Abstract
Introduction:
Mycosis fungoides (MF) is the most common cutaneous T-cell lymphomas. Despite extensive studies, etiopathogenesis of MF is unknown. Environmental, infectious and genetic factors have been proposed as potential risk factors of MF. Herpes virus family members, especially Epstein-Barr virus (EBV), have been among the viral factors of interest in recent years. The aim of this study was to investigate the possible association of EBV infection with MF.
Materials and Methods:
This case-control study was performed on skin biopsy samples of 57 MF patients referred to Pathology Department of Mashhad Emam Reza Hospital from 2000 to 2011 and also on 57 melanocytic nevus samples matched with patients for age and sex. The presence of EBV in samples was evaluated by polymerase chain reaction. Statistical analysis of the data was conducted with the Statistical Package for the Social Sciences version 11.5 (SPSS Inc., Chicago, IL, USA).
Results:
In this study, out of 57 MF samples, there were 34 male and 23 female patients, with male:female ratio of 1.04. Mean patient age was 51.4 years. There were 22 and 4 positive cases of EBV in the case and control groups, respectively. Chi-square statistical test showed that EBV was significantly higher in case group than control (P = 0.000). There was no correlation between the presence of EBV in samples with lesion type, age and gender of the patients.
Conclusion:
According to our study results, EBV is a likely etiologic agent or potential promoter in the pathogenesis of MF.
Keywords: Cutaneous T-cell lymphoma, Epstein-Barr virus, mycosis fungoides, polymerase chain reaction
What was known?
Although several studies have indicated the relationship between mycosis fungoides (MF) and viral factors, the results still remain contradictory. In recent years, retroviruses such as human T-cell leukemia virus type 1 and herpes virus family members like Epstein-Barr virus have been discussed in connection with cutaneous T-cell lymphomas. There was no previous study regarding this point in Iranian population with MF.
Introduction
Cutaneous T-cell lymphomas (CTCL) form a heterogeneous group of peripheral extranodal non-Hodgkin lymphomas (NHL). Approximately, 25-40% of NHL cases occur in extranodal sites, with skin being the second most common extranodal site after gastrointestinal system.[1] Mycosis fungoides (MF) is the most common form of CTCL resulting from clonal expansion of epidermotropic CD45RO+/CD4+ T helper cells.[2] In the United States, 1000 new cases of MF occur in each year, accounting for 0.5% of the total NHL cases.[1]
Although the etiology of MF is still unknown, infectious agents, genetic and environmental factors have been proposed as potential etiologic factors.[3] MF is supposed to be caused by chronic antigenic stimulation, which can exacerbate and/or stimulate skin inflammation and T-cell clonal expansion and result in the accumulation of T-cells residing in the skin and lacking Fas-mediated apoptosis.[2,4] A number of viral and bacterial agents have been known to play a role in this chronic antigenic stimulation and are thought to play an important role in the etiology of MF. Although several studies have indicated the relationship between MF and viral factors, the results still remain contradictory. In recent years, retroviruses such as human T-cell leukemia virus type 1 (HTLV-1), HTLV-2 and human immunodeficiency virus and herpes virus family members like Epstein-Barr virus (EBV), human herpesvirus 8 and cytomegalovirus have been discussed in connection with CTCL.[3,4,5,6] The purpose of this study was to investigate the probable etiopathologic role of EBV in MF patients.
Materials and Methods
In this case-control study, microscopic slide and paraffin blocks of 125 MF patients were selected and reviewed. Definite cases of MF confirmed by immunohistochemistry were isolated. Exclusion criteria included incomplete records, lack of or scarcity of tissue in paraffin blocks and negative β-globin test result. Accordingly, a number of cases were excluded from the study and finally 57 skin biopsy samples of MF patients entered the study.
A total of 57 normal skin samples around the melanocytic nevuses eliminated for cosmetic reasons and matched for age and sex with patients were selected as the control group.
Then, using a microtome with disinfected blade, six 5-μm sections were prepared and placed in sterile 1.5 ml microcentrifuge tubes. The following steps were performed for deoxyribonucleic acid (DNA) extraction and polymerase chain reaction (PCR).
