Abstract
Background and Purpose
Lysophosphatidylinositol (LPI), a lipid signalling molecule, activates GPR55 and elevates intracellular Ca2+. Here, we examine the actions of LPI in the rat resistance mesenteric artery and Ca2+ responses in endothelial cells isolated from the artery.
Experimental Approach
Vascular responses were studied using wire myographs. Single-cell fluorescence imaging was performed using a MetaFluor system. Hypotensive effects of LPI were assessed using a Biopac system.
Key Results
In isolated arteries, LPI-induced vasorelaxation was concentration- and endothelium-dependent and inhibited by CID 16020046, a GPR55 antagonist. The CB1 receptor antagonist AM 251 had no effect, whereas rimonabant and O-1918 significantly potentiated LPI responses. Vasorelaxation was reduced by charybdotoxin and iberiotoxin, alone or combined. LPI decreased systemic arterial pressure. GPR55 is expressed in rat mesenteric artery. LPI caused biphasic elevations of endothelial cell intracellular Ca2+. Pretreatment with thapsigargin or 2-aminoethoxydiphenyl borate abolished both phases. The PLC inhibitor U73122 attenuated the initial phase and enhanced the second phase, whereas the Rho-associated kinase inhibitor Y-27632 abolished the late phase but not the early phase.
Conclusions and Implications
LPI is an endothelium-dependent vasodilator in the rat small mesenteric artery and a hypotensive agent. The vascular response involves activation of Ca2+-sensitive K+ channels and is not mediated by CB1 receptors, but unexpectedly enhanced by antagonists of the ‘endothelial anandamide’ receptor. In endothelial cells, LPI utilizes PLC-IP3 and perhaps ROCK-RhoA pathways to elevate intracellular Ca2+. Overall, these findings support an endothelial site of action for LPI and suggest a possible role for GPR55 in vasculature.
Tables of Links
| TARGETS |
|---|
| GPCRsa |
| CB1 receptors |
| GPR55 |
| Enzymesb |
| ERK |
| PLC |
| ROCK, Rho-associated kinase |
| Ion channelsc |
| BKCa, large conductance Ca2+-activated K+ channels (KCa1.1) |
| IKCa, intermediate conductance Ca2+-activated K+ channels (KCa3.1) |
| IP3 receptors |
| SKCa, small conductance Ca2+-activated K+ channels (KCa2.1, 2.2, 2.3) |
| LIGANDS | |
|---|---|
| 2 APB, 2-aminoethoxydiphenyl borate | L-NAME |
| 4-aminopyridine | LPI, lysophosphatidylinositol |
| AM251 | Methoxamine |
| AM630 | Oleamide |
| Apamin | Oleoylethanolamide |
| Carbachol | Rimonabant |
| Charybdotoxin | Thapsigargin |
| CID 16020046 | TRAM-34 |
| Glibenclamide | U46619 |
| Iberiotoxin | U73122 |
| Indomethacin | Y27632 |
| JWH 015 |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,b,cAlexander et al., 2013a,b,c).
Introduction
There is increasing evidence that long-chain lipid signalling molecules have vasoactive actions. Much activity has focused on the actions of the endocannabinoid, anandamide (arachidonoyl ethanolamide), which relaxes blood vessels through both endothelium-dependent and endothelium-independent mechanisms (Wagner et al., 1999) and may also have cardioprotective actions (see Hiley and Ford, 2004). More recently, other derivatives of long-chain fatty acids have also been shown to have vasodilator actions, and they show complex pharmacology in terms of overlapping sensitivity to antagonists, which does not show consistency between the different agents. For example, O-1918 was introduced as an antagonist at the ‘endothelial anandamide receptor’ (Offertaler et al., 2003) at which the cannabinoid antagonist rimonabant was also an antagonist. This pattern of sensitivity is shared with vasorelaxation to oleamide (Hoi and Hiley, 2006), but not with oleoylethanolamide-induced relaxation of rat mesenteric arteries which is sensitive to O-1918 but not rimonabant (AlSuleimani and Hiley, 2013). This has led to the conclusion that there are several receptors, apart from cannabinoid CB1 and CB2 receptors, involved in response to these fatty acid derivatives (Hiley and Kaup, 2007; Bondarenko, 2014).
One receptor that has attracted particular attention is the orphan G protein-coupled receptor GPR55. This, with its distinct intracellular signalling cascades in endothelial cells, has recently been hypothesized to form part of a novel signalling system with lysophosphatidylinositol (LPI), an endogenous cell membrane-derived lipid, as its primary ligand (Waldeck-Weiermair et al., 2008; Bondarenko et al., 2010; 2011b,). When LPI was investigated as a ligand for GPR55, it initiated responses, which were either absent in untransfected cells, diminished by small interfering RNA (siRNA) knock-down of GPR55 or enhanced by GPR55 overexpression (Oka et al., 2007; 2010,; Waldeck-Weiermair et al., 2008; Henstridge et al., 2009; 2010,; Bondarenko et al., 2010; Ford et al., 2010). For instance, in HEK293 cells expressing a human GPR55 receptor, LPI induced Ca2+ mobilization (Henstridge et al., 2009), caused phosphorylation of ERK (Oka et al., 2007; 2009,) and activated RhoA and the transcription factor NFAT (Henstridge et al., 2009). Additionally, LPI initiates Ca2+ mobilization in mice dorsal root ganglion neurons (Lauckner et al., 2008) and human endothelial cells naturally expressing GPR55 (Waldeck-Weiermair et al., 2008; Bondarenko et al., 2010). In addition, knock-down of GPR55 in human endothelial cells partly inhibited the angiogenesis and endothelial wound healing induced by N-arachidonoyl serine (Zhang et al., 2010). In HUVEC, LPI induced both GPR55-dependent and GPR55-independent signals (Waldeck-Weiermair et al., 2008; Bondarenko et al., 2010) and acted as an intracellular messenger modulating K+ channels (Bondarenko et al., 2011a), suggesting a vascular function for this lipid.
It was initially suggested that GPR55 might be the proposed ‘endothelial anandamide receptor’ (Offertaler et al., 2003). However, there is now evidence against this, as mesenteric arteries of both wild-type and GPR55 knockout mice show vasorelaxation in the presence of abnormal cannabidiol and O-1602 (Johns et al., 2007). However, Johns et al. (2007) found that GPR55 knockout mice had elevated baseline mean arterial pressure. Furthermore, a recent oral communication indicated that GPR55 knockout mice showed reduced vasorelaxation to anandamide, providing a further evidence of a possible role for GPR55 in vasculature (McNaughton and Ho, 2013). Stimulation of GPR55 by LPI results in activation of the large conductance Ca2+-activated K+ channels (BKCa) and subsequent membrane hyperpolarization in endothelial cells (Bondarenko et al., 2010), a key pathway involved in vasorelaxation, although Fukao et al. (1996) had previously shown that LPI inhibited ACh-induced vascular hyperpolarization in rat mesenteric artery. Another early study showed that LPI enhanced the Ca2+ sensitivity of smooth muscle contractile proteins in α-toxin permeabilized rat mesenteric arteries by a PKC-based mechanism (Jensen, 1996).
