnormal responses.13,14(p. 205) The underlying neurobiological basis for central sensitization relates to the fact that most synaptic input to neurons is subthreshold, acting subliminally either because synaptic input is too weak, or membrane excitability is restrained by inhibitory inputs.13 These subthreshold inputs can be elevated to suprathreshold action potentials by increasing synaptic response to the transmitter, by reducing inhibition, or by increasing membrane excitability.13 Central sensitization has been observed during cutaneous inflammation as well as during inflammation of a joint, muscle or viscera.15 Typical changes of the individual neurons include, but are not limited to: 1) increased response to noxious stimulation of inflamed tissue; 2) lowered threshold of nociceptive specific spinal cord neurons; 3) increased response to stimuli applied to non‐inflamed tissue surrounding the inflamed site; and 4) expansion of the receptive field.15 Furthermore, it has recently been appreciated that in addition to activity‐dependent synaptic plasticity, changes to microglia, astrocytes, gap junctions, membrane excitability, and gene transcription all can contribute to the continuation of central sensitization.13 This “ramped up” nervous system perpetuates chronic muscular hypertonicity, and the development of MTrPs.
In contrast to central sensitization, peripheral sensitization occurs due to an increase in responsiveness, and reduced threshold of activation of the peripheral ends of nociceptors.13,14,16,17 Sensitization arises secondary to inflammatory mediators released around the site of tissue damage.16 Specifically, peripheral nociceptive terminals become ‘‘sensitized” after injury, secondary to an influx of neutrophils. The neutrophils create Cox‐2 enzyme, which leads to the production and secretion of prostaglandin E2 (PGE2).16 The PGE2 acts as a sensitizer, thus altering pain sensitivity. This hypersensitivity is localized to the site of injury, also known as the zone of primary hyperalgesia.13,16,17 Because peripheral sensitization represents a form of pain elicited by activation of nociceptors, it generally requires ongoing peripheral pathology in order for it to continue.16
It has been estimated that myofascial pain is responsible for 30‐85% of patients who present to a primary care setting or pain clinic with a complaint of pain.18-21 Gerwin et al5 noted that MTrPs were the primary source of pain in 71 of 96 patients who were referred to a neurologist with musculoskeletal pain. Similar results have been found in patients with chronic head and neck pain seeking dental care.20 In a study of musculoskeletal disorders in rural Thailand, it was found that pain arising from one or more myofascial trigger points was the primary cause for 36% of 431 individuals who had pain in the previous seven days.22 Despite the prevalence of MTrPs causing musculoskeletal pain, they often go undiagnosed, and therefore untreated.
Recent advances in medical imaging allow for the visualization of MTrPs.23 Sikdar et al24 recently introduced sonoelastography, a unique ultrasound application, which allows visualization of MTrPs. They noted that MTrPs in the upper trapezius were elliptically shaped focal areas of hypoechogenicitiy (ultrasound waves did not reflect back to sound head) that corresponded with the palpable nodule (Figure 1).23,24 The authors were able to identify retrograde blood flow during diastole which suggests a highly restrictive vascular bed. Additionally, magnetic resonance elastography (MRE) has recently been used to quantify asymmetries in muscle tone, and localize MTrPs.23,25 MRE has the ability to measure stiffness of soft tissues by measuring the propagation of shear waves introduced by a standard MRI.
Figure 1.
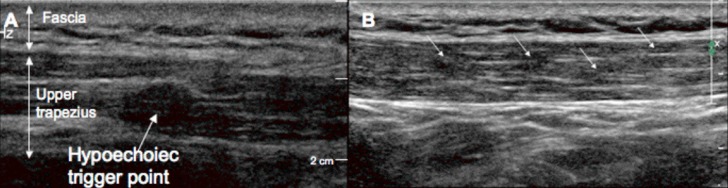
Gray scale imaging of a trigger point in the upper trapezius. (A) An isolated MTrP appears as a well‐defined focal hypoechoic nodule. (B) A series of four hypoechoic MTrPs in the upper trapezius. Reproduced with permission from Sikdar et al., 2009
While particular imaging methods can assist in identifying specific locations of MTrPs, their availability and cost currently prohibit their widespread use. Thus, a systematic approach to palpating trigger points is vital prior to treatment. Not only is it essential to palpate MTrPs in the primarily affected muscle, but also in the synergist and antagonist as well (secondary MTrPs). Baldry26 suggests practitioners first draw their palpating finger perpendicular to the muscle using a flat finger. A pincer palpation is not advised. In addition, if the practitioner does not palpate with sufficient pressure (approximately 4 kilograms), it is very difficult to elicit the characteristic ‘jump’ sign (involuntary flexion withdrawal) that confirms the presence of the MTrP.26 If the lesion is superficial, the MTrP should feel like a taut band; this can be confirmed by “snapping” the trigger point similar to how one would pluck a violin string. A local twitch response that frequently produces the associated referred pain will confirm the presence of the trigger point; both responses are not necessary, however, as either is sufficient for diagnosis.26
As Lucas et al27(p. 80) note: “pivotal to the appropriate and accurate prescription of any treatment is accurate diagnosis.” Therefore, both intra and inter‐rater reliability should be established. Various techniques have been employed attempting to establish a reference standard for identifying MTrPs, though none have been accepted as definitive.27 Microdialysis, biopsy, imaging, and electromyography all fall short of qualifying as a gold standard in MTrP identification.6,9,12,25 Gerwin et al28 attempted to establish inter‐rater reliability with identifying MTrPs in the neck and upper quarter. The first phase of their study failed to establish a high degree of agreement between therapists when palpating MTrPs for tenderness, taut bands, referred pain, local twitch response, or reproduction of pain. The authors extended the study into a second phase, which included a three‐hour training session prior to patient examination. The features of a trigger point were reviewed during this training in order to be certain that the physicians were interpreting their findings similarly. Following Phase II, the authors concluded that the training period significantly increased their inter‐rater reliability in the diagnosis of MTrPs. Additionally, they noted that inter‐rater reliability of different clinical features tended to vary, with the local twitch response being the most difficult to identify and that reliability with all characteristics varied depending on the muscle being palpated.
The need for well‐trained examiners in the identification of MTrPs was further supported by several studies. Wolf et al29 found poor reliability between clinicians (k=0.38), which was similar to the results of Nice et al.30 However, in both studies, there was a lack of training standardization amongst clinicians. Njoo and Van der Does31 reported a kappa of 0.49 with well‐trained examiners. Bron et al32 investigated reliability between three well‐trained examiners as they assessed 40 subjects. The authors noted good reliability for referred pain and the jump sign.32 These improvements in reliability are consistent with the results from Phase II Gerwin et al28 suggesting that inter‐rater reliability of trigger point identification is adequate as long as the clinicians are properly trained.
There is a marked paucity of research related to intra‐rater reliability of MTrP palpation. One of the only well designed studies investigating this utilized an experienced therapist (>10 years clinical practice, with extensive training with MTrP palpation) using the upper trapezius of 24 subjects with neck pain.33 Using the acromion angle of the scapula, and the C7 spinous process, a Cartesian coordinate system was utilized to record the locations of the MTrPs. Interclass correlation coefficient (ICC) for the observed values revealed a moderate to high correlation for both the x and y axis (ICC = 0.62 with a 95% CI of 0.30‐0.81 for the x axis; ICC = 0.81 with a 95% CI of 0.61‐0.91 for the y axis).33 This research must be reproduced with various muscle groups, and with varied levels of clinician training, before any clinical inferences should be drawn.
