Abstract
Linked Editorials
This Editorial is part of a series. To view the other Editorials in this series, visit: http://onlinelibrary.wiley.com/doi/10.1111/bph.12956/abstract; http://onlinelibrary.wiley.com/doi/10.1111/bph.12954/abstract; http://onlinelibrary.wiley.com/doi/10.1111/bph.12955/abstract and http://onlinelibrary.wiley.com/doi/10.1111/bph.12856/abstract.
Video
To view the video on the IUPHAR/BPS Guide to PHARMACOLOGY, visit: https://www.youtube.com/watch?v=Qhy3q33VtRI
In order to link articles in BJP to the State of the Art in Pharmacology and the growing network of online databases we are introducing hyperlinks for the key drug targets and key drugs in each article to the authoritative Guide to PHARMACOLOGY database (GtoPdb) of the International Union of Basic and Clinical Pharmacology (IUPHAR), which is now produced with the support of the British Pharmacological Society (BPS).
This builds on citing the Concise Guide to PHARMACOLOGY (CGTP, formerly GRAC), which is done mainly to ensure correct pharmacological nomenclature and classification. In addition to ensuring nomenclature, GtoPdb is further linked to other biological and chemical databases, placing the work of each article first in a pharmacological, then in a broader scientific, context.
This is one of a series of editorials discussing updates to the BJP Instructions to Authors (McGrath and Curtis, 2015). The other key updates are McGrath and Lilley (2015), Curtis et al. (2015) and McGrath et al. (2015).
BJP is already inserting these hyperlinks at the Production stage as can be seen by consulting recent issues of the journal. They appear as Tables of Links near the start of the article. From April 2015, authors will be asked to insert hyperlinks to the key drug targets and ligands (drugs) featured in their study, when they submit the manuscript. Editors will be expected to check that the items linked are appropriate to the article and that the links work. The submission system will be tweaked to facilitate this early in 2015.
It is important that information that a journal article links to is both current and permanent. GtoPdb is a dynamic database that is curated and kept up to date, so provides the immediate current information; but precisely because it is dynamic it will contain different information when the article is consulted in the future. A permanent record is also needed and this is provided by the biennial CGTP published in conjunction with BJP. So for drug targets we ask authors first to link to the database electronically, which goes straight to the molecule of interest; and secondly to cite the relevant part of the CGTP, which covers the broad category, such as GPCR or Transporter, and will contain the state of knowledge on which the article was based no matter how long into the future it is consulted.
Why are we doing this?
A major feature of current biological science is the creation and curation of databases documenting the genome, proteins and phenotypes and their interlinkage. Pharmacology increasingly uses molecules and techniques that rely on these databases but has not been effective, particularly in publications, in linking in the pharmacological ‘data’, especially functional information on drug targets and the drugs (ligands) that act upon them. There is also a perceived need for nomenclature standards, without which linkages between databases become impossible.
This is now all possible by employing GtoPdb, an expert-driven guide to pharmacological targets and the substances that act on them. Major strengths are the classification of all human genome-encoded drug targets and nomenclature thereof and the inclusion of functional pharmacological data in which other databases are very deficient. By linking drug targets and ligands to GtoPdb journal readers can link through to other databases and place the work in the paper firmly in the context of the greater world of current knowledge as contained in these databases.
BJP is seeking to enhance the content of the articles that we publish in line with the growing trend of enriching articles with links to further sources of information, which enhances their usability and may also increase citations, a benefit for authors. This is also in tune with contemporary attitudes about providing more transparency in scientific reporting in general, as illustrated by the UK Concordat on Openness on Animal Research and USA NIH Principles and Guidelines for Reporting Preclinical Research. More specifically there are attempts by agencies or groups to provide guidelines for the publication of research involving animals. This gives a framework for transparent reporting, e.g. the ARRIVE Guidelines initiated by the UK National Centre for the Replacement, Refinement & Reduction of Animals in Research (NC3Rs), and the Basel Declaration, an international consensus calling for more trust, transparency and open communication on animal research.
The two resources
1. The International Union of Basic and Clinical Pharmacology/British Pharmacological Society (IUPHAR/BPS) Guide to PHARMACOLOGY database (GtoPdb) (http://www.guidetopharmacology.org; see Pawson et al., 2015)
GtoPdb is an open access resource that has been online since December 2011, providing pharmacological, chemical, genetic, functional and pathophysiological data on the targets of approved and experimental drugs. It is developed at the University of Edinburgh under the auspices of the IUPHAR Committee on Receptor Nomenclature and Drug Classification (NC-IUPHAR; http://www.guidetopharmacology.org/nciuphar.jsp) and the BPS. The database is constantly expanding and is the result of curation and integration of data previously contained in two separate but overlapping resources, the IUPHAR Database (IUPHAR-DB) and the published BPS ‘Guide to Receptors and Channels’ (GRAC) compendium (e.g. Alexander et al., 2011).