The lack of clarity in the effects of LPI on the vasculature was the stimulus for the present study of its actions and, in particular, the role of GPR55 in the vasculature. The haemodynamic effects of LPI in vivo, its vascular actions on isolated arteries and on freshly isolated endothelial cells were studied. The effect of LPI on BP in vivo was examined in rats (see the Supporting Information), and vascular tone was assessed in the third generation of the rat superior mesenteric artery. Furthermore, the effects of LPI on intracellular Ca2+ signals was investigated in endothelial cells isolated from the rat mesenteric arterial bed. In addition, as a majority of recent studies on GPR55 link this receptor to LPI actions, expression of GPR55 in this arterial bed was investigated with the real-time PCR and compared with the expression of the cannabinoid CB1 and CB2 receptors.
Methods
Animals
All animal care and experimental procedures for the in vitro experiments were in accordance with the UK Animal (Scientific Procedures) Act 1986. The in vivo procedures reported in Supplementary Material were approved by the Animal Ethical Committee of Sultan Qaboos University. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A total of 151 rats were used to provide the tissue samples for the in vitro experiments and seven rats for the in vivo experiments
Myograph studies
Male Wistar rats (250–400 g; Charles River UK Ltd, Kent, UK) were killed with an overdose of sodium pentobarbital (120 mg·kg−1, i.p.; Sagatal, Rhône Mérieux, Harlow, Essex, UK). The mesentery was rapidly removed and placed in ice-cold, gassed (95% O2/5% CO2), Krebs–Henseleit solution (pH 7.4) of the following composition (mM): NaCl, 118; KCl, 4.7; MgSO4, 1.2; KH2PO4, 1.2; NaHCO3, 25; CaCl2, 2.5; d-glucose, 5.5. Unless otherwise stated, the solution also contained indomethacin (10 μM). Small branches (328 ± 4 μm diameter, 180 vessels) of the rat superior mesenteric artery were mounted in a Mulvany–Halpern wire myograph maintained at 37°C in gassed (95% O2/5% CO2) Krebs–Henseleit solution. The mean vessel diameter after normalization was 480 ± 6 μm (180 vessels) and the mean resting force of contraction generated following this process was 3.6 ± 0.1 mN (150 vessels). Vessels were precontracted with 10 μM methoxamine and the mean force of contraction generated was 12.9 ± 0.4 mN (127 vessels), and they were designated as endothelium-intact when carbachol (10 μM) relaxed them >90%.
After the endothelial integrity test, vessels were left to re-equilibrate for 30–40 min and were then used to assess the actions of the lysophospholipids. When the contractile effect of these lipids was examined, they were added in a cumulative manner to vessels under basal tone. The possible vasorelaxant effects of these compounds were investigated by cumulative addition to vessels precontracted with methoxamine (10 μM). In some experiments, they were precontracted with 60 mM KCl. The mean force of contraction generated by 60 mM KCl was 12.14 ± 1.2 mN (n = 11). In those experiments where intracellular signalling pathways were explored, the arteries were precontracted with either U46619 (3 μM) alone or in combination with methoxamine.
Any receptor antagonist, channel blocker or enzyme inhibitor being examined was added to the bathing solution 30 min before, and was present during construction of the concentration–response curve. Experiments were conducted in a paired manner, with control and test experiments carried out on arteries from the same animal, and each preparation was only exposed to a single agonist.
Isolation and culture of rat mesenteric arterial bed endothelial cells (MAECs)
Isolation of MAECs was performed by a modification of the method of Santos et al. (2003). Male Wistar rats (250–400 g; Charles River UK Ltd) were killed with an overdose of sodium pentobarbital (120 mg·kg−1, i.p.; Sagatal, Rhône Mérieux). A polyethylene (PE50) cannula was inserted into the superior mesenteric artery at its origin from the abdominal aorta. The entire mesentery and small intestine were then removed and placed in a Petri dish containing PBS (Invitrogen Life Technology, Paisley, Scotland) at 37°C. The mesenteric vascular bed was then carefully detached from the intestine and perfused through the mesenteric artery, first with PBS at 1 mL·min−1 for 15 min at 37°C, to ensure thorough removal of all blood cells and then with 0.2% collagenase type A (Roche, Mannheim, Germany) at 1 mL·min−1 at 37°C for 40 min. Perfusate was collected every 10 min and centrifuged at 1200× g for 10 min. Pellets were resuspended in endothelial cell basal medium-2 (Lonza, Cologne, Germany) supplemented with 10% FBS (Invitrogen Life Technology), penicillin (100 U·mL−1; Sigma Chemical Company, Poole, Dorset, UK), streptomycin (100 μg·mL−1; Sigma Chemical Company), 2 mM l-glutamine (Sigma Chemical Company), 80 μg·mL−1 heparin (Sigma Chemical Company), 5 μg·mL−1 ascorbic acid (Sigma Chemical Company) and endothelial growth supplement (75 μg·mL−1; First Link, Birmingham, UK). Isolated cells were directly seeded onto round glass coverslips (22 mm, No. 0; VWR International, Radnor, PA, USA) coated with 0.5% bovine gelatine (Sigma Chemical Company) and incubated in a humidified 5% CO2 atmosphere for 3–5 days before being used for experiments.
Single-cell Ca2+ imaging
Isolated MAECs on 22-mm-diameter coverslips coated with 0.5% gelatine were used for Ca2+ imaging 3–5 days following seeding. Initially, they were washed twice at 20°C with HEPES-buffered saline (HBS) of the following composition (mM): NaCl, 135; KCl, 5.9; MgCl2, 1.2; HEPES, 11.6; glucose, 11.5; CaCl2, 1.5 (this was omitted in Ca2+-free HBS), pH 7.3. The cells were then loaded with fura-2-acetoxymethyl ester (fura-2 AM; 2 μM; Invitrogen Life Technology) in HBS + 2.5 mM probenecid (Sigma Chemical Company) at 20°C for 1 h, then washed twice with HBS and incubated for a further 30 min period to allow de-esterification of the indicator (Rosker et al., 2009).
Fluorescence ratios (excitation at 340 and 380 nm; emission at 510 nm) from single cells were recorded at 20°C using an Olympus IX71 inverted fluorescence microscope (Olympus, Tokyo, Japan) and a Luca EMCCD camera (Andor Technology, Belfast, Northern Ireland) and collected at 5 s intervals. At the end of each experiment, cells were exposed to a quench solution composed of 1 mM MnCl2 and 1 μM ionomycin in HBS to correct for autofluorescence. The compounds under investigation were added 10–15 min before and were then present throughout the period when LPI effects were assessed.
Detection of mRNAs for GPR55, CB1 and CB2 receptors in the rat mesenteric artery
Male Wistar rats weighing 250–400 g were killed as above and the entire mesentery was rapidly removed and placed in Krebs solution. The mesenteric arterial bed, cleaned of blood content and surrounding fatty tissue, was then quickly placed in TRIZOL® reagent (Invitrogen Life Technology). Similarly, parts of the testis were removed and placed in the reagent. The tissues were then rapidly used for RNA isolation.