Trigger Point Dry Needling History & Theory
Modern trigger point dry needling has its origins in the work of Karel Lewit of Czechoslovakia.23,34,35 In his classic work, he examined the short and long‐term effects of dry needling in the treatment of myofascial pain in 241 patients with 312 painful MTrP sites. He reported an immediate analgesic affect without hypesthesia in 86% of cases when the most painful location was engaged by the needle. He popularized the phrase “needle effect,” where the analgesic affect of the needle is distinct from that of the injectable substance.23,35 This is similar to research published ∼40 years prior (1941) by Kelly.36 Kelly36 noted that injections of local anesthetics did not achieve any better effect than the introduction of normal saline when treating myofascial pain.
To some, trigger point dry needling may appear synonymous with Traditional Chinese Acupuncture (TCA); nonetheless, the two are uniquely different. TCA is based on the theory that the workings of the human body are controlled by a vital force or energy called “Qi” (pronounced “chee”), which circulates between organs along channels called meridians.37 These meridians are networks of channels inside the body with acupoints (high density sites of polymodal and specific nociceptive receptors near neurovascular structures and/or lymphatic vessels) on the skin and deeper tissues.38,39 TCA suggests that there are 12 primary meridians, each corresponding to major functions or organs of the body.37 In theory, these meridian channels provide migratory tracks for mast cells, fibroblasts, and other cells to carry out various physiological functions;38 Qi must flow in the correct strength and quality through each meridian in order to maintain optimal homeostasis. Therefore, if an acupuncturist detects any abnormal flow or quality of Qi about a meridian, he or she would needle the respective acupoint, theoretically normalizing the flow of Qi in the body.38 Acupuncturists utilize this philosophy to treat not only musculoskeletal dysfunction, but also problems with fertility, smoking cessation, allergies, depression, and other non‐musculoskeletal and neuromuscular conditions.
A more modern and alternate model to acupuncture recognizes that inserting a needle into the skin (not necessarily into a MTrP) stimulates A‐delta nerve fibers, consequently releasing opioid peptides from interneurons in the dorsal horns.40 These peptides inhibit intradorsal horn transmission of nociceptive information conveyed to the cord via group IV sensory afferents from the MTrP.40 A‐delta fibers are also stimulated with needle insertion secondary to a low‐intensity monophasic current of injury being created secondary to the difference in electrical potential between the needle and the skin.40 The combination of the mechanical and electrical activation of A‐delta fibers is what likely drives the inhibitory pain response noted with TCA.41
While there are several philosophies of practice that differ between acupuncture institutes, all TCA is based on the Daoist concept of yin and yang.42 Daoism refers to a “philosophical system developed by Lao‐tzu and Chuang‐tzu advocating a simple honest life and noninterference with the course of natural events.”42 Yin and yang refers to two principles in Chinese philosophy and religion; yin is negative, dark and feminine, while yang is positive, bright and masculine.43 It is thought that the interaction between the two influences the destinies of man. TCA promotes diagnoses related to meridians, such as “kidney‐yang deficiency, water overflowing” or “damp heat in the bladder.”37
While traditional Chinese Acupuncturists typically perform a multi‐system case history, the focus of their evaluation is on the shape, coating and color of the tongue, as well as the color of the face, and the strength, rhythm and quality of the pulse.37 The quality of these markers is thought to be an indicator of the patient's state of health. Typically between four and ten acupoints are needled during a session, with the needles being left in anywhere from 10‐30 minutes.37 Traditional Chinese Acupuncturists often augment their practice with various adjunctive therapies as well, including the use of electrical current between the needles, moxibustion (burning of an herb just above the surface of the skin), massage, cupping, and herbal preparations.37 A typical course of acupuncture will span 6‐12 sessions over a three month period, followed by “maintenance” treatments approximately every 3‐6 months.37
Several authors have noted that the scientific basis regarding pain neurophysiology and the mechanisms employed with dry needling supports its use.44-47 This technique is based on a different model than that of acupuncture, and is commonly broken down into three typical schemes: 1) a radicular model; 2) a spinal segmental sensitization model; and 3) a trigger point model.44 The radicular model is based on the empirical observations by Chan Gunn, a Canadian physician and early pioneer of dry needling.48,49 This technique is based on the hypothesis that myofascial pain is always the result of neuropathy or radiculopathy.48,49 This model is founded on the “Law of Denervation,” as written by Cannon & Rosenbluth.50 According to this law, the health and integrity of innervated structures is dependent upon the unhindered flow of nervous impulses providing a regulatory or trophic affect.48 When this free flow of impulses is inhibited in a series of efferent neurons, “an increased irritability to chemical agents develops in the isolated structure or structures, the effect being maximal in the part directly denervated.”50(p. 185) That being said, Gunn noted that treatment points are always located close to the muscle motor points, or musculotendinous junctions, and the distribution is myotomal in nature, and thus, MTrPs do not play a vital role.48
The second model is called the spinal segmental sensitization model, and was developed by Andrew Fischer.51 He proposed that paraspinal muscle spasm is frequently responsible for compression of a nerve root, narrowing of a foraminal space, and a sprain of the supraspinous ligament.48 Hence, Fischer51 contends that the most effective treatment for musculoskeletal pain includes preinjection blocks, dry and/or wet needling, infiltration (injection) of tender spots and trigger points, somatic blocks, spray and stretch methods, and relaxation exercises. Fischer51 contends that use of the needle and infiltration of a local anesthetic is optimal for achieving long term relief of muscle pain and normalization of tenderness.51 Several key differences distinguish the spinal segmental sensitization model and the radicular model. These differences include, but are not limited to: 1) the use of injection needles by Fischer vs. acupuncture needles by Gunn; 2) Fischer's recognition of the MTrPs vs. Gunn who minimizes their importance; and 3) the integration of new research into Fischer's model vs. Gunn's which has not been developed much beyond its inception in 1973.48
The last, and most frequently utilized model for dry needling, is the trigger point model. This model was birthed from the research and observations of Janet Travell (1901‐1997) (Figure 2).34 Clinicians who subscribe to this model specifically target myofascial trigger points in hopes of relieving the sensory, motor and autonomic abnormalities that can occur secondary to myofascial trigger points. The trigger point model advocates that inactivation of the MTrPs via dry needling is the fastest and most effective means to reduce pain, as compared to other conventional interventions. While the actual mechanism of dry needling continues to be debated, the localized twitch response commonly evoked with dry needling may interrupt motor end‐plate noise, thus inducing an analgesic effect.52 This localized twitch response, when coupled with stretching, helps to relax the actin‐myosin bonds restricting the tight bands.53 Additionally, dry needling of the MTrPs will help to normalize muscle tone and the neurological interface, and improve the flow of acetylcholinesterase, thus correcting bradykinin, calcitonin gene‐related peptide, and substance P levels in the affected muscle.52-54 Advocates of the trigger point model believe that treatment of the MTrPs should only be one facet of a patients plan of care: stretching, joint mobilizations, neuromuscular reeducation, strengthening, and other related interventions should still be employed. It is this model of trigger point dry needling that the remainder of this commentary will address.
