Abstract
Context:
Pityriasis versicolor is a superficial, chronically recurring fungal infection caused by Malassezia species. Recently it has been revised taxanomically into 14 species, in that only 7 species have been well studied in relation to pityriasis versicolor.
Aims:
To identify Malassezia species isolated from patients with pityriasis versicolor and to find out any correlation between the species with clinical presentation of lesions.
Settings and Design:
Prospective study comprising of 100 clinically diagnosed cases of pityriasis versicolor attending Dermatology Outpatient Department over a period of 1 year.
Materials and Methods:
The clinical specimens were collected under aseptic precautions and subjected to culture on Sabouraud's Dextrose Agar overlaid with olive oil and modified Dixon agar. The isolates were identified by biochemical tests.
Statistical Analysis Used:
Statistical analysis was done using proportion, mean and chi-square test.
Results:
Of the 100 cases, 73% were males, 26% were females and predominant age group was 21-30 years. Out of 100 samples, 70 yielded growth. The most common isolate was M. sympodialis (50%), followed by M. furfur (32.86%), M. globosa (14.28%) and M. slooffiae (2.86%). Among 100 cases, 74% had hypopigmented and 26% had hyperpigmented lesions. M. sympodialis and M. furur were predominantly isolated from hypopigmented lesions and M. globosa and M. slooffiae were found to be more common in hyperpigmented lesions.
Conclusions:
M. sympodialis was the most common isolate, followed by M. furfur, M. globosa and M. slooffiae. There was no significant difference in distribution of different species in patients with hypo or hyper pigmented lesions
Keywords: Hyperpigmentation, hypopigmentation, Malassezia, pityriasis versicolor
What was known?
World wide it is believed that the causative agent of the common disease pityriasis versicolor is M globosa then M furfur.
Introduction
Pityriasis versicolor (PV) is a superficial, chronically recurring fungal infection of stratum corneum. The lesions are characterized by scaly, hypo or hyper pigmented irregular macules, most often seen on lipid-rich areas of the body, including upper part of trunk, neck, face and upper aspect of arms.[1] Clinically, the disease is asymptomatic, usually patient seeks medical attention for cosmetic purposes.[1] It has a worldwide distribution, though it is more frequent in tropical region (40%) due to relatively high temperature and humidity.[2,3,4]
The etiological agents of this superficial surface infection belong to the genus Malassezia. The Malassezia is lipophilic dimorphic fungus, occurs as yeast form in culture media and mycelia forms in the skin lesions.[5] It has been recognized as a member of normal skin flora as well as organisms involved with superficial cutaneous infections.[6] Under the influence of certain exogenous and endogenous factors, the commensal yeasts transform into filamentous pathogenic forms.[7] The normal human skin flora consists predominantly the yeast phase where as mycelia phase predominates in skin lesions.[8] Pathological specimens consists predominantly hyphae with clusters of spherical yeasts described as “spaghetti and meat ball” appearance.[9]
Malassezia species have been associated with diverse dermatological lesions like pityriasis versicolor, seborrheic dermatitis, atopic dermatitis, pityrosporum folliculitis, psoriasis, onychomycosis and blepharitis. Though generally associated with very mild superficial infection, it is emerging as an opportunistic fungal pathogen in immunocompromised patients and as nosocomial pathogen in patients with central line vascular catheter, particularly in low birth weight neonates who receive lipid emulsions.[10]
Currently genus Malassezia includes 14 species, namely M. furfur, M. sympodialis, M. globosa, M. restricta, M. slooffiae, M. obtusa, M. dermatis, M. japonica and M. yamatoensis associated with normal human flora and can cause skin lesions, M. pachydermatis, M. nana, M. equina, M. caprae and M. cuniculi are associated with animals.[11,12]
Although there is evidence to suggest variation in geographical distribution of different species, not much data is available from our country. Therefore, this study was undertaken to study the distribution of different Malassezia species in Kolar region, Karnataka.