Total RNA was extracted using TRIZOL (Invitrogen Life Technology), as recommended by the manufacturer with slight modification. Briefly, tissues were cut into small pieces with a scalpel blade on a Petri dish and then placed in dry ice. They were then ground using a mortar and pestle and homogenized in 1 mL of TRIZOL with a needle and syringe. The homogenate was transferred to a fresh tube and incubated for 5 min at room temperature to permit dissociation of nucleoprotein complexes. RNA was separated from the other components by chloroform and precipitated by isopropyl alcohol (Sigma Chemical Company), as described in the manufacturer's protocol. The pellet was washed with 75% ethanol, air dried and then resuspended in RNase-free water (20–100 μL; 65°C for 10 min). The concentration of isolated RNA was determined using a Nanovue spectrophotometer (GE Healthcare Life Sciences, Amersham, UK) and the obtained A260/280 ratios were 2–2.2.
About 1 μg of RNA from each sample was then treated with RQ1 RNase-free DNase (Promega, Southampton, Hampshire, UK) to remove genomic DNA contamination using the manufacturer's protocol. Treated RNA samples were immediately used for complementary DNA (cDNA) synthesis. First-strand cDNA was synthesized using SuperScript II reverse transcriptase (Invitrogen Life Technology). The primers shown in Table 1 (Hoi, 2007) were used to amplify cDNA corresponding to the messenger RNA (mRNA) transcripts for rat GPR55, the rat CB1 and CB2 receptors, and rat GAPDH (glyceraldehyde-3-phosphate dehydrogenase; used as a reference gene) as expressed in the mesenteric artery and testis. Real-time PCR was carried out using the SensiMix™ SYBR & Fluorescein Kit (Bioline, London, UK). The mastermix contains the SYBR Green I dye, dNTPs, stabilizers and enhancers. The cDNA samples (20 μL) were diluted fourfold in RNase/DNase-free water and the following reaction mix was prepared before proceeding to the real-time PCR: 2× SensiMix SYBR & Fluorescein (11 μL); forward and reverse primers (1 μL of 500 nM solution of each); MgCl2 (0.6 μL of 3 mM); cDNA or non-reverse-transcribed RNA (7 μL). Amplification was allowed to proceed in a Rotor-Gene 3000™ (Corbett Research, Sydney, NSW, Australia) using the following cycle conditions: initial denaturation (10 min); followed by 45 cycles of 94°C (30 s), 55°C (30 s), 72°C (30 s; fluorescence detection) and 2 min final extension at 72°C. Melt curve analysis was performed from 72 to 95°C in 1°C steps, 45 s for the first step and 5 s for each step thereafter.
Table 1.
Primers used to amplify cDNA from rat mesenteric artery and testis
| GPR55 | Forward | 5′-TTGTGGAGTGCCTCTACTTC-3′ |
| Reverse | 5′-CCGTAGAATGTGAATGCTCC-3′ | |
| CB1 receptor | Forward | 5′-ACAAGTCTCTCTCGTCGTTC-3′ |
| Reverse | 5′-TTGTGAAGGAGGCTGTAACC-3′ | |
| CB2 receptor | Forward | 5′-TCTTTGCCTGCAACTTCGTC-3′ |
| Reverse | 5′-ACTAGGACAACAAGTCCACC-3′ | |
| GAPDH | Forward | 5′-AGTCTACTGGCGTCTTCACC-3′ |
| Reverse | 5′-CCATCACGCCACAGCTTTCC-3′ |
To ensure that specific products corresponding to the genes under investigation were amplified, the samples (20 μL each) were subjected to electrophoresis in a 2% agarose gel in Tris–acetate–EDTA buffer and visualized with ethidium bromide. The expected sizes (as base pairs) of the amplified fragments were as follows: GPR55, 341; CB1 cannabinoid receptor, 357; CB2 cannabinoid receptor, 275; GAPDH, 307. Control reactions with reverse transcriptase omitted from the reverse transcription reaction were also included and shown not to produce any specific amplification products, ruling out any possible genomic DNA contamination.
Data analysis
In myograph studies, relaxation responses are expressed as the percentage relaxation of the tone induced by either 10 μM methoxamine, 3 μM U46619 or 60 mM KCl. Data are shown as mean ± SEM, and n represents the number of rats. The mean relaxation achieved at the highest concentration used (R10 μM) is provided.
In single-cell Ca2+ imaging experiments, data are expressed as Δratio (F340/F380), which indicates the difference in the 340–380 nM fluorescence ratios between basal and peak Ca2+. n represents the number of cells from at least two independent isolations.
In experiments where expression of mRNAs was investigated, the authenticity of each product was assessed by melting curve analysis. Results were analysed using the ‘comparative quantitation’ feature of the Rotor-Gene software (Version 6; Corbett Research), which compares the relative expression of samples to control (here the housekeeping gene GAPDH). For each sample, the cycle number at which the second derivative of the amplification curve reaches 20% of its maximum level, and indicates the end of the noise and the transition into the exponential phase, is calculated and used as cycle threshold (CT). The expression relative to that of a gene selected as the calibrator (i.e. GAPDH) is then calculated as follows:
The ‘amplification’ is the average increase in fluorescence for four cycles after CT and is determined from the empirical slope of the amplification curves (Lim et al., 2008). Each reaction was performed in duplicate with samples obtained from three rats.
Concentration–response curves were analysed using two-way anova of the whole dataset. In experiments where the effect of single concentrations of LPI was assessed, statistical comparisons between individual groups were carried out using one-way anova followed by the Dunnett's or Bonferroni's multiple comparison tests, and where only two groups were compared, unpaired two-tailed Student's t-test was used. P values of <0.05 were set as criterion of statistical significance.
Materials
Methoxamine, carbachol, l-NAME, 4-aminopyridine, l-α-lysophosphatidyl-inositol (all from Sigma Chemical Company), apamin, charybdotoxin, iberiotoxin and Y-27632 (all from Tocris Cookson, Bristol, UK) were dissolved in distilled water. U46619 (Tocris) was dissolved in PBS. O-1918, AM 251, TRAM 34, JWH 015, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) and 2-aminoethoxydiphenyl borate (2-APB; all from Tocris) were dissolved in 100% ethanol as was rimonabant (SR141716A; a generous gift from Sanofi-Synthélabo, Montpellier, France) and glibenclamide (Hoechst UK Ltd, Hounslow, Middlesex, UK). AM 630, CID 16020046, U73122 (all from Tocris) and thapsigargin (Sigma Chemical Company) were dissolved in 100% dimethyl sulfoxide. Indomethacin (Sigma Chemical Company) was dissolved in 5% (w/v) NaHCO3 solution. All compounds were further diluted with distilled water and used within a maximum of 2 days of preparation.
Results
Myograph studies
Effects of LPI on vascular tone
LPI produced concentration- and endothelium-dependent vasorelaxation (Figure 1A). Figure 1B illustrates original traces of the vasorelaxation to LPI in rat small mesenteric arteries in the presence and absence of functional endothelium.