Figure 2.
Janett Travell, MD (1901‐1997). Compliments of Bachrach Studios. Used with permission from Virginia Street, daughter of Dr. Janet Travell
Trigger Point Dry Needling Technique
Proper DN technique begins with identifying the appropriate patients, and eliminating those in whom it may lead to adverse affects. DN should not be administered in the following patient scenarios: 1) a patient with needle phobia; 2) an unwilling patient; 3) a patient who is unable or unwilling to give consent; 4) a patient with a history of abnormal reaction to needling or injection; 5) in a medical emergency; 6) a patient who is on anticoagulant therapy, or who has thrombocytopenia; and 7) into an area or limb with lymphoedema.2,55,56 Relative contraindications include, but are not limited to, abnormal bleeding tendencies, a severely compromised immune system (eg. cancer, HIV, hepatitis, etc.), vascular disease, diabetes mellitus, pregnancy, frail patients, epilepsy, allergy to metals or latex, children, and individuals taking certain prescriptive medications (eg. significant mood altering medication, blood thinning agents, etc.). Additional relative contraindications include an altered psychological status, anatomic considerations (extreme caution must be taken over the pleura and lungs, blood vessels, nerves, organs, joints, prosthetic implants, implantable electrical devices, etc.), needling near a surgical site within four months of the surgical procedure, and a decreased ability to tolerate the procedure.2,55,56
An ideal candidate for DN should possess the following qualities: 1) a physical therapy diagnosis that will reasonably improve with DN; 2) the ability to understand what is being done and why; 3) the ability to effectively communicate his or her own response to treatment; 4) the ability to lie still during treatment; and 5) the ability to provide informed consent according to clinical guidelines.2,57 Once indications, contraindications and precautions have been examined, it is vital to obtain signed informed consent from the patient. This comes after discussion regarding the indication and aim of the treatment, a brief explanation of how the intervention works, and an open discussion concerning the risks involved.2,57
Treatment is commenced with the patient positioned in a relaxed posture suitable to expose the muscles being treated. Positions may include supine, prone, or sidelying, and pillows and bolsters may be utilized to help with patient positioning. Completion of DN in a seated position is not recommended given the risk of syncope. Ideally, the practitioner would be able to view the patient's face, so as to receive regular feedback during the intervention, though treating the patient in prone is acceptable. According to the work of several authors, routine disinfection of visibly clean skin before needling is not necessary.26,55,56,58,59 However, current standards of care in the United States recommend preparing the skin with 70% isopropyl alcohol prior to needling, as well the practitioner utilizing gloves during the intervention.1 The trigger point is then identified using palpation methods previously described. A pincer grip technique is employed to gently lift the skin. Additionally, flat palpation can be utilized to take up the slack of the skin. A high quality, sterile, disposable, solid filament needle is inserted directly through the skin, or using a guide tube that is then removed (Figure 3).2 The depth of needle penetration must be sufficient to engage the MTrP. Once the needle has penetrated the skin and is inserted into the muscle, techniques vary: the practitioner may utilize a slow, steady, lancing or pistoning motion in and out of the muscle (termed dynamic needling), he or she may leave the needle in situ (termed static needling), or the needle may be rotated several revolutions in order to draw the fascia or soft tissues.2 Baldry26 recommends leaving the needle in situ for 30‐60 seconds for “average responders,” or up to 2‐3 minutes in “weak responders.” While there is no consensus as to which technique is ideal, it is the opinion of the author that dynamic needle is superior to static needling (without intramuscular electrical stimulation) in most cases.
Figure 3.
Needle being inserted into the upper trapezius
If a static technique is utilized, it can be augmented by intramuscular electrical stimulation (IES) as well.26,49,56 Since electrotherapy has been shown to elicit muscle relaxation and increase local blood circulation, utilizing the modality in conjunction with dry needling can be used to further decrease muscle tone and improve motor recruitment.60 While there is very little research to support specific parameters, typically IES (often with an asymmetric biphasic square waveform) is utilized at the motor level of a muscle with the frequency set at a level sufficient to elicit repeated muscular contractions; this typically corresponds to between 2 and 4 Hz with as high intensity as tolerable.48 If the goal is to reduce neuropathic pain, frequencies between 80 and 100Hz are recommended, which can enhance the release of gamma‐aminobutyric acid, galanin, and dynophin, which will ultimately function by modulating the pain response.61 While all standard precautions and contraindications should be followed for DN, unique contraindications must be followed when electrical stimulation is delivered via dry needling. Contraindications include, but are not limited to: 1) a patient who is not comfortable or phobic to electrical stimulation or needling;2 2) it is not recommended to connect needles across the spinal column; 56 3) patients with implanted electrical devices; 56 4) in the vicinity of the mid or low back, pelvis or abdomen during pregnancy;2 5) in the vicinity of the carotid sinus or near the recurrent laryngeal nerve;40 and 6) in an area of sensory denervation.56 The aforementioned contraindications are synonymous with those for all electrical stimulation, and are not exclusive when used with dry needling.
Whichever techniques are employed, the intensity of the treatment must suit the tolerance of the patient, and their pathologic presentation. After a needle is withdrawn, the tissue should be compressed for 5‐10 seconds, or for 30‐60 seconds using a cotton swab if there is any bleeding; this will help to ensure adequate hemostasis.2
It is important to note that gauge and length of needles vary (Figure 4). A 0.30 × 50mm needle is appropriate for most muscles. The 0.30 corresponds to the gauge, or diameter, of the needle, and the 50 corresponds to length. .30 × 60mm is often utilized for the quadratus lumborum, and a .30 × 75mm for the psoas or for other muscles of similar depth. Smaller gauge needles are utilized for smaller tissues, including a .20 × 25mm for the forearm, .14 × 25mm for the face/head, and .12 × 25mm for the hands or feet. Please note that these are simply guidelines, and not standards; choosing the gauge and length of needles should be left to the discretion of the treating practitioner.
Figure 4.
Various lengths of dry needles within guide tubes
The effectiveness of DN is largely dependent upon the skill of the therapist, and his or her own ability to accurately palpate MTrPs. Not only is superficial palpation key, but also the ability to picture the trigger point in 3‐dimensions. This kinesthetic awareness helps assist in better localization of needling, and improved outcomes. Several authors have noted that a trained clinician should be able to perceive the end of the needle, the pathway or trajectory the needle takes inside the patient's body and be able to decipher between skin, subcutaneous tissue, and the anterior and posterior lamina of the aponeurosis of the rectus abdominis, for example.44,62,63
Practitioners often inquire as to how many muscles should be treated in one session. This is highly dependent on the patients’ history, location of pain, reservations with needling, and chronicity of their symptoms. For example, if a patient consents to treatment, but displays obvious apprehension, then treating 1‐2 muscles in the first session may be appropriate. After the patients’ reservations begin to decrease, then treating 3‐4 muscles, or more, may be appropriate. As young practitioners will learn, every muscle will respond uniquely different to DN. For example, the medial gastrocnemius often becomes tonic and dysfunctional in young athletes. Obtaining more than one or two twitch responses of this muscle will undoubtedly cause excessive post needling soreness; hence, this muscle is often the only muscle needled in a session. Other muscles, such as muscles of the rotator cuff or the upper trapezius tend to produce less post‐needling soreness, and more can be treated in the same session. Rarely will the author needle more than 4‐5 muscles in a given session.