The database covers a range of established and potential drug targets (currently 2708 distinct human UniProt accessions), divided into sections on G protein-coupled receptors, ion channels, nuclear hormone receptors, catalytic receptors, enzymes, transporters, and other targets. Within each section, targets are divided into families, with each having a summary page including an overview, recommended literature references, target nomenclature and a list of the key selective ligands and probes. For a subset of the most important targets more detailed information is given on separate database pages.
The data are derived through manual curation of the primary literature by a global network of NC-IUPHAR subcommittees and other voluntary expert contributors, and linked to relevant databases, including ChEMBL, DrugBank, Ensembl, HGNC, PubChem, UniProt and PubMed. Each of the >7000 small molecule and peptide ligands is annotated with manually curated 2D chemical structures or amino acid sequences, nomenclature and links to external resources for further information. Current work is focused on expanding the resource to include all the primary targets of approved drugs and likely future candidate targets, alongside educational resources to guide scientists and students in pharmacological principles and techniques.
2. The Concise Guide to PHARMACOLOGY 2013/14 (CGTP) (see Alexander et al., 2013)
This is a permanent, citable publication that contains a view of the database at one point in time. The version current at the time of an article's publication thus contains the same information as the database if consulted immediately after the article's publication. A new version is produced every two years.
‘It provides concise overviews of the key properties of over 2000 human drug targets with their pharmacology, plus links to entries for drug targets and their ligands contained in GtoPdb, thereby providing more detailed views of target and ligand properties. Direct links to citations in PubMed are also provided. The full contents of The Concise Guide to PHARMACOLOGY 2013/14 can be found at http://onlinelibrary.wiley.com/doi/10.1111/bph.12444/full.’ (Alexander et al., 2013)
This compilation of the major pharmacological targets is divided into several areas of focus reflecting the organisation of GtoPdb (see above and Figure 1). It is produced in conjunction with NC-IUPHAR, and provides the official IUPHAR classification and nomenclature for human drug targets, where appropriate, alongside summary information on the best available pharmacological tools, key references and suggestions for further reading. A new landscape format has easy to use tables comparing related targets. It is a condensed version of material contemporary to late 2013, which is presented in greater detail and is constantly updated on the GtoPdb website, thus superseding data presented in previous editions of GRAC.
Figure 1.
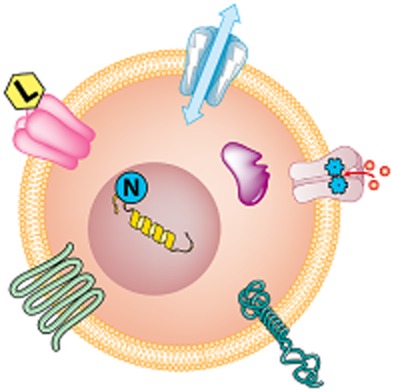
The logo of The Concise Guide to PHARMACOLOGY 2013/14 illustrates the seven chapters into which the major pharmacological targets have been compiled: clockwise from bottom left then inside cell; G protein-coupled receptors, ligand-gated ion channels, voltage-gated ion channels, transporters catalytic receptors, nuclear hormone receptors and enzymes (Alexander et al., 2013; http://onlinelibrary.wiley.com/doi/10.1111/bph.12444/full).
How will we do this?
BJP are working with our publishers (Wiley) to facilitate the implementation of links from BJP articles to target and ligand entries in GtoPdb. Links to the database will take the form of ‘Tables of Links’ for targets and ligands, appearing at the beginning of the manuscript before the introduction. From April 2015, authors will be instructed on how to provide the correct citations to both the database and published CGTP, and the necessary information in order to create the tables of links to the database. The process will begin at the point of online submission of the manuscript, prior to the peer review process. Both reviewers and authors will be required to check that links are pointing to the correct entries in the database. There will also be a mechanism in place to alert the database curators where key entities are missing in the GtoPdb, which can then be passed to the curation pipeline for inclusion in a future version of the resource.
Instructions for authors
Table of links to drugs targets and key ligands
A list of the drug targets that are the topic of the article and of the key ligands employed, with hyperlinks to the IUPHAR/BPS Guide to PHARMACOLOGY (where available) is also required in the form of a Table of Links (see below), which will be positioned within the article as a first table and called ‘Table of Links’. Use the ‘Table of Links’ templates for Targets and Ligands provided on submission. (Instructions will be given within the submission process ‘Scholar One’.)