Figure 1.
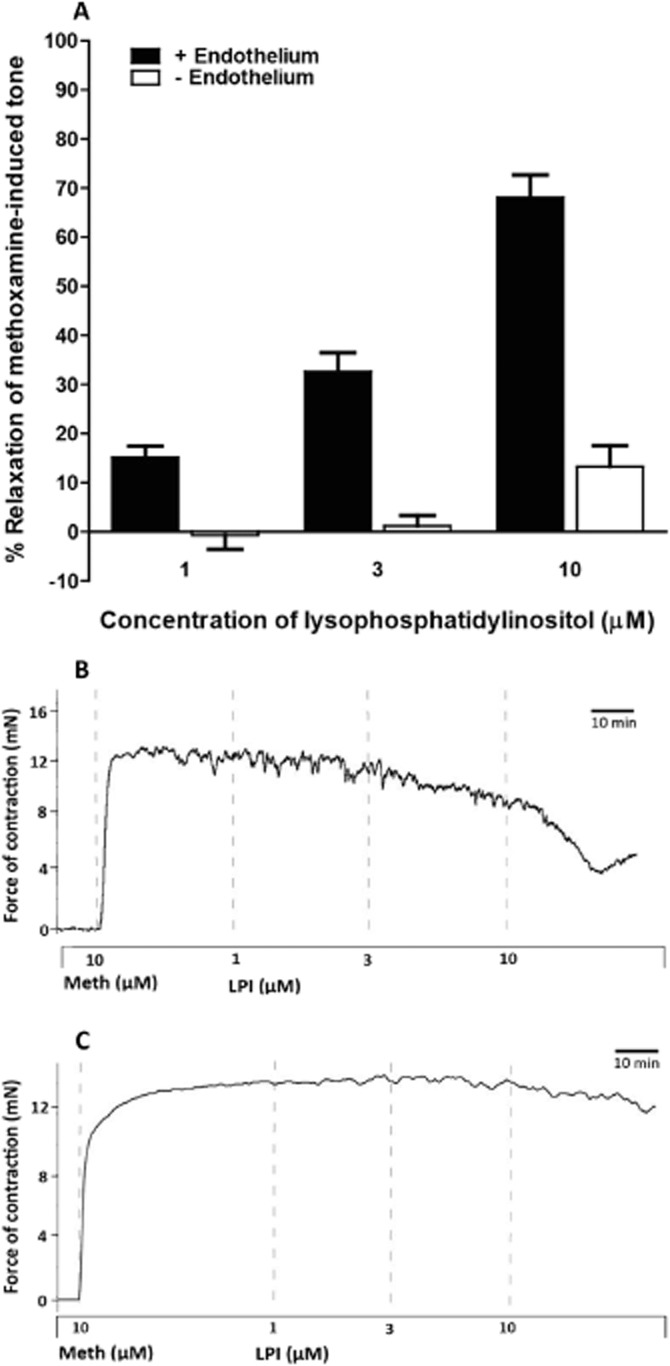
LPI-induced vasorelaxation of the rat small mesenteric artery precontracted with 10 μM methoxamine (Meth) in the presence and absence of the endothelium. (A) Relaxation was determined in the presence (n = 10) and absence (n = 6) of endothelium. Values are shown as means ± SEM. (B, C) Original traces demonstrating LPI-induced relaxation of rat small mesenteric arteries precontracted with methoxamine. (A) shows a vessel with an intact endothelium and (B) represents an endothelium-denuded vessel. Vertical lines denote addition of drugs at the concentrations indicated.
Effects of indomethacin and ODQ on vasorelaxation induced by LPI
In endothelium-intact vessels precontracted with methoxamine (see Figure 2A), omission of indomethacin (10 μM) from the bathing solution had no significant effect on vasorelaxation to LPI. Similarly, the selective inhibitor of NO-sensitive guanylyl cyclase, ODQ, at 10 μM did not inhibit the relaxation to LPI (Figure 2B).
Figure 2.
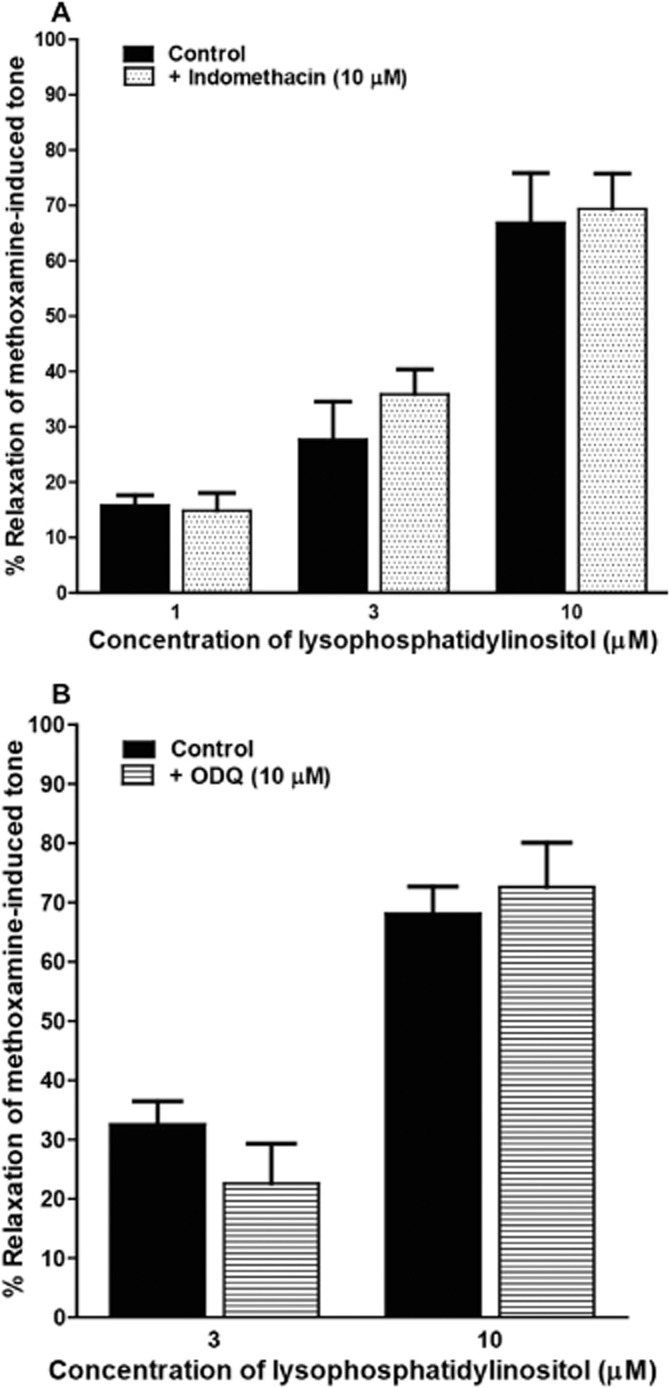
Concentration-dependent relaxation to LPI of methoxamine-induced tone in rat mesenteric resistance arteries. Responses were determined in the presence of functional endothelium. (A) Vessels were relaxed by LPI alone (n = 4), or in the presence of 10 μM indomethacin (n = 6). (B) Vessels were relaxed by LPI in the absence (n = 10) or presence (n = 4) of ODQ. Data are presented as means + SEM.