Deparaffinization
The xylol/ethanol method was used. First, 1 ml xylol was added to 1.5 ml microtubes containing histological section and the microtubes were constantly stirred in laboratory temperature for ½ h. Then, the microtubes were centrifuged in 13,000 rpm for 10 min and the supernatant was discarded. These two steps were performed in duplicate. In the next step, 500 μl 100% ethanol was added to the sediment. After several inversions, the microtubes were centrifuged in 13,000 rpm for 10 min and the supernatant was discarded. This step was repeated and the sediment was incubated in laboratory temperature to completely evaporate the ethanol.
DNA extraction
The procedure was performed manually. Extraction buffer was 100 mM Tris-Cl with pH = 7.5 and 0.05% Tween 20. A total volume of 400 μl extraction buffer and 20 μl proteinase K was added to each microtube. The microtubes were then placed in 100°C dry block to inactivate proteinase K. The microtubes were centrifuged in 5000 rpm for 10 min and the supernatant was used for PCR.
PCR procedure
After DNA extraction from paraffin blocks, the quality of extracted DNA samples was evaluated using GH20 and PC04 β-globin gene primers. These primers amplify a 260 bp fragment with the following sequence:
GH20: 5’ GAA GAG CCA AGG ACA GGT AC 3’
PC04: 5’ CAA CTT CAT CCA CGT TCA CC 3’
PCR was conducted using the above-mentioned primers to amplify β-globin gene segment. The samples producing the 260 bp fragment using the desired primers were considered to be optimum.
EBV14/20 primers were used to evaluate the presence or absence of EBV sequences in extracted DNA samples. The sequence is as follows and the fragment length was 240 b.
EBV14: 5′ CTC TGG TAG TGA TTT GGA CC3′
EBV20: 5′ GTG AAG TCA CAA ACA AGC CC3′
After amplification, 5 μl of each sample was electrophoresed on 2% agarose gel and stained using Green viewer dye and was used to track 240 bp EBV fragment [Figure 1].
Figure 1.
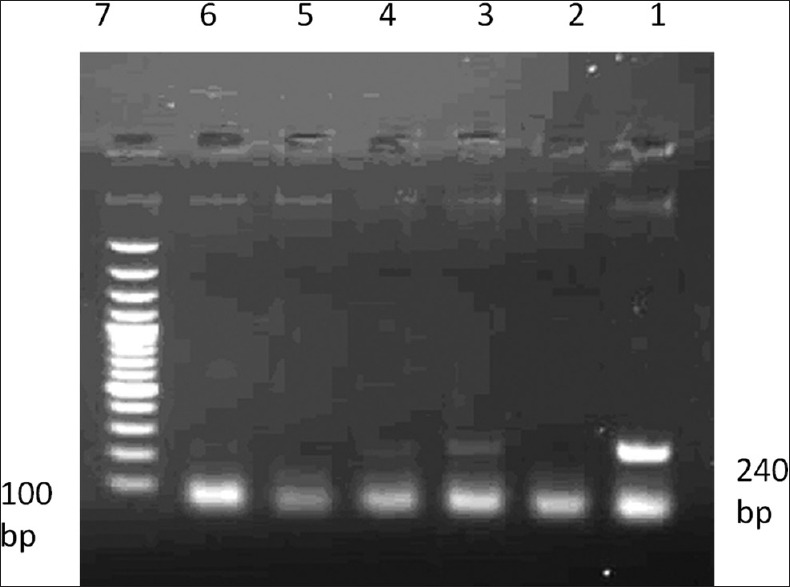
Gel electrophoresis of polymerase chain reaction performed to identify Epstein-Barr virus: 1 - Positive control; 2 - Negative control; 3 - Positive patient; samples 4, 5 and 6 - Negative patient; sample 7 - Weight marker of each 100 bp band
The data were analyzed using the Statistical Package for the Social Sciences 11.5 SPSS Inc., 233 South Wacker Drive, 11th Floor, Chicago, IL 60606-6412. Frequency diagram along with mean, median and standard deviation (SD) indices were used to describe data and Chi-square and Fisher's exact test were used to analyze data.
Results
A total of 114 samples from 57 MF patients together with 57 normal skin samples around melanocytic nevuses of individuals matched for age and sex with patients entered this study. Nearly 50.7% of the patients were male and 48.9% female. Mean and SD of patients’ age was 51.43 ± 16.34 years and they were in the range of 22-80 years. The majority of patients (40.4%) were over 60 years of age.