Materials and Methods
This study comprises of 100 clinically diagnosed cases of PV attending Dermatology Outpatient Department over a period of 1 year at R. L. Jalappa Hospital and Research Centre, Kolar, Karnataka. A detailed history with reference to name, age, sex, place and clinical details like site of lesion and whether hypo or hyperpigmented were recorded. All patients clinically diagnosed as PV showing positive for presence of yeast in KOH (Potassium Hydroxide) mount were included in the study. Patients who have already received topical antifungal therapy within last 3 months and oral therapy within the last 6 months were excluded.
After taking informed consent skin scrapings from affected area are collected.[10] Those samples that showed the typical “spaghetti and meat balls” appearance on 10% KOH examination were subjected to culture. The samples were inoculated into SDA (Sabourad's Dextrose Agar) slant overlaid with sterile olive oil, plain SDA and MDA (modified Dixon agar) plates and all the cultures were incubated at 32°C. The culture plates and slants were examined every day for growth and culture negatives were discarded only after 3 weeks of incubation.[4,13]
The morphological features like budding pattern, shape of yeast cell were studied by observing Gram's stained smears on 5th day after colonies were noticed on culture media. Speciation of Malassezia was done using catalase test, esculin hydrolysis and assimilation of Tween 20, 40, 60 and 80.[3,14,15,16] Statistical analysis was done with descriptive tools like proportion and mean. Proportional difference observed within groups was being compared using the Chi-square test.
Results
Of the 100 cases of PV, (73%) were males and (27%) were females, with a high male preponderance. The age of the patients with PV ranged from 15 to 60 years. Majority of them were in the age group of 21-30 years (57%), followed by 31-40 years (27%), 11-20 years (10%), 41-50 years (4%) and 51-60 years (2%). Seventy-four (74%) had hypopigmented lesions and 26 (26%) had hyperpigmented lesions.
Distribution of PV lesions on diferent sites of the body showed that chest and trunk (30%) was the most common site involved, followed by back (27%), multiple sites (21%), these patients showed involvement of two or more sites, neck (12%), arm (8%) and axilla (2%).
Among 100 samples only 70 yielded growth on both SDA with olive oil and MDA. None of them showed any growth on plain SDA. The growth on SDA with olive oil appeared after 8 to 10 days of inoculation and on MDA within 5 days of inoculation.
Of the 70 culture positives, 35 (50%) yielded M. sympodialis, 23 (32.86%) M. furfur, 10 (14.28%) M. globosa, 2 (2.86%) M. slooffiae. The isolation of M. sympodialis is significantly (P < 0.001) higher than the isolation of other species.
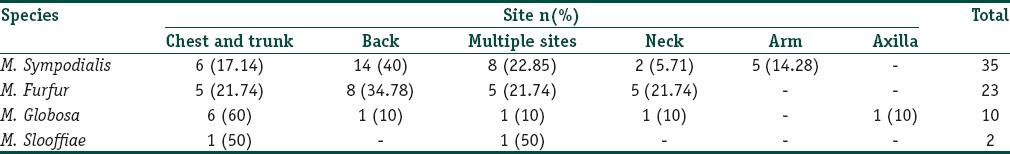
Distribution of different species on different sites of the body shown in Table 1. M. sympodialis and M. furfur was isolated predominantly from lesions on the back, M. globosa from the chest and trunk, M. slooffiae was isolated from chest and trunk and also from lesions on neck and arm.
Table 1.
Isolation of Malassezia species from different sites of the body
Of the 70 culture-positive patients, 52 (74.27%) had hypopigmented lesions and 18 (25.71%) had hyperpigmented lesions. Different species isolated with respect to type of lesion is shown Figure 1. M. sympodialis and M. furur were predominantly isolated from hypopigmented lesions and M. globosa and M. slooffiae were found to be more common in hyperpigmented lesions.
Figure 1.