Effects of K+ channel blockers on LPI-induced vasorelaxation
In endothelium-intact vessels, precontraction with 60 mM KCl, instead of methoxamine, abolished the vasorelaxation to LPI over the concentration range used (n = 4; Figure 3A).
Figure 3.
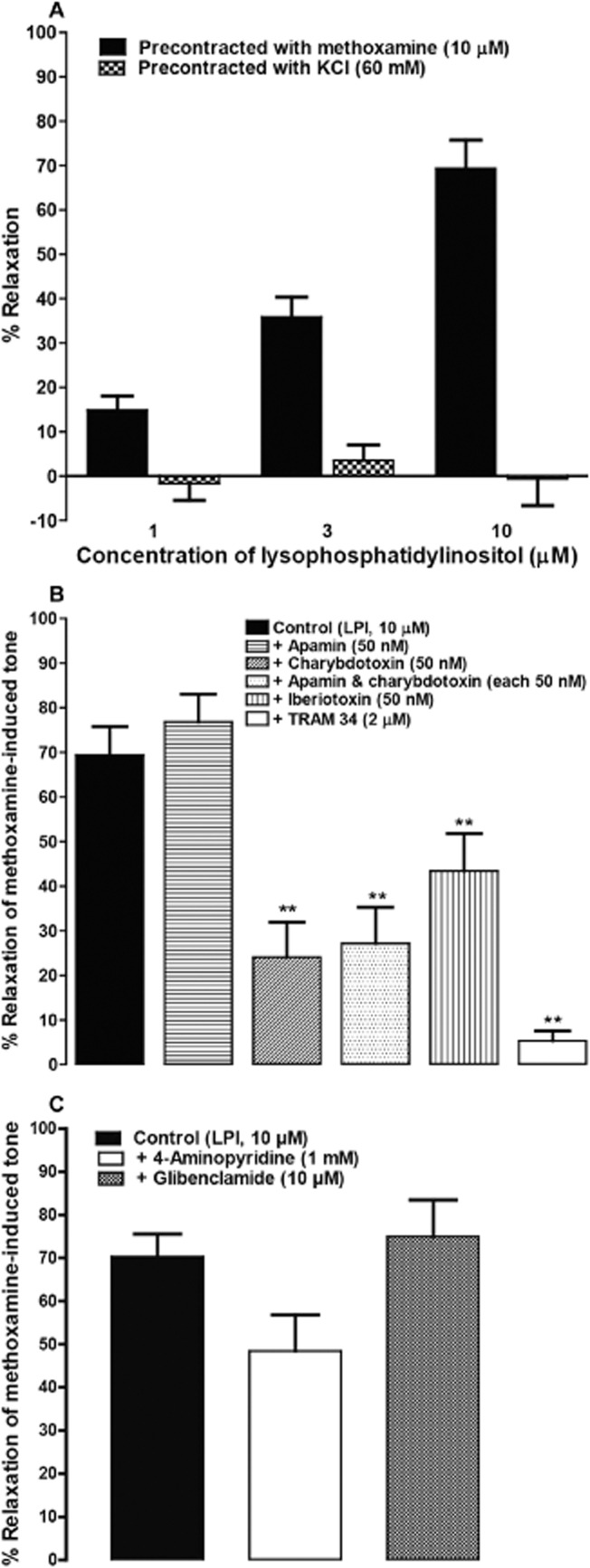
Role of K+ channels in LPI-induced vasorelaxation. (A) Relaxation by LPI of either methoxamine- or KCl-induced tone in rat isolated small mesenteric arteries with an intact endothelium. Tone was induced by either 10 μM methoxamine (n = 7) or 60 mM KCl (n = 6). (B) Relaxation was produced by LPI either alone (n = 7) or in the presence of either apamin (n = 4), charybdotoxin (n = 4), apamin plus charybdotoxin (n = 4), iberiotoxin (n = 4) or TRAM 34 (n = 5). (C) Relaxation was elicited by LPI alone (n = 6), or in the presence of 4-aminopyridine (n = 6) or glibenclamide (n = 4). **P < 0.01, significantly different from control; one-way anova with Bonferroni's post hoc test. Data are presented as means ± SEM.
Therefore, we investigated the possible involvement of K+ channels in response to LPI. As shown in Figure 3B, pretreatment with apamin [a blocker of small conductance Ca2+-sensitive K+ channels (SKCa); 50 nM] had no significant effect on LPI-induced vasorelaxation. On the contrary, charybdotoxin [a blocker of intermediate (IKCa) and large (BKCa) conductance Ca2+-sensitive K+ channels; 50 nM], given alone or in combination with apamin, produced a marked reduction of the response. Figure 3B also indicates that TRAM 34 (a selective blocker of IKCa; 2 μM) attenuated the response, leaving only a very small residual relaxation. In addition, iberiotoxin (a blocker of BKCa; 50 nM) significantly inhibited the relaxation to LPI, whereas blockade of ATP-dependent K+ channels (KATP) by glibenclamide (10 μM) or voltage-dependent K+ channels (Kv) by 4-aminopyridine (1 mM) had no significant effect on the response (Figure 3C).
Effect of receptor antagonists on vasorelaxation to LPI
The CB1 receptor antagonist AM 251, used at 10 μM, had no noticeable effect on relaxation to LPI (Figure 4A). Furthermore, antagonists reported to act against anandamide at the ‘endothelial anandamide receptor’, rimonabant (3 μM) and O-1918 (10 μM), caused a significant enhancement in vasorelaxation to LPI (Figure 4B). In contrast, the newly introduced antagonist of GPR55, CID 16020046, significantly reduced the relaxation to 10 μM LPI (Figure 4C).
Figure 4.