Among 57 samples of MF patients, 22 cases (36.8%) along with 4 cases in the control group (7%) were positive for EBV DNA using PCR. Using Chi-square test, EBV infection was significantly higher in case group compared with the control (P ≤ 0.001) [Figure 2].
Figure 2.
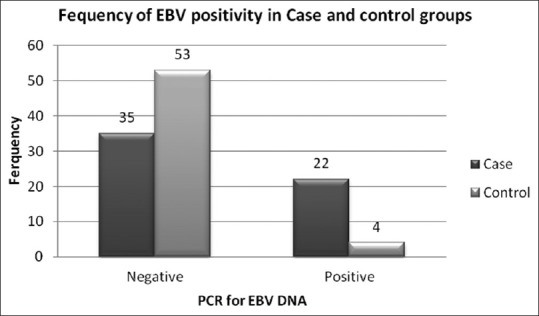
Epstein-Barr virus infection frequency in the samples of the two groups under study
Twenty out of 22 MF patients (90.9%) in whom EBV virus was detected had clinical lesion in the form of papule and plaque and there was one case of patch and nodular lesions. Based on Smirnov-Kolmogorov test, patient age followed normal distribution. T student test has shown that there is no significant difference between the mean age of the EBV positive and EBV negative patients (P = 0.579). There was no significant relationship between EBV infection and gender in patients according to Chi-square test (P = 0.627).
Discussion
In this study, skin biopsy samples of 125 MF patients were evaluated in a 12 year period from 2000 to 2012 and 57 samples were included in the study according to exclusion criteria. There were 34 male and 23 female patients. Male to female ratio was 1.47. It was between 0.8 and 4 in other studies.[3,7,8,9,10] The age range of patients was 22-80 with the average age of 50.9 years. The age range and average age of the patients was 14-82 and 48.5 in the study of Erkek et al.,[3] 13-84 and 61 in the study of Novelli et al.,[8] 60 in the study of Francesco et al.,[7] 32-91 and 62 in the study of Bonin et al. and 29-82 and 60.4 in the study of Angel et al.[10]
In this study, samples from MF patients showed the EBV virus genome considerably higher than the control group (P ≤ 0.001), suggesting a possible etiologic role of this virus in MF.
In the study of Lee on 21 CTCL patients, antibody against EBV Ag was seen in serum of 12 patients compared with 5 out of 21 in the control group (psoriasis) and it was concluded that EBV may be a trigger for initiation of the malignant process.[11] In a study on 25 CTCL cases (including MF and Sézary's syndrome [SS]) and 12 cases of reactive inflammatory dermatosis as the control group, Dreno et al. showed 32% and 0% prevalence of EBV ribonucleic acid (RNA) in case and control groups, respectively and concluded that EBV can be a long-term etiologic agent or trigger for CTCL. On the other hand, infection by EBV can induce production of tumor necrosis factor alpha, interleukin (IL)-6 and IL-1a in keratinocytes, which is likely involved in tumoral infiltrate event.[12] Kim showed the incidence of EBV in 15 out of 43 CTCL patients (35%) using PCR.[13] Shimakage et al. performed a study on 7 MF and 5 CTCL patients of other types to evaluate the presence of EBV using in situ hybridization (ISH), PCR and indirect fluorescence staining. All the 12 cases were positive for EBV, but none of the 3 control cases (dermatitis) were positive for it.[14] Novelli et al. studied 30 patients with SS, 71 MF and 18 inflammatory dermatosis patients along with 25 normal skin samples as the control group. The prevalence of EBV DNA was 27% in SS, 10% in MF and 11% in inflammatory dermatosis, but it was not detected in 25 normal skin samples and prevalence of EBV DNA in skin and peripheral blood was considerably consistent with patients’ survey. They showed that detection of EBV DNA does not indicate its etiologic role in CTCL, but the presence of EBV DNA has negative prognostic value in MF and SS and highlights the clinical value of monitoring CTCL patients for EBV.[8] Noorali et al. detected EBV genome in 3 out of 6 (50%) MF samples using PCR.[1] In the study of Francesco et al., 6 out of 20 MF patients were positive for EBV and it was concluded that EBV positive patients had a worse prognosis, faster diseases progress and higher mortality than EBV negative patients. These findings showed that EBV may be a promoter causing acceleration of disease progress. In this study, average age of EBV positive patients (65 years) was higher than EBV negative patients (58 years), but there was no significant difference in age between EBV negative and positive patients in our study. In the study of Jumbou et al., patients with SS and MF had a higher titer of antibody against viral capsid (an average of 1200) compared with the control group (an average of 320). They concluded that EBV can directly affect lymphocytes, or more probably it can be indirectly involved by chronic antigenic stimulation.[15] Erkek et al. showed that the incidence of EBV is likely to increase with disease stage. In other words, rapid lymphocyte proliferation resulting from EBV can cause a faster progression to end-stage clinical disease. In addition, the proliferation of T-cells may be faster in EBV positive cases of CTCL than EBV negative CTCL.[3] In the study of Bonin et al. on 83 MF patients and 83 normal skin samples, EBV was found in 19% of MF patients and 5% of controls. Although this result was statistically significant, regarding the fact that most EBV infection cases accompanied other viruses like HTLV-1, they concluded that EBV may play the role of a cofactor than being the primary cause of antigenic stimulation for lymphoma.[9] Anagnostopoulos et al. indicated the presence of EBV in 9 out of 42 cases of MF (21.4%) using PCR, 4 cases of which were positive for Epstein-Barr viral encoded RNA (EBER) by ISH.[16] Chang et al. showed the presence of EBV in 2 out of 10 MF cases.[17] Mouly et al. reported two cases of fatal MF, which were both positive for EBV.[18]
In contrast, there are studies indicating no important etiologic role for EBV in Erkek et al. showed EBV in 9 cases (8.9%) among 92 MF samples. They showed no strong etiologic role for EBV. No primary role of EBV in the skin lesions of MF was found, but it may play the role of promoter in etiopathogenesis of MF.[3] Nagore et al. concluded that EBV is not involved in the pathogenesis of primary cutaneous lymphomas, although PCR could detect EBV DNA in 7 out of 29 cases of MF.[19] In the study of SU et al., no EBV genome was detected in 12 cases of classic CTCL/MF (CTCL Type I) or in three CTCL cases associated with HTLV-1 (type IV) using ISH to identify EBV genome.[20] Kanavaros et al. did not detect EBV in any of the 14 samples of early stage MF, 21 cases of advanced MF and three cases of SS.[21] Peris et al. found EBV DNA by PCR in one of the two cases of MF and 2 out of 22 normal skin samples and did not suggest an important role of EBV in incidence of cutaneous lymphoma.[22] In the study of Angel et al., none of the 25 MF cases were positive for 1EBER or 2EBER by ISH.[10] Iwatsuki et al. found no EBV in their survey of 19 patients with MF/SS.[23]
EBV is the etiologic agent of infectious mononucleosis and after the initial infection; it causes predominantly latent lifelong infection of B-cells. In a few infected individuals, EBV is associated with lymphoproliferative and epithelial proliferative disorders including Burkitt's lymphoma, nasopharyngeal carcinoma and Hodgkin's disease. In immunosuppressed patients, whether normal or iatrogenic, EBV is a risk factor for lymphoproliferative disorders including immunoblastic B-cell lymphoma.[14,24] Increasing evidence suggests that EBV may be able to infect T-cells as well and it has also been shown that this virus is involved in three groups of T-cell lymphomas including lymphoma associated with hemophagocytic syndrome, nasal T-cell lymphoma and peripheral T-cell lymphoma of pleomorphic angioimmunoblastic lymphadenopathy type.[14] EBV may function as a promoter in a multi-step process of tumor formation.[3] Shimakage et al. showed that both lymphoma cells and keratinized squamous cells present in infiltration area of lymphoma cells in MF express EBV.[14] EBV has been recognized to be associated with B-cell transformation from resting B-cells to ever growing lymphocytic cell line in the laboratory environment. Two virus related peptides of Epstein-Barr nuclear antigen and latent membrane protein play an essential role in cell transformation. Gene polymorphisms in these EBV genes may be associated with their ability to cause a transformation of cells.[7] In addition to direct oncogenic capacity, these viruses appear to function as stable chronic antigen within the skin and indirectly cause the occurrence of CTCL. In addition, ability of these viruses to directly infect T-cells and cause latency in host tissues has made them new research foci to assess CTCL.[3] ISH has shown early immediate EBV transcripts in CD4+ and CD8+ T-cells, indicating the contribution of EBV to T-cell proliferation and malignancy.[9]
Conclusion
Based on the results of this study, EBV is possibly involved in the pathogenesis of MF as a potential etiologic factor or promoter. It is recommended to assess positive cases of EBV by ISH or T-cell receptor gene rearrangement. These cases should also be evaluated for other viruses such as HTLV-1. The relationship between disease stage and prognosis with EBV status should be assessed in MF patients.
What is new?
EBV is possibly involved in the pathogenesis of MF as a potential etiologic factor or promoter in Iranian population with this disease.
Acknowledgments
The authors express their profound gratitude for research deputy of Mashhad University of Medical Sciences for financial support and approval of the research proposal related to thesis of Mitra Hesamifard with code of 6620.
Footnotes
Source of support: Research Deputy of Mashhad University of Medical Sciences
Conflict of Interest: Nil.
References
- 1.Noorali S, Yaqoob N, Nasir MI, Moatter T, Pervez S. Prevalence of mycosis fungoides and its association with EBV and HTLV-1 in Pakistanian patients. Pathol Oncol Res. 2002;8:194–9. doi: 10.1007/BF03032394. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RS, Pandolfino T, Guitart J, Rosen S, Kuzel TM. Primary cutaneous T-cell lymphoma: Review and current concepts. J Clin Oncol. 2000;18:2908–25. doi: 10.1200/JCO.2000.18.15.2908. [DOI] [PubMed] [Google Scholar]
- 3.Erkek E, Sahin S, Atakan N, Kocagöz T, Olut A, Gököz A. Examination of mycosis fungoides for the presence of Epstein-Barr virus and human herpesvirus-6 by polymerase chain reaction. J Eur Acad Dermatol Venereol. 2001;15:422–6. doi: 10.1046/j.1468-3083.2001.00309.x. [DOI] [PubMed] [Google Scholar]
- 4.Nagasawa T, Takakuwa T, Takayama H, Dong Z, Miyagawa S, Itami S, et al. Fas gene mutations in mycosis fungoides: Analysis of laser capture-microdissected specimens from cutaneous lesions. Oncology. 2004;67:130–4. doi: 10.1159/000080999. [DOI] [PubMed] [Google Scholar]
- 5.Mirvish JJ, Pomerantz RG, Falo LD, Jr, Geskin LJ. Role of infectious agents in cutaneous T-cell lymphoma: Facts and controversies. Clin Dermatol. 2013;31:423–31. doi: 10.1016/j.clindermatol.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Mirvish ED, Pomerantz RG, Geskin LJ. Infectious agents in cutaneous T-cell lymphoma. J Am Acad Dermatol. 2011;64:423–31. doi: 10.1016/j.jaad.2009.11.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Francesco MA, Gargiulo F, Esteban P, Calzavara-Pinton PG, Venturini M, Perandin F, et al. Polymorphism analysis of Epstein-Barr virus isolates of lymphoblastoid cell lines from patients with mycosis fungoides. J Med Microbiol. 2004;53:381–7. doi: 10.1099/jmm.0.05439-0. [DOI] [PubMed] [Google Scholar]
- 8.Novelli M, Merlino C, Ponti R, Bergallo M, Quaglino P, Cambieri I, et al. Epstein-Barr virus in cutaneous T-cell lymphomas: Evaluation of the viral presence and significance in skin and peripheral blood. J Invest Dermatol. 2009;129:1556–61. doi: 10.1038/jid.2008.396. [DOI] [PubMed] [Google Scholar]
- 9.Bonin S, Tothova SM, Barbazza R, Brunetti D, Stanta G, Trevisan G. Evidence of multiple infectious agents in mycosis fungoides lesions. Exp Mol Pathol. 2010;89:46–50. doi: 10.1016/j.yexmp.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Angel CA, Slater DN, Royds JA, Nelson SN, Bleehen SS. Absence of Epstein-Barr viral encoded RNA (EBER) in primary cutaneous T-cell lymphoma. J Pathol. 1996;178:173–5. doi: 10.1002/(SICI)1096-9896(199602)178:2<173::AID-PATH428>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 11.Lee PY, Charley M, Tharp M, Jegasothy BV, Deng JS. Possible role of Epstein-Barr virus infection in cutaneous T-cell lymphomas. J Invest Dermatol. 1990;95:309–12. doi: 10.1111/1523-1747.ep12485017. [DOI] [PubMed] [Google Scholar]