Occasionally the practitioner will be unable to elicit a twitch response, commonly occurring when treating deep musculature (eg. gluteus minimus). Often with these deeper muscles, the patient will still receive a therapeutic effect, without a twitch response, if the needles are left in place for 5‐10 minutes, with or without IES. If a twitch response is not elicited in a more superficial muscle, it is advised that the practitioner utilize more dynamic needling techniques, including twirling of the needle, or repeated lancing motions. If the twitch is still not elicited, then the needle should be withdrawn and second attempt made. It is opinion of the author that if the twitch is not elicited after the second needle is inserted, the practitioner may not have correctly palpated the trigger point, the needle did not engage the palpated trigger point, or the trigger point will require IES in order to engage it.
Another frequent question relates to how many trigger point sessions should be utilized with patients. In order to answer this, it is imperative that the practitioner sees dry needling within the larger picture of an entire plan of care. Dry needling is often followed by stretching the affected muscle groups, coupled with neuromuscular re‐education of new movement patterns. In subsequent visits (not the same day the DN was performed), strengthening of the once inhibited or painful muscle groups can then be initiated. Therefore, the dry needling itself should be seen as a “springboard” in order to facilitate a rapid decrease in pain, thus facilitating improvements in mobility and function. It is this author's opinion that most sub‐acute conditions will improve after two to three needling sessions, with chronic MTrP's requiring five to six sessions. Rarely will the author needle an individual fitting these parameters for more than six sessions, although the remainder of the rehabilitation program may still be in progress.
Risk Management
Despite the proven efficacy of DN when treating myofascial pain, utilization of the procedure must be balanced by the inherent risk that comes with employing the technique; this is especially true given the fact that the skin is violated. While a paucity of research currently exists describing the risk of infection with dry needling, extensive data has been reported on infections and acupuncture. Considering that both techniques employ dermal penetration with a solid filament to varying depths within the body for therapeutic indications, it appears reasonable to correlate the data.2 However, readers are encouraged to remember the key philosophical differences between acupuncture and DN, noting that many of the locations that a Traditional Chinese Acupuncturist would needle, a practitioner utilizing DN would not.
Vulfsons et al23 summarized several adverse effects associated with dry needling, including post‐needling soreness, hemorrhages at the needling site, syncopal responses, and acute cervical epidural hematoma. On a catastrophic level, McCutcheon & Yelland64 recently documented several cases of pneumothorax secondary to acupuncture or dry needling. Despite the relatively low incidence reported (<1/10,000), the authors did note over 100 cases of pneumothorax, with four subsequent deaths; all were secondary to acupuncture treatment.64 There is also a risk of damage to the central nervous system as well. In a review of the literature, Peuker and Gronemeyer65 noted ten cases of injuries to the spinal cord or spinal nerve roots. In four cases, fragmented needles were responsible for the lesions, whereas six were caused from direct injury. The authors also describe several cases of arachnoiditis and subarachnoid hemorrhage as well.65 It is vital to note, however, that these cases were secondary to deep needling of BL11 to B20 (inner line of the bladder meridian), which are not typical locations for dry needling.65
There have been rare and isolated cases of serious bacterial skin infection associated with acupuncture, which have even led to death.66 Walsh67 reviewed several outbreaks of Hepatitis B in England, Wales, Germany, Israel and the United States between 1976 and 1997; he notes that nearly all infections could be attributed to negligence on the behalf of the administering practitioner.67 The author goes on to note that as of 2001, there have been no cases reported in the UK of human immunodeficiency virus (HIV) transmission through acupuncture; the same is true with regards to Hepatitis C, and Variant Creutzfeldt‐Jacob Disease.67
The incidence of infectious diseases with acupuncture has decreased dramatically since the 1980's.67 Greater emphasis has been placed on the utilization of single use, disposable acupuncture needles, which is now the standard of care. The risk of infection continues to decrease with the optimization of sharps containers, latex gloves, and universal precautions, including regular hand washing.68 Two reviews investigating the risk of infections associated with acupuncture noted that the risk is “extremely low.”69,70 Furthermore, Vulfsons et al23 notes that “dry needling provided by trained physicians or physical therapists can be considered a safe treatment. Serious adverse effects of dry needling are very rare.”23(p. 411)
In order to place risk in perspective, one could compare the aforementioned data to that describing the risk of taking nonsteroidal anti‐inflammatory drugs (NSAIDs). These drugs range from over the counter aspirin, ibuprofen or naproxen, to a whole host of prescription brands (Indocin®, Daypro®, Celebrex®, etc). Rarely do patients think twice about taking one of these medications. However, data suggests that patients are significantly more likely to have a serious adverse effect, or even die, after taking one of these medications, as compared to receiving trigger point dry needling.71 Another perspective can compare the risk of DN to driving to a physical therapy clinic. According to the Department of Transportation, the annual risk of dying in a transportation‐related accident is 1 in 6,800.72 This is 32% higher than the risk of catastrophic injuries noted by McCutcheon & Yelland64 associated with acupuncture or DN. Therefore, while there is a risk to any physical therapy intervention, the risk associated with DN is minute in the hands of a skilled practitioner.73
Trigger Point Dry Needling Outcomes
Considering the invasive nature of DN, it is very difficult to execute a double blinded, randomized, controlled clinical trial.44,62,63 Nonetheless, there have been several case reports, review articles, and research studies that support the benefit of DN. A 2005 Cochrane review investigated the effects of DN in the treatment of myofascial pain syndrome in the lumbar spine.74 While the authors noted that there is a lack of high‐quality literature related to DN, they also reported that “dry‐needling appears to be a useful adjunct to other therapies for chronic low back pain.”74(p. 961)
Several systematic reviews have also been published related to needling therapies for the management of myofascial trigger point pain. Cummings & White4 reviewed 23 randomized controlled clinical trials investigating needling of myofascial trigger points with the use of various injectable medications (known as “wet needling”). They noted that nearly all the studies revealed that the beneficial effect of the intervention was independent of the injectable substance.4 They concluded by stating that marked improvement was noted in all groups under investigation in which trigger points were directly needled. However, the hypothesis that this has any efficacy beyond placebo is “neither supported nor refuted by the evidence from clinical trials.”4(p. 986)
A second systematic review was performed by Teasdale10 and focused on DN in athletes. The study examined two systematic reviews, one meta‐analysis, one case summary, four randomized clinical trials, and two clinical trials all published after 2000. Teasdale10 investigated four comparisons: 1) DN vs. placebo or no treatment; 2) DN vs. standard care; 3) DN vs. standard acupuncture; and 4) DN vs. wet needling. She concluded that DN in athletes was more beneficial than sham acupuncture or no treatment, and that no safety problems were reported.10 She also noted no statistically significant benefit with dry needling compared to standard care. However, when comparing dry needling to standard acupuncture, Teasdale10 found a statistically significant benefit to dry needling, and noted that dry needling has been shown to reduce pain, increase quality of life, and increase range of motion beyond that produced with standard acupuncture.10 She concluded, “For athletes, this treatment has the ability to have a positive impact on pain, performance, and quality of life,” especially if used in conjunction with stretching, exercise therapy, and other non‐invasive treatments.10(p.7)