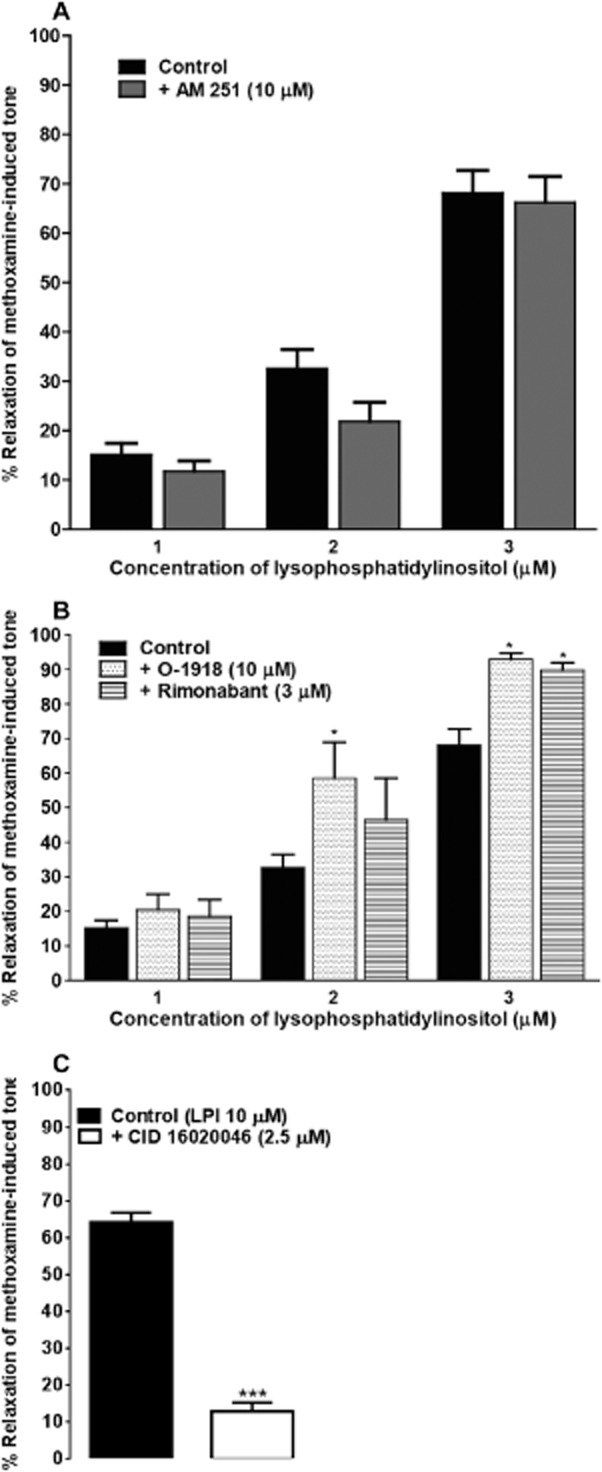
Relaxation by LPI of methoxamine-induced tone in rat small mesenteric arteries. All responses were determined in the presence of functional endothelium. (A) Vasorelaxation was elicited by LPI either alone (n = 10), or in the presence of AM 251 (n = 5). (B) Vasorelaxation was elicited by LPI alone (n = 10), or in the presence of either O-1918 (n = 4) or rimonabant (n = 8). (C) Vessels were relaxed by LPI alone (n = 4), or in the presence of 2.5 μM of the GPR55 antagonist CID 16020046 (n = 4). Data are presented as means + SEM. In (A) and (B), *P < 0.05, significantly different from control; two-way anova with Bonferroni's post hoc test. In (C), ***P < 0.001, significantly different from control; two-tailed Student's t-test.
Effects of 2-APB, U73122 and Y-27632 on relaxation to LPI
The downstream signalling cascade by which LPI initiates its effect was investigated by using selective blockers of PLC (U73122) and RhoA-ROCK (Rho-associated kinase) (Y-27632) as well as an antagonist of IP3 receptors [2-aminoethoxydiphenyl borate (2-APB)]. Figure 5 shows that vasorelaxation induced by LPI was largely reduced by U73122 (10 μM) and Y-27632 at 50 μM, and abolished by 100 μM 2-APB. In each case, care was taken to ensure that relaxation in the presence of an inhibitor was measured from a level of precontraction that was the same as that obtained in the test for endothelial integrity; if necessary, the concentration of the precontracting agent was increased to reach this level, or a combination of methoxamine and U46619 was used as stated in the Methods section. Generated force of contraction before and after exposure to inhibitors is given in the Supporting Information.
Figure 5.
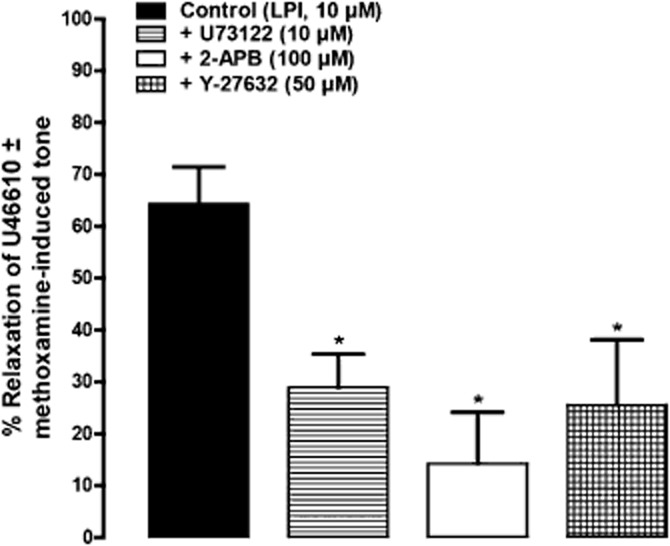
Involvement of the PLC-IP3 and RhoA-ROCK pathways in LPI-induced vasorelaxation of rat mesenteric resistance arteries. Vessels were precontracted with U46619 (3 μM) alone or in combination with methoxamine (10 μM). Relaxation was elicited by LPI alone (n = 6), or in the presence of U73122 (n = 8), 2-APB (n = 7) or Y-27632 (n = 5). *P < 0.05, significantly different from control; one-way anova with Dunnett's multiple comparison test. Data are shown as means + SEM.
Actions on endothelial cells isolated from the rat mesenteric arterial bed
The current study shows that LPI initiates vasorelaxation of rat small mesenteric arteries solely by endothelium-dependent mechanisms, probably involving elevation of intracellular Ca2+. We therefore attempted to further clarify this by conducting Ca2+ imaging studies in endothelial cells isolated from the same vessel. The characterization of the cells is described in the Supporting Information and AlSuleimani (2011). They had a very slow population doubling rate and they substantially changed their morphology by 6 days after isolation. Furthermore, trypsinization also stopped the cells dividing and changed their morphological characteristics. Therefore, isolated cells were grown in cultures for 3–5 days for use in experiments.
Single-cell calcium imaging
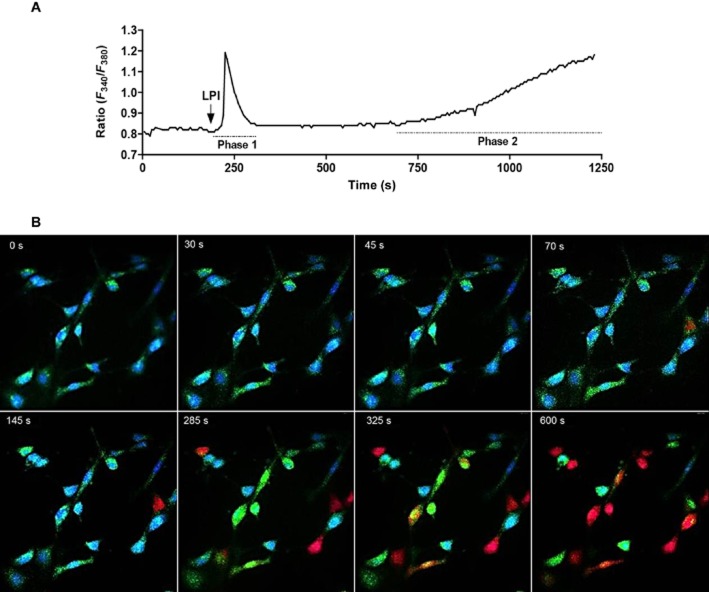
In Ca2+-free HBS, LPI (10 μM) evoked biphasic mobilization of cytosolic Ca2+ (Figure 6A). The initial phase was rapid (20 s after agonist stimulation) and transient (lasting for 100 s), whereas the late phase was characterized by a slow (observed 250 s after the first phase) and sustained Ca2+ elevation. Figure 6B shows images of the endothelial cells while being observed for Ca2+ signalling.