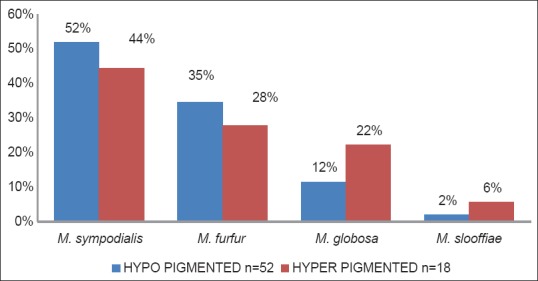
Distribution of Malassezia species in patients with hypo and hyper pigmented lesions
Discussion
Yeasts of the genus Malassezia cause various skin lesions, among which PV is the most common and worldwide in occurrence.[4] The genus Malassezia has undergone several taxonomic revisions. In the last reclassification, 14 distinct species are recognized, in that only 7 species have been well studied in relation to PV.[11,12]
The incidence of PV was more common in males (73%) than in females (27%). Similar to our study Gosh et al., Rao et al. and Krishnan et al. also found male predominance.[1,2,17] The higher incidence of PV in males may be due to their outdoor activities.[18]
Hypopigmented lesions were seen in 74% and hyperpigmented lesions in 26% of patients. Other studies from India, Gosh et al. (81% hypopigmented), Rao et al. (75% hypopigmented) and Krishnan et al. (84% hypopigmented) also showed predominance of hypopigmented lesions in PV.[1,2,17] In general, PV is thought to have a tendency to be hypopigmented in dark skinned individuals and hyper pigmented in fair skinned individuals. However, Aljabre et al. studied pigmentary changes in patients and concluded that PV is not necessarily hypopigmented in dark-skinned individuals and there is no correlation between pigment variation and the type of skin, sex and age of patient and site of lesion.[19]
The occurrence of PV was more common in adult age group than in the pediatric age group. In adults it was found to be more common in 21-30 year age group (57%). This association was found to be statistically significant with P < 0.001. A similar finding was also reported by Rao et al. (21-30yr), Krishnan et al. (15-29yr), Dutta et al. (11-30yr) and Tarazooie et al. (20-30yr).[2,17,20,21] In contrast, el-Hefnawi et al. reported highest prevalence in the third and fourth decade of life.[22] In the study by Akapata et al. it was found that majority of cases occurred during adolescence.[23] This was believed to be due to hormonal changes and increase in sebum secretion duing this period.[23]
The most common site of involvement was chest and trunk, followed by back, multiple sites involving two or more sites, neck, arm and axilla. Similar findings are also noted by Dutta et al., Rao et al. and Tarazooie et al. with chest and trunk, back and neck as most commonly involved sites. Possibly because of the distribution of sebaceous glands is higher in these areas.[2,20,21]
The isolation rate of Malassezia species in patients with pityriasis versicolor in the current study is 70%, which is comparable to that of Kindo et al. (68.57%) from south India. In contrast to our study it is higher in Chaudhary et al. (96.66%) from central India and Shokoshi et al. (88.4%) from Iran.[13,15,24] The growth on ordinary media like SDA with olive oil appeared after 8 to 10 days of inoculation and growth on special media like MDA appeared early, within 5 days of inoculation. Preparation of these special media is difficult and they are expensive when compared to SDA. Use of simple media like SDA overlaid with olive oil for isolation of lipid-dependent Malassezia species is cost effective and can be used in any routine diagnostic laboratory.[3]
The distribution of Malassezia species varies with different geographical locations. In the present study, the most common Malassezia species isolated was M. sympodialis. Kindo et al. from South India also reports M. sympodialis as the predominant species.[15] Similar reports also available from other parts of world such as Canada and Indonesia, where they found M. sympodialis as predominant species in PV.[3,8,25] In contrast, Chaudhary et al. from Central India and Dutta et al., Kaur et al. from North India reported M. globosa as the most common isolate.[4,13,20] M. furfur was the second most frequent species isolated from PV in this study, which is comparable to Dutta et al.[20] Our study showed M. globosa as third common isolate, which is contrary to other studies from India which report it as the most common species causing PV.[13,20] In our study M. slooffiae was isolated from two (2.86%) cases of PV which is almost similar to Tarazooie et al. (4%) from Iran; however, none of the other Indian studies have isolated M. slooffiae from PV.[21] M. globosa and M. restricta are predominant species isolated in non culture based epidemiological studies.[26]
Distribution of Malassezia species in lesions from different sites of the body showed that M. sympodialis and M. furfur were more common in lesions on the back, M. globosa from lesions on the chest and trunk, M. slooffiae was isolated from chest and trunk and also from lesions on neck and arms.