A recent meta‐analyses conducted by Tough et al75 reviewed seven randomized clinical trials including DN and acupuncture for the management of MTrPs.75 The authors noted that only one study suggested that DN was effective in reducing pain, when compared with no intervention. Four studies revealed that DN is superior to non‐penetrating interventions aimed at decreasing myofascial trigger points.75 Lastly, two studies provided contradictory results when comparing outcomes with dry needling placed into the trigger point itself, versus another location in the muscle.75 However, Tough et al75 reported significant methodological flaws with the literature under investigation. The authors noted that the source of patients pain was not controlled in any of the studies, that sample sizes were small (thus increasing the risk of making a Type II error), and that there was poor consistency between specific parameters of intervention (eg. depth of needle penetration, length of time needles were left in the skin, the number of needles being utilized, etc.).75 The authors concluded: “Whilst the result of the meta‐analysis of needling compared with placebo controls does not attain statistical significance, the overall direction could be compatible with a treatment effect of dry needling on myofascial trigger point pain.”75(p. 3)
Most recently, Rainey76 described the case of a 30‐year female on active military duty who injured her low back while weight lifting. 76 She was diagnosed with a lumbar segmental instability along with right hip stability dysfunction. 76 She was treated for two sessions with DN and IES to the gluteus maximus and medius, as well as the bilateral L3 and L5 multifidus muscles.76 After two sessions, the patient reported no existing pain or disability on the Numerical Pain Rating Scale or the Oswestry Disability Questionnaire, and a large improvement on the Global Rating of Change. 76
Several case series have also been documented demonstrating the benefits of DN. Fernandez‐Carnero et al77 found that the application of dry needling into active MTrPs in the masseter muscle of 12 females significantly increased their pressure pain threshold, as well as jaw active range of motion.77 Edwards78 conducted a pragmatic, single blind, randomized, controlled trial of 40 patients in order to assess if dry needling coupled with active stretching was more effective than stretching alone at deactivating trigger points and reducing myofascial pain. They concluded that dry needling followed by active stretching is more effective than stretching alone in reducing the sensitivity to pressure of MTrPs.78 They also noted that stretching without prior deactivation of the MTrP may actually increase pain and MTrP sensitivity.78
In summary, dry needling research is still in its infancy. However, there is mounting evidence that the procedure can be effective at decreasing pain, improving range of motion, reducing the sensitivity of MTrPs, and ultimately improving quality of life.
Scope of Practice & Reimbursement
As of March 2014, State Boards regulating the practice of physical therapy in 32 jurisdictions have determined that DN does indeed fall within a physical therapists scope of practice. This view is shared by Canada, the United Kingdom, Ireland, the Netherlands, Norway, Switzerland, Belgium, Spain, Chile, South Africa, Australia, and New Zealand, among other nations.79 Nine states have prohibited the practice by physical therapists.1 Arizona and Pennsylvania are unique, as their state boards are legally prohibited from issuing an interpretive statement about their respective practice acts.79 In many states, the jurisdiction has made no definitive statements on the issue.
Several organizations have taken a stance on the sensitive issue of dry needling and physical therapy practice. The American Academy of Orthopaedic and Manual Physical Therapists (AAOMPT) released a position statement in October of 2009 stating:
Physical therapists are well trained to utilize dry needling in conjunction with manual physical therapy interventions. Research supports that dry needling improves pain control, reduces muscle tension, normalizes biochemical and electrical dysfunction of motor endplates, and facilitates an accelerated return to active rehabilitation.3(p. 1)
The APTA shares in this opinion, and supports the practice of trigger point dry needling by licensed physical therapists.2 In fact, the 3rd Edition of the Guide to Physical Therapy Practice includes dry needling as part of manual therapy techniques employed by physical therapists in order to “prevent, minimize, or eliminate impairments of body functions and structures, activity limitations, and participation restrictions.”80
Not all organizations share this view, however. The American Association of Acupuncture & Oriental Medicine stated that dry needling is, by definition, an acupuncture technique.81 This implies that the technique is outside of a physical therapists scope of practice. The statement also notes: “Trigger Point Dry Needling and Intramuscular Manual Therapy are re‐titlings and re‐packaging's of a subset of the acupuncture techniques described in the field of Acupuncture as “ashi point needling.”81(p. 1) The organization goes on to state that “no standards of education have been validly determined to assure that Physical Therapists (PT) using DN are providing the public with a safe and effective product,” again implying that physical therapists should not perform the intervention.81(p. 1)
In order to understand the complex issues related to a physical therapists’ scope of practice, and the “turf‐battles” that cloud the issue, it is imperative to have a robust understanding of the many issues surrounding DN. These issues include, but are not limited to:
Understanding what is included in entry‐level physical therapy education
Identifying the similarities and differences between trigger point dry needling and TCA
Defining clinical competence
Exploring the dynamics related to reimbursement practices
Is DN an Entry‐level Skill?
In the United States (US), DN is not commonly included in the physical therapy entry‐level curriculum.44 As of 2011, Georgia State College is the only physical therapy program in the US that has DN included in their entry‐level coursework.44 However, Mercer University and the University of St. Augustine for Health Sciences have both made significant strides towards adding intramuscular manual therapy to the curricula of their entry‐level educational programs.1 Therefore, given the paucity of entry‐level programs that include DN in their curricula, DN is not typically considered an entry‐level skill; hence, DN should not be utilized without appropriate entry‐level or post‐graduate training.
The lack of training at the entry‐level will likely continue, given The Federation of State Boards of Physical Therapy's (FSBPT) recently released report.1 The report notes:
…it appears that there is a historical basis, available education and training as well as an educational foundation in the CAPTE criteria, and supportive scientific evidence for including intramuscular manual therapy in the scope of practice of physical therapists. The education, training and assessment within the profession of physical therapy include the knowledge base and skill set required to perform the tasks and skills with sound judgment. It is also clear; however, that intramuscular manual therapy is not an entry‐level skill and should require additional training.1(p. 15)
Is Trigger Point Dry Needling the Same as Acupuncture?
Within practitioners or disciplines, a particular group does not own, or have the rights to, a particular technique. Such restrictions, especially in medicine, would ultimately be disadvantageous to patients. For example, chiropractors do not possess an exclusive domain over the skill of manipulation; physical therapists and osteopathic physicians commonly utilize the skill as well, since they too have the prerequisite training necessary to effectively use the skill. Neither naturopathic physicians nor homeopathic physicians “own” herbal remedies, but they instead use them autonomously for the purpose of improving patient outcomes. Both a carpenter and a surgeon utilize a hammer; should one own the tool to the exclusion of the other? The vast difference between the two professionals relates to their underlying philosophy, thought processes, and decision making; the only thing they really have in common is the tool.73 The same argument applies to acupuncture versus dry needling: Traditional Chinese Acupuncturists and physical therapists utilizing DN use the same needles. However, just like the surgeon has a completely different thought process compared to the carpenter, despite having the same tool, a physical therapist diagnoses and treats pain and dysfunction completely differently than an acupuncturist. Therefore, to prevent confusion on the part of the patient, it is imperative that physical therapists clearly communicate they are not performing acupuncture. This is often done through utilizing consent forms, as well as during discussions with the patient.