Figure 6.
Ca2+ signals evoked by 10 μM LPI in endothelial cells isolated from the rat mesenteric arterial bed. Responses were obtained in Ca2+-free HBS. (A) A representative recording from a single cell showing the characteristic biphasic Ca2+ mobilization. (B) Images of the endothelial cells at 40× while being recorded for Ca2+ signalling using the MetaFluor system. Blue indicates basal calcium levels, whereas green and red demonstrate the increase in cytosolic Ca2+. Zero seconds represents the point where LPI was added.
Effect of thapsigargin, U73122, 2-APB and Y-27632 on LPI-evoked Ca2+ signals
In Ca2+-free HBS, as noted earlier, the lysolipid initiated biphasic Ca2+ signals. Therefore, the effects of thapsigargin (inhibitor of sarcoplasmic reticulum Ca2+-ATPases), U73122 (PLC inhibitor), 2-APB (the IP3 receptor antagonist) and Y-27632 (RhoA-ROCK inhibitor) were assessed in both phases. Figure 7A shows that the initial phase of Ca2+ release was abolished by pretreating the cells with thapsigargin (1 μM), U73122 (10 μM) and 2-APB (100 μM). The figure also indicates that 50 μM Y-27632 significantly reduced the response. The late phase, on the contrary, was completely inhibited by thapsigargin, 2-APB and the ROCK inhibitor (Figure 7B). Interestingly, U73122 did not inhibit the late phase, instead, it shortened the time to peak (Figure 7C).
Figure 7.
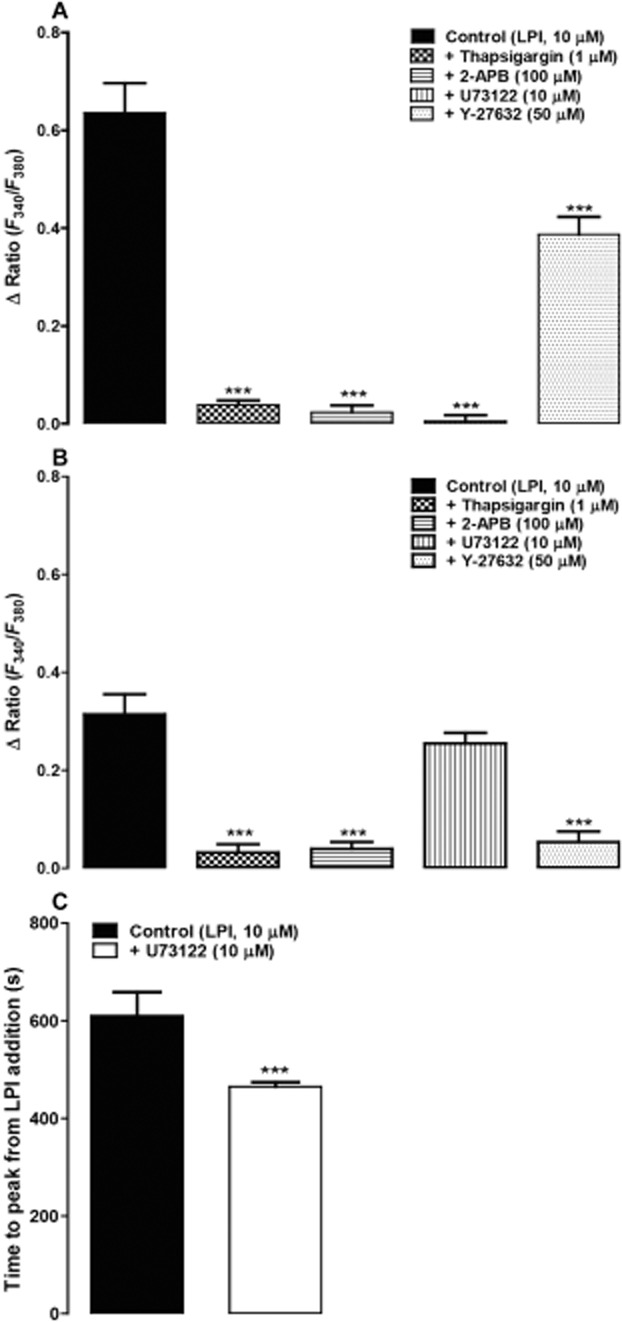
Ca2+ signals evoked by LPI in endothelial cells isolated from the rat mesenteric arterial bed. Responses were recorded in Ca2+-free HBS. (A) The initial rapid phase (control, n = 27) which was abolished by thapsigargin (n = 15), 2-APB (n = 14) and U73122 (n = 39). The response was also significantly reduced by Y-27632 (n = 43). The late slow phase (control, n = 42) as shown in (B) was attenuated by thapsigargin (n = 31), 2-APB (n = 14) and Y-27632 (n = 49), whereas U73122 (n = 48) did not reduce the peak response. (C) The time to peak of the late phase was significantly shortened by U73122 (control, n = 28; U73122, n = 39). Drugs were pre-incubated for 10–15 min before the addition of LPI and were present throughout the period of the experiment. n indicates the number of cells obtained from two to three independent isolations. Data are presented as means + SEM. In A and B, ***P < 0.001, significantly different from control; one-way anova with Bonferroni's post hoc test. In C, ***P < 0.001, significantly different from control; unpaired two-tailed t-test.
Expression profile of GPR55, CB1 and CB2 receptors
The expression of GPR55 as well as CB1 and CB2 receptors was investigated in the rat mesenteric artery using quantitative PCR. For comparison, the expression of these receptors was also assessed in extracts of rat testis. Melting curve analysis revealed single specific peaks beyond 80°C. Figure 8 shows that mRNA transcripts for all the receptors were detected in both tissues. In the artery, mRNA levels for GPR55 were similar to those for CB1 receptors, and there was about sixfold more expression of CB2 receptors as compared to other receptors. In addition, there was abundant expression of all the receptors in rat testis. Compared with the mesenteric artery, there was approximately a 1000 times more expression of GPR55 and CB1 receptors and nearly a 100-fold greater expression of CB2 receptors.
Figure 8.