In our study, M. sympodialis and M. furur were predominantly isolated from hypopigmented lesions, whereas M. globosa and M. slooffiae were found to be more common in hyperpigmented lesions. But this distribution is statistically not significant, may be because of lower sample size.
Use of simple media like SDA overlaid with olive oil for isolation of lipid dependent Malassezia species is cost effective and can be used in any routine diagnostic laboratory.[3] Speciation of Malassezia is based on simple biochemical tests such as catalase reaction, esculin hydrolysis and Tween assimilation. These tests are simple to perform and cost effective and can be used in routine diagnostic laboratory. Molecular methods such as RFLP (restriction fragment length polymorphism), nested-PCR (polymerase chain reaction) or PCR-REA (polymerase chain reaction-restriction endonuclease analysis) are being developed to hasten the identification process and resolve the difficulty in interpretation of some physiological patterns. Although molecular techniques are most sensitive methods for identification, they are expensive and technically demanding.[13]
What is new?
This study shows that M. sympodialis was the predominant isolate followed by M. furfur, M. globosa and M. slooffiae.
Footnotes
Source of support: Nil
Conflict of Interest: Nil.
References
- 1.Gosh SK, Dey SK, Saha I, Barbhuiya JN, Ghosh A, Roy AK. Pityriosis versicolor: A clinico-mycological and epidemiological study from a tertiary care hospital. Indian J Dermatol. 2008;53:182–5. doi: 10.4103/0019-5154.44791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rao GS, Kuruvilla M, Kumar P, Vinod V. Clinicoepidemiological studies on Tinea versicolor. Indian J Dermatol Venereal Leprol. 2002;68:208–9. [PubMed] [Google Scholar]
- 3.Erchiga VC, Gueho E. 10th ed. Edward Arnold: London; 2005. Topley and Wilson's Microbiology and microbial infections – Medical Mycology; pp. 202–19. [Google Scholar]
- 4.Kaur M, Narang T, Bala M, Gupte S, Aggarwal P, Manhas A. Study of the Malassezia species in patients with pityriasis versicolor and healthy individuals in Tertiary care hospital, Punjab. Indian J Med Microbiol. 2013;31:270–4. doi: 10.4103/0255-0857.115636. [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharyya T, Edward M, Cordery C, Richardson MD. Colonization of living skin equivalents by Malassezia furfur. Med Mycol. 1998;36:15–9. [PubMed] [Google Scholar]
- 6.Gueho E, Midgley G, Guillot J. The genus Malassezia with description of four new species. Antonie Van Leeuwenhoek. 1996;69:337–55. doi: 10.1007/BF00399623. [DOI] [PubMed] [Google Scholar]
- 7.Faergemann J. Pityriasis versicolor. SeminDermatol. 1993;12:276–9. [PubMed] [Google Scholar]
- 8.Gupta AK, Kohli Y. Prevalence of Malassezia species on various body sites in clinically healthy subjects representing different age groups. Med Mycol. 2004;42:35–42. doi: 10.1080/13693780310001610056. [DOI] [PubMed] [Google Scholar]
- 9.Inamadar AC, Palit A. The genus Malassezia and human disease. Indian J Dermatol Venereal Leprol. 2003;69:265–70. [PubMed] [Google Scholar]
- 10.Chander J. Text book of Medical Mycology. 3rd ed. New Delhi: Mehta Publishers; 2009. Malassezia infections; pp. 92–105. [Google Scholar]