Defining Clinical Competence
Even though DN is not synonymous with acupuncture, acupuncturists often argue that physical therapists lack sufficient training in order to safely perform the technique. The American Association of Acupuncture & Oriental Medicine reports that acupuncturists must complete 3000 hours of education prior to being licensed; they contend that anything less is a risk to the general public. Nonetheless, this argument is fundamentally flawed. The hours acupuncturists gain are not exclusive to the performance of acupuncture. Significant time must be spent on anatomy, physiology, diagnosis, as well as studying Eastern and Western theory long before a student ever inserts a needle into a patient.73 A similar, rigorous preparation is required of entry‐level physical therapists. Entry‐level physical therapist education includes anatomy, histology, physiology, biomechanics, kinesiology, neuroscience, pharmacology, pathology, clinical sciences, clinical interventions, medical screening and differential diagnosis. Much of the basic anatomical, physiological, and biomechanical knowledge that dry needling uses is taught as part of the core or entry‐level physical therapist education; the specific dry needling skills are supplemental to that knowledge.82
Currently there is no profession‐wide standard that defines initial competence before being allowed to dry needle. To date, each state has been forced to define its own requirements. States have taken vastly different approaches to this. Some states have treated dry needling the same as any other tool that a therapist might use, and therefore require professionals to perform only what they are trained and competent to do. Other states require that therapists have a predetermined number of years of experience before utilizing the technique. Still, others require a specific number of continuing education hours in order to be deemed “competent.” Whatever the requirement, the physical therapist is held to the practice act and laws in their respective state, and thus he or she must comply.
Reimbursement Concerns
Currently there is no CPT code dedicated to dry needling. It appears as though CPT 20552 and 20553 (both for trigger point injection) would be appropriate. However, according to Medicare guidelines, this code requires that an injectable substance be administered. Since dry needling is not acupuncture, CPT codes 97780‐97781 (acupuncture codes) are not appropriate either. The APTA's 2014 Official Statement titled, “Billing of Dry Needling by Physical Therapists” recommends that practitioners check the payer's coverage policy to determine if the policy specifies which code should be used to report the service.”83
It is clear that the issue of reimbursement for dry needling is unresolved, and varies widely from state to state. It is also clear that third‐party payer policies are rapidly changing with regards to DN. As such, therapists are encouraged to review these policies on a regular basis in order to accurately bill for the technique.
CONCLUSION
Trigger point dry needling is a technique rooted in medical science, and can be utilized to treat various musculoskeletal pathologies. It has been deemed safe, often effective, and consistent with the general scope of practice for a physical therapist. DN is not synonymous with acupuncture, which is a discipline and licensed profession. The technique of DN should be available to any profession provided they prove sufficient knowledge and training. As physical therapy moves forward as a profession, therapists must be able to engage in professional conversations with both colleagues and adversaries, in order to elevate the standard of care, in an ongoing attempt to improve patient outcomes.
REFERENCES
- 1.Federation of State Boards of Physical Therapy. Dry Needling Resource Paper (Intramuscular Manual Therapy): 4th Ed. Alexandria, VA: Federation of State Boards of Physical Therapy; 2013. [Google Scholar]
- 2.American Physical Therapy Association. Physical Therapists & the Performance of Dry Needling: An Educational Resource Paper. Alexandria, VA: American Physical Therapy Association; 2012. [Google Scholar]
- 3.American Academy of Orthopaedic Manual Physical Therapists. Position Statement: Dry Needling. Baton Rouge, LA: American Academy of Orthopaedic Manual Physical Therapists; 2009. [Google Scholar]
- 4.Cummings TM White AR. Needling therapies in the management of myofascial trigger point pain: A systematic review. Arch Phys Med Rehabil. 2001;82:986‐992. [DOI] [PubMed] [Google Scholar]
- 5.Gerwin RD. A review of myofascial pain and fibromyalgia – factors that promote their persistence. Acupunct in Med. 2005;23:121‐134. [DOI] [PubMed] [Google Scholar]
- 6.Simons DG. Diagnostic criteria of myofascial pain caused by trigger points. J Musculoskel Pain. 1999;7:111‐120. [Google Scholar]
- 7.Yap E. Myofascial pain – an overview. Ann Academy of Med. 2007;36:43‐48. [PubMed] [Google Scholar]
- 8.Bron C Dommerholt JD. Etiology of myofascial trigger points. Curr Pain Headache Rep. 2012;16:439‐444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah JP Danoff JV Desai MJ, et al. Biochemicals associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points. Arch Phys Med Rehabil. 2008;89:16‐23. [DOI] [PubMed] [Google Scholar]
- 10.Teasdale T. Safety, effectiveness and impact of dry needling trigger points in athletes: Asystematic review. SIRC. Available online from http://old.sirc.ca/research_awards/documents/TTeasdale.pdf.