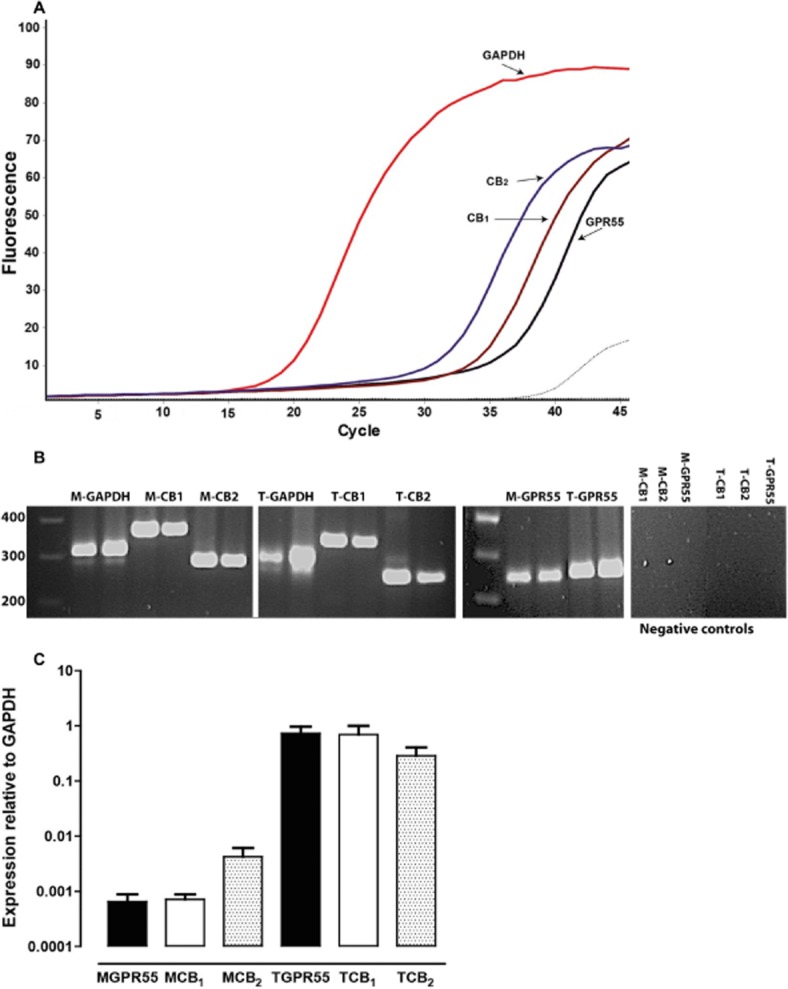
Expression of mRNA transcripts for GPR55, CB1 and CB2 receptors in the rat mesenteric artery. (A) Representative real-time PCR measurement of the mRNA levels in the rat mesenteric artery for a housekeeping gene (GAPDH), and for GPR55, the CB1 and the CB2 receptors. Each curve is an average of duplicate readings from the same isolation. The dashed lines represent samples where reverse transcriptase was omitted (negative control). (B) Real-time PCR products of specific gene fragments from two separate samples of mRNA isolated from mesenteric artery (M) and testis (T). The expected sizes of amplicons were 341 bp for rat GPR55, 357 bp for the rat CB1 receptor, 275 bp for the rat CB2 receptor and 307 bp for rat GAPDH. Also shown are negative control samples where RNA was not reverse-transcribed to cDNA. (C) Quantified mRNA expression levels in both rat mesenteric artery and testis. Values are presented as mean expression, relative to the housekeeping gene, + SEM.
Discussion
The present study demonstrates some of the vascular actions of LPI, which is considered to be an endogenous agonist at GPR55. GPR55, with GPR18 and GPR119, shares sensitivity with endogenous agonists, and some synthetic or natural exogenous ligands, which are active at cannabinoid receptors (Alexander et al., 2013a, p. 1519). Our investigation has shown that LPI relaxes resistance arteries and causes concentration-dependent relaxation of the rat small mesenteric artery that is mediated by an endothelial site of action. The mechanism involved elevation of intracellular Ca2+ through the PLC-IP3 and RhoA-ROCK pathways and subsequent activation of IKCa. In addition, the response showed some sensitivity to blockade of BKCa. LPI also decreased mean arterial pressure in anaesthetized rats. Moreover, this study has provided evidence for the expression of GPR55 as well as CB1 and CB2 receptors in the rat mesenteric artery.
Preliminary data (not shown here) showed that LPI (0.01–30 μM) had no contractile effects in rat small mesenteric arteries at resting tension. However, when the vessels were precontracted with methoxamine, LPI caused concentration-dependent relaxation, which was entirely endothelium-dependent. It is noteworthy that the concentrations of LPI used (1–10 μM) are far below the critical micellar concentration (∼75 μM; Falasca et al., 1995), ruling out physicochemical actions through a detergent-like effect. It might also be noted that the concentrations of LPI used in this study are generally higher than those used to stimulate GPR55 in recombinant systems. However, here we are investigating a receptor expressed at native levels, which are likely to be much less than those in cells induced to express the receptor, and increased receptor expression will increase the sensitivity of the system to agonists like LPI.
The vasorelaxation was not mediated by COX metabolites, as the presence or absence of indomethacin had no significant effect on the response. In addition, responses to LPI were unlikely to result from the formation of active metabolites as, when LPI was added to normal fibroblasts, it was metabolically stable and most of it was recovered unchanged after 20 h of incubation, with no significant conversion to metabolites (Falasca et al., 1998). It is also unlikely that the NO-cGMP pathway contributes to this vasorelaxation because the potent and selective inhibitor of NO-sensitive guanylyl cyclase, ODQ (10 μM; Garthwaite et al., 1995), did not inhibit the response. Thus, relaxation to LPI is mediated by mechanisms other than prostanoids and cGMP.
The next step in this study was to examine if the relaxation was elicited by K+ fluxes, such as underlie endothelium-dependent hyperpolarization. Precontraction of the vessel with high K+ solution abolished relaxation to LPI. As elevated extracellular K+ inhibits the electrochemical gradient for K+ efflux, this means that the LPI response is mediated by K+ channels, such as KCa channels. Apamin, which blocks SKCa, given alone, had no effect on the relaxation to LPI, whereas the response was sensitive to blockade by charybdotoxin (a blocker of IKCa and BKCa at the concentration used) given alone, or in combination with apamin. In addition, the highly selective blocker of IKCa, TRAM 34 (used at 100 times its Kd value; Wulff et al., 2000), abolished the relaxation. This collectively indicates that IKCa channels are the main target for LPI-induced vasorelaxation of rat small resistance mesenteric arteries. Our finding that there was a relaxation of ∼20%, resistant to charybdotoxin, whereas it was completely blocked by TRAM 34, may indicate that the concentration of charybdotoxin used was not sufficient to block all IKCa.
The physiological importance of IKCa in the vasculature is highlighted by the greater mean arterial BP recorded in IKCa knockout mice (Brahler et al., 2009). Furthermore, inhibition of IKCa alone abolished endothelial hyperpolarization in human mesenteric (Kohler et al., 2000) and rat cerebral arteries (Marrelli et al., 2003). Immunohistochemical approaches show IKCa channels to localize exclusively at the endothelial projections, which pass through the internal elastic lamina to come into contact with smooth muscle cells (Sandow et al., 2006; Dora et al., 2008; Ledoux et al., 2008). At these projections, ACh elicits