Drug targets and nomenclature
For each Target under investigation, perform the following steps
Go the IUPHAR/BPS Guide to PHARMACOLOGY at http://www.guidetopharmacology.org/;
Perform a search or browse the Target list and navigate to the relevant target family page;
Note that the quick search box at the top right of the page returns predictive search results as you start to type the name of the Target;
On the family page, locate the Targets under investigation and click the ‘Show summary’ link;
Enter the standard Nomenclature (found at the top of each summary) for each Target, listed in alphabetical order under their appropriate target class heading (i.e. GPCRs, Ion channels, Enzymes, etc.), into the template as defined in the database, and use this nomenclature throughout your manuscript, e.g. M1 receptor, IP3R1, Nav1.1; alternatively, define an abbreviation for this nomenclature as described above;
Enter the corresponding Target ID into the template;
Locate the ‘How to cite this family’ box at the bottom of each Target family page (or section if there is more than one family per page);
Enter the ‘Database page citation’ as it appears, e.g. Acetylcholine receptors (muscarinic). Accessed on 26/02/2014. IUPHAR/BPS Guide to PHARMACOLOGY, http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=2. This allows a hyperlink to the database to be entered in the final paper and allows readers to understand the context now and in the immediate future;
Enter the ‘Concise Guide to PHARMACOLOGY citation’ as it appears, e.g. Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, Peters JA and Harmar AJ, CGTP Collaborators. (2013) The Concise Guide to PHARMACOLOGY 2013/14: G Protein-Coupled Receptors. Br J Pharmacol. 170: 1459–1581. This provides a permanent record (DOI: digital object identifier) that will survive database updates and allows future readers to understand the contemporary context.
If you cannot find the Target studied in your investigation used, please enter details into the relevant box so that the database Curators can note and, if possible, correct the omission. The database holds information on human, mouse and rat targets.
Drugs and other chemicals
A list of Ligands used in the study should be provided in a table in the manuscript template. For each key ligand important for the study, perform the following steps:
Go to the IUPHAR/BPS Guide to PHARMACOLOGY at http://www.guidetopharmacology.org/;
Perform a search or browse the Ligand list and navigate to the Ligand page;
Note that the quick search box at the top right of the page returns predictive search results as you start to type the name of the Ligand;
Enter each Ligand name into the template, listed in alphabetical order, as it appears at the top of the ligand page in the database;
Enter the Ligand ID into the template as provided above the Ligand name
Enter the International Non-proprietary Names (INN) if this is given on the ligand page if provided; the INN is provided beneath the IUPAC name on the summary tab of the ligand page, e.g. for acetylcholine, the INN is acetylcholine chloride;
If a Ligand has no INN, its full chemical name (IUPAC name) must be provided;
Cumbersome chemical names should be suitably abbreviated for later reference in the paper;
If you cannot find the Ligand used in your study, please enter details into the relevant box so that the database Curators can note and, if possible, correct the omission. The manuscript template provides a table to enter this information. This procedure links the Ligand to its known targets, and also to further data on its pharmacological properties and chemical structure in the IUPHAR/BPS Guide to PHARMACOLOGY;
Enter the name, city and country of the suppliers.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, McGrath JC, et al. CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: Overview. Br J Pharmacol. 2013;170:1449–1458. [Google Scholar]
- Alexander S, Mathie A, Peters J. Guide to Receptors and Channels (GRAC), 5th edition. British Journal of Pharmacology. 2011;164:1–324. doi: 10.1111/j.1476-5381.2011.01649_1.x. Issue Supplement s1, Pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, et al. Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol. 2015;172 doi: 10.1111/bph.12856. . doi: 10.1111/bph.12856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JC, Curtis MJ. BJP is changing its requirements for scientific papers to increase transparency. Br J Pharmacol. 2015;172:2671–2674. [Google Scholar]
- McGrath JC, Lilley E. Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol. 2015;172 doi: 10.1111/bph.12955. . doi: 10.1111/bph.12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JC, McLachlan EM, Zeller R. Transparency in Research involving Animals: The Basel Declaration and new principles for reporting research in BJP manuscripts. Br J Pharmacol. 2015;172:2427–2432. doi: 10.1111/bph.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucleic Acids Res. 2014;42(Database issue):D1098–1106. doi: 10.1093/nar/gkt1143. 2014 Jan;. doi: 10.1093/nar/gkt1143. Epub 2013 Nov 14. PMID: 24234439. [DOI] [PMC free article] [PubMed] [Google Scholar]