- 11.Mense S. The pathogenesis of muscle pain. Curr Pain Headache Rep. 2003;7:419‐425. [DOI] [PubMed] [Google Scholar]
- 12.Shah JP Danoff JV Desai MJ, et al. Biochemicals associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points. Arch Phys Med Rehabil. 2008;89:16‐23. [DOI] [PubMed] [Google Scholar]
- 13.Woolf CJ. Central sensitization: Implications for the diagnosis and treatment of pain. Pain. 2011; S2‐S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dommerholt J Bron C Franssen J. Myofascial trigger points: An evidence‐informed review. J Man Manip Ther. 2006;14:203‐221. [Google Scholar]
- 15.Schaible HG. Peripheral and central mechanisms of pain generation. HEP. 2006; 177:3‐28. [DOI] [PubMed] [Google Scholar]
- 16.Latremoliere A Woolf C. Central Sensitization: A generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vranken JH. Mechanisms and treatment of neuropathic pain. Central Nervous System Agents in Med Chem. 2009:9:71‐78. [DOI] [PubMed] [Google Scholar]
- 18.Kalichman K Vulfsons S. Dry needling in the management of musculoskeletal pain. J Am Board Fam Med. 2010;23:640‐646. [DOI] [PubMed] [Google Scholar]
- 19.Fishbain DA Goldberg M Meagher BR, et al. Male and female chronic pain patients categorized by DSM‐III psychiatric diagnostic criteria. Pain. 1986;26:181‐197. [DOI] [PubMed] [Google Scholar]
- 20.Fricton JR Kroening R Haley D, et al. Myofascial pain syndrome of the head and neck: a review of clinical characteristics of 164 patients. Oral Surg Oral Med Oral Pathol. 1985;60:615‐623. [DOI] [PubMed] [Google Scholar]
- 21.Skootsky SA Jaeger B Oye RK. Prevalence of myofascial pain in general internal medicine practice. West J Med. 1989;151:157–160. [PMC free article] [PubMed] [Google Scholar]
- 22.Chaiamnuay P Darmawan J Muirden KD, et al. Epidemiology of rheumatic disease in rural Thailand: a WHO‐ILAR COPCORD study. Community oriented programme for the control of rheumatic disease. J Rheumatol. 1998;25:1382‐1387. [PubMed] [Google Scholar]
- 23.Vulfsons S Ratmansky M Kalichman L. Trigger point needling: Techniques and outcome. Curr Pain Headache Rep. 2012;16:407‐412. [DOI] [PubMed] [Google Scholar]
- 24.Sikdar S Shah JP Gebreab T, et al. Novel applications of ultrasound technology to visualize and characterize myofascial trigger points and surrounding soft tissue. Arch Phys Med Rehabil. 2009;90:1829‐1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Q Bensamoun S Basford JR, et al. Identification and quantification of myofascial taut bands with magnetic resonance elastography. Arch Phys Med Rehabil. 2007;88:1658‐1661. [DOI] [PubMed] [Google Scholar]
- 26.Baldry PE. Acupuncture, Trigger Points and Musculoskeletal Pain. Edinburgh: Churchill Livingstone; 2005. [Google Scholar]
- 27.Lucas N Macaskill P Irwig L, et al. Reliability of physical examination for diagnosis of myofascial trigger points. A systematic review of the literature. Clin J Pain. 2009;25:80‐89. [DOI] [PubMed] [Google Scholar]
- 28.Gerwin RD Shannon S Hong CZ, et al. Interrater reliability in myofascial trigger point examination. Pain. 1997;69:65‐73 [DOI] [PubMed] [Google Scholar]
- 29.Wolfe F Simons DG Fricton J, et al. The fibromyalgia and myofascial pain syndromes: a preliminary study of tender points and trigger points in persons with fibromyalgia, myofascial pain syndrome and no disease. J Rheumatol. 1992;19:944‐951. [PubMed] [Google Scholar]
- 30.Nice DA Riddle DL Lamb RL, et al. Intertester reliability of judgments of the presence of trigger points in patients with low back pain. Arch Phys Med Rehabil. 1992;73:893–898. [PubMed] [Google Scholar]
- 31.Njoo KH Van der Does E. The occurrence and inter‐rater reliability of myofascial trigger points in the quadratus lumborum and gluteus medius: A prospective study in non‐specific low back pain patients and controls in general practice. Pain.1994; 58:317–323. [DOI] [PubMed] [Google Scholar]
- 32.Bron C Franssen J Wensing M, et al. Interrater reliability of palpation of myofascial trigger points in three shoulder muscles. J Man Manip Ther. 2007;15:203‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barbero M Bertolia P Cescon C, et al. Intera‐rater reliability of an experienced physiotherapist in locating myofascial trigger point in the upper trapezius muscle. J Man Manip Ther. 2012;20:171‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Travell G Simons DG. Myofascial Pain and Dysfunction: The Trigger Point Manual. Baltimore, MD: Williams & Wilkins; 1992. [Google Scholar]
- 35.Lewit K. The needle effect in the relief of myofascial pain. Pain. 1979;6:83–90. [DOI] [PubMed] [Google Scholar]
- 36.Kelly M. The treatment of fibrositis and allied disorders by local anaesthesia. Med J Aust. 1941;1:294‐298. [Google Scholar]
- 37.Vickers A Zollman C. ABC of complementary medicine: Acupuncture. BMJ. 1999; 319:973‐976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fung PCW. Probing the mystery of Chinese medicine meridian channels with special emphasis on the connective tissue interstitial fluid system, mechanotransduction, cells durotaxis and mast cell degranulation. Chin Med. 2009:4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Madsen MV Gotzsche PC Hrobjartsson A. Acupuncture treatment for pain: systematic review of randomized clinical trials with acupuncture, placebo acupuncture, and no acupuncture groups. BMJ. 2009;338:a3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baldry P. Management of Myofascial Trigger Point Pain. Acupuncture in Med. 2002;20:2‐10. [DOI] [PubMed] [Google Scholar]
- 41.Gunn CC. Acupuncture and the Peripheral Nervous System. A Radiculopathy Model. 1998. Churchill Livingstone. Institute for the Study and Treatment of Pain ‐ 2002.
- 42.“Daoism.” thefreedictionary.com/Daoism. Accessed May 12, 2014.
- 43.“Yin and Yang.” thefreedictionary.com/yin+and+yang. Accessed May 12, 2014.
- 44.Dommerholt J Mayoral del Moral O Grobli C. Trigger point dry needling. J Man Manip Ther. 2006;14:70‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carlsson C. Acupuncture mechanisms for clinically relevant long‐term effects – reconsideration and a hypothesis. Acupunct in Med. 2001;20:82‐99. [DOI] [PubMed] [Google Scholar]
- 46.Filshie J Cummings M. Western medical acupuncture. In Ernst E & White A (Eds.) Acupuncture: A Scientific Appraisal. Oxford: Butterworth Heinemann; 1999:31‐59. [Google Scholar]
- 47.Bradnam L. A proposed clinical reasoning model for western acupuncture. New Zealand J of Physio. 2003;31:4‐45. [Google Scholar]
- 48.Dommerholt J. Dry needling in orthopedic physical therapy practice. Orthop Practice. 2004;16:15‐20. [Google Scholar]
- 49.Gunn CC. The Gunn Approach to the Treatment of Chronic Pain. 2nd ed. New York, NY: Churchill Livingstone; 1997. [Google Scholar]
- 50.Cannon WB Rosenblueth A. The Supersensitivity of Denervated Structures: A Law of Denervation. New York, NY: MacMillan; 1949. [Google Scholar]
- 51.Fischer AA. Treatment of myofascial pain. J Musculoskeletal Pain. 1999;7:131‐142. [Google Scholar]
- 52.Kietrys DM Palombaro KM Azzaretto E, et al. Effectiveness of dry needling for upper‐quarter myofascial pain: A systematic review and meta‐analysis. J Orthop Sports Phys Ther. 2013;43:620‐634. [DOI] [PubMed] [Google Scholar]