- 11.Gonzalenz A, Sierra R, Cardens ME, Grajales A, Restrepo S, Gracia MC, et al. Physiological and molecular characterization of atypical isolates of M furfur. J Clin Microbiol. 2009;47:48–53. doi: 10.1128/JCM.01422-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cabanes FJ, Vega S, Castlla G. Malassezia cuniculi sp Nov, a novel yeast species isolated from rabbit skin Med Mycol. 2011;49:40–8. doi: 10.3109/13693786.2010.493562. [DOI] [PubMed] [Google Scholar]
- 13.Chaudhary R, Singh S, Banerjee T, Tilak R. Prevalence of different Malassezia species in Central India. Indian J Dermatol Venereol Leprol. 2010;76:159–64. doi: 10.4103/0378-6323.60566. [DOI] [PubMed] [Google Scholar]
- 14.Kaneko T, Makimura K, Abe M, Shiota R, Nakamua Y, Kano R, et al. Revised cultue-based system for identification of Malassezia species. J Clin Microbiol. 2007;45:3737–42. doi: 10.1128/JCM.01243-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kindo AJ, Sophia SK, Kalyani J, Anandan S. Identification of Malassezia species. Indian J Med Microbiol. 2004;22:179–181. [PubMed] [Google Scholar]
- 16.Sohnle PG, Collis-Lech C. Cell-mediated immunity to Pityrosporum orbiculare in Tineaversicolor. J Clin Invest. 1978;62:45–53. doi: 10.1172/JCI109112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krishnan A, Thapa DM. Mophological and pigmentary variation in tineaversicolor in south Indian patients. Indian J Dematol. 2003;48:83–6. [Google Scholar]
- 18.Vijaya D, Nagarathnamma T, Anand Kumar BH, Rajesh R, Satish N, Savitha G, et al. Study of Pityriasisversicolor. The Antiseptic. 1998;95:133. [Google Scholar]
- 19.Aljebre SH, Alzayir AA, Abdulghani M, Osman OO. Pigmentary changes of tineaversicolor in dark-skinned patients. Int J Dermatol. 2001;40:273–5. doi: 10.1046/j.1365-4362.2001.01201.x. [DOI] [PubMed] [Google Scholar]
- 20.Dutta S, Bajaj AK, Basu S, Dikshit A. Pityriasis versicolor: Socioeconomic and clinic-mycologic study in India. Int J Dermatol. 2002;41:823–4. doi: 10.1046/j.1365-4362.2002.01645.x. [DOI] [PubMed] [Google Scholar]
- 21.Tarazooie B, Kordbacheh P, Zaini F, Zomorodian K, Saadat F, Zeraati H, et al. Study of distribution of Malassezia species in patients with pityriasisversicolor and healthy individuals in Tehran, Iran. BMC Dermatol. 2004;4:1–6. doi: 10.1186/1471-5945-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.el-Hefnawi H, el-Gothamy Z, Refai M. Studies on pityriasis versicolor in Egypt: I. Incidence. Mycoses. 1971;14:225–231. doi: 10.1111/j.1439-0507.1971.tb03041.x. [DOI] [PubMed] [Google Scholar]
- 23.Akapata LE, Gugnani HC, Utsalo SJ. Pityriasisversicolor in school children in cross river state of Nigeria. Mycoses. 1990;33:549–51. doi: 10.1111/myc.1990.33.11-12.549. [DOI] [PubMed] [Google Scholar]
- 24.Shokohi T, Afshar P, Barzgar A. Distribution of Malassezia species in pityriasis versicolor in Northern Iran. Indian J Med Microbiol. 2009;27:321–4. doi: 10.4103/0255-0857.55445. [DOI] [PubMed] [Google Scholar]
- 25.Gupta AK, Kohli Y, Faegemann J, Summerbells RC. Epidemiology of Malassezia yeasts associated with pityriasis versicolor in Ontario, Canada. Med Mycol. 2001;39:199–206. doi: 10.1080/mmy.39.2.199.206. [DOI] [PubMed] [Google Scholar]
- 26.Gaitanis G, Magiatis P, Hantschke M, Bassukas ID, Velegraki A. The Malassezia genus in skin and systemic diseases. ClinMicrobiol Rev. 2012;25:106–41. doi: 10.1128/CMR.00021-11. [DOI] [PMC free article] [PubMed] [Google Scholar]