- 12.Dreno B, Celerier P, Fleischmann M, Bureau B, Litoux P. Presence of Epstein-Barr virus in cutaneous lesions of mycosis fungoides and Sézary syndrome. Acta Derm Venereol. 1994;74:355–7. doi: 10.2340/0001555574355357. [DOI] [PubMed] [Google Scholar]
- 13.Kim JE, Huh J, Cho K, Kim CW. Pathologic characteristics of primary cutaneous T-cell lymphoma in Korea. J Korean Med Sci. 1998;13:31–8. doi: 10.3346/jkms.1998.13.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimakage M, Sasagawa T, Kawahara K, Yutsudo M, Kusuoka H, Kozuka T. Expression of Epstein-Barr virus in cutaneous T-cell lymphoma including mycosis fungoides. Int J Cancer. 2001;92:226–31. doi: 10.1002/1097-0215(200102)9999:9999<::aid-ijc1172>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 15.Jumbou O, Mollat C, N’Guyen JM, Billaudel S, Litoux P, Dréno B. Increased anti-Epstein-Barr virus antibodies in epidermotropic cutaneous T-cell lymphoma: A study of 64 patients. Br J Dermatol. 1997;136:212–6. [PubMed] [Google Scholar]
- 16.Anagnostopoulos I, Hummel M, Kaudewitz P, Korbjuhn P, Leoncini L, Stein H. Low incidence of Epstein-Barr virus presence in primary cutaneous T-cell lymphoproliferations. Br J Dermatol. 1996;134:276–81. [PubMed] [Google Scholar]
- 17.Chang YT, Liu HN, Chen CL, Chow KC. Detection of Epstein-Barr virus and HTLV-I in T-cell lymphomas of skin in Taiwan. Am J Dermatopathol. 1998;20:250–4. doi: 10.1097/00000372-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Mouly F, Baccard M, Rybojad M, Lebbé C, Morinet F, Morel P. Aggressive cutaneous T-cell lymphoma associated with the presence of Epstein-Barr virus.2 cases. Ann Dermatol Venereol. 1996;123:574–6. [PubMed] [Google Scholar]
- 19.Nagore E, Ledesma E, Collado C, Oliver V, Pérez-Pérez A, Aliaga A. Detection of Epstein-Barr virus and human herpesvirus 7 and 8 genomes in primary cutaneous T- and B-cell lymphomas. Br J Dermatol. 2000;143:320–3. doi: 10.1046/j.1365-2133.2000.03657.x. [DOI] [PubMed] [Google Scholar]
- 20.Su IJ, Tsai TF, Cheng AL, Chen CC. Cutaneous manifestations of Epstein-Barr virus-associated T-cell lymphoma. J Am Acad Dermatol. 1993;29:685–92. doi: 10.1016/0190-9622(93)70231-h. [DOI] [PubMed] [Google Scholar]
- 21.Kanavaros P, Ioannidou D, Tzardi M, Datseris G, Katsantonis J, Delidis G, et al. Mycosis fungoides: Expression of C-myc p62 p53, bcl-2 and PCNA proteins and absence of association with Epstein-Barr virus. Pathol Res Pract. 1994;190:767–74. doi: 10.1016/S0344-0338(11)80423-1. [DOI] [PubMed] [Google Scholar]
- 22.Peris K, Niedermeyer H, Cerroni L, Radaskiewicz T, Chimenti S, Höfler H. Detection of Epstein-Barr virus genome in primary cutaneous T and B cell lymphomas and pseudolymphomas. Arch Dermatol Res. 1994;286:364–8. doi: 10.1007/BF00371794. [DOI] [PubMed] [Google Scholar]
- 23.Iwatsuki K, Ohtsuka M, Harada H, Han G, Kaneko F. Clinicopathologic manifestations of Epstein-Barr virus-associated cutaneous lymphoproliferative disorders. Arch Dermatol. 1997;133:1081–6. [PubMed] [Google Scholar]
- 24.Stevens SJ, Verschuuren EA, Verkuujlen SA, Van Den Brule AJ, Meijer CJ, Middeldorp JM. Role of Epstein-Barr virus DNA load monitoring in prevention and early detection of post-transplant lymphoproliferative disease. Leuk Lymphoma. 2002;43:831–40. doi: 10.1080/10428190290016971. [DOI] [PubMed] [Google Scholar]