- 53.Chu J. Does EMG (dry needling) reduce myofascial pain symptoms due to cervical nerve root irritation? Electromyogr Clin Neurophysiol. 1997;37:259‐272. [PubMed] [Google Scholar]
- 54.Kash H Qerama E Kongsted A, et al. Clinical assessment of prognostic factors for long‐term pain and handicap after whiplash injury: A 1‐year prospective study. Eur J Neurol. 2008;15:1222‐1230. [DOI] [PubMed] [Google Scholar]
- 55.Australian Society of Acupuncture Physiotherapists Inc. Guidelines for safe acupuncture and dry needling practice. Lismore, NSW: Australian Society of Acupuncture Physiotherapists; 2007. [Google Scholar]
- 56.White A Cummings M Filshie J. An Introduction to Western Medical Acupuncture. Edinburgh: Churchill Livingstone; 2008. [Google Scholar]
- 57.College of Physical Therapists of Alberta. Dry needling competency profile for the physical therapist. Alberta, Canada: College of Physical Therapists of Alberta; 2007. [Google Scholar]
- 58.Hoffman P. Skin disinfection and acupuncture. Acupunct Med. 2001;19:112‐116. [DOI] [PubMed] [Google Scholar]
- 59.Hutin Y Hauri A Chiarello L, et al. Best infection control practices for intradermal, subcutaneous, and intramuscular needle injections. Bull World Health Org. 2003;81:491‐500. [PMC free article] [PubMed] [Google Scholar]
- 60.Cameron MH. Physical Agents in Rehabilitation: From Research to Practice. 3rd ed. St. Louis, MO: Elsevier. Saunders; 2008. [Google Scholar]
- 61.Lundeberg T Lund I. Is there a role for acupuncture in endometriosis pain, or ‘endometrialgia.” Acupunct Med. 2008;26:94‐110. [DOI] [PubMed] [Google Scholar]
- 62.Dincer F Linde K. Sham interventions in randomized clinical trials of acupuncture: A review. Complement Ther Med. 2003;11:235‐242. [DOI] [PubMed] [Google Scholar]
- 63.White P Lewith G Hopwood V, et al. The placebo needle: Is it a valid and convincing placebo for use in acupuncture trialsϿ. A randomised, single‐blind, cross‐over pilot trial. Pain. 2003;106:401‐409. [DOI] [PubMed] [Google Scholar]
- 64.McCutcheon LJ Yelland M. Iatrogenic pneumothorax: safety concerns when using acupuncture or dry needling in the thoracic region. Phys Ther Rev. 2011;16:126‐132. [Google Scholar]
- 65.Peuker E Gronemeyer D. Rare but serious complications of acupuncture: Traumatic lesions. Acupunct in Medicine. 2001;19:103‐108. [DOI] [PubMed] [Google Scholar]
- 66.Pierik MG. Fatal Staphylococcal septicemia following acupuncture: Report of two cases. Rhode Isl Med J. 1982;65:251‐253. [PubMed] [Google Scholar]
- 67.Walsh B. Control of infection in acupuncture. Acupunct in Med. 2001;19:109‐111. [DOI] [PubMed] [Google Scholar]
- 68.British Acupuncture Council. The British Acupuncture Council Code of Safe Practice. London, England: British Acupuncture Council; 2010. [Google Scholar]
- 69.MacPherson H Thomas K Walters S, et al. The York acupuncture safety study: Prospective survey of 34 000 treatments by traditional acupuncturists. BMJ. 2001; 323:486‐487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.White A Hayhoe S Hart A, et al. Adverse events following acupuncture: Prospective survey of 32 000 consultations with doctors and physiotherapists. BMJ. 2001;323:485‐486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singh G. Recent considerations in nonsteroidal anti‐inflammatory drug gastropathy. Am J Med. 1998;105:31S‐38S. [DOI] [PubMed] [Google Scholar]
- 72.Savage I. Comparing the fatality risks in United States transportation across modes and over time. Res in Transport Econ. 2013;43:9‐22. [Google Scholar]
- 73.Dommerhold J. The dry needling issue. Qi‐Unity Report, AAAOM. July 2008. [Google Scholar]
- 74.Furlan AD van Tulder M Cherkin D, et al. Acupuncture and dry‐needling for low back pain: An updated systematic review within the framework of the Cochrane Collaboration. Spine. 2005;30:944‐963. [DOI] [PubMed] [Google Scholar]
- 75.Tough EA White AR Cummings TM, et al. Acupuncture and dry needling in the management of myofascial trigger point pain: a systematic review and meta‐analysis of randomized controlled trials. Eur J Pain. 2009;13:3–10. [DOI] [PubMed] [Google Scholar]
- 76.Rainey CE. The use of trigger point dry needling and intramuscular electrical stimulation for a subject with chronic low back pain: A case report. IJSPT. 2013; 8:145‐161. [PMC free article] [PubMed] [Google Scholar]
- 77.Fernandez‐Carnero J LaTouche R Ortega‐Santiago R, et al. Short‐term effects of dry needling of active myofascial trigger points in the masseter muscle in patients with temporomandibular disorders. J Orofacial Pain. 2010;24:106‐112. [PubMed] [Google Scholar]
- 78.Edwards J Knowles N. Superficial dry needling and active stretching in the treatment of myofascial pain – A randomised controlled trial. Acupunct in Med. 2003;21:80‐86. [DOI] [PubMed] [Google Scholar]
- 79.FitzGibbon S. Should dry needling for myofascial pain be within the scope of practice for physical therapists? Ortho Practice. 2011;23:212‐218. [Google Scholar]
- 80.American Physical Therapy Association. Guide to Physical Therapist Practice (3rd ed). Alexandria, VA: American Physical Therapy Association; 2014. [Google Scholar]
- 81.American Association of Acupuncture & Oriental Medicine. American Association of Acupuncture and Oriental Medicine (AAAOM) Position Statement on Trigger Point Dry Needling (TDN) and Intramuscular Manual Therapy (IMT). Roseville, MN: American Association of Acupuncture & Oriental Medicine; 2011.
- 82.Edelman, George Edelman. On Behalf of Delaware Physical Therapy Association Letter to Grant, Jerry. Received 7 May 2014.
- 83.American Physical Therapy Association. Billing of Dry Needling by Physical Therapists. Official Statement. APTA Public Policy, Practice, and Professional Affairs Unit. Alexandria, VA: American Physical Therapy Association; 2014.
- 84.Borg‐Stein J Simons DG. Focused review: Myofascial pain. Arch Phys Med Rehabil. 2002;83:S40‐S47. [DOI] [PubMed] [Google Scholar]
- 85.Hong CZ Simons DG. Pathophysiologic and electrophysiologic mechanisms of myofascial trigger points. Arch Phys Med Rehabil. 1998;79:863‐872. [DOI] [PubMed] [Google Scholar]
- 86.Friction JR. Myofascial pain. Baillieres Clin Rheumatol. 1994;8:857‐880. [DOI] [PubMed] [Google Scholar]
- 87.Gerwin RD. The management of myofascial pain syndrome. J Musculoskel Pain. 1993;1:83‐94. [Google Scholar]
- 88.Letchuman R Gay RE Shelerud RA, et al. Are tender points associated with cervical radiculopathy? Arch Phys Med Rehabil. 2005;86:1333‐1337. [DOI] [PubMed] [Google Scholar]
- 89.Hsueh TC Yu S Kuan TS, et al. Association of active myofascial trigger points and cervical disc lesions. J Formos Med Assoc. 1998;97:174‐180. [PubMed] [Google Scholar]
- 90.Dimitriadis Z Kapreli E Strimpakos N, et al. Hypocapnia in patients with chronic neck pain: association with pain, muscle function, and psychologic states. Am J Phys Med Rehabil. 2013;92:746‐754. [DOI] [PubMed] [Google Scholar]
- 91.McLaughlin L Goldsmith CH Coleman K. Breathing evaluation and retraining as an adjunct to manual therapy. Man Ther. 2011;16:51‐62. [DOI] [PubMed] [Google Scholar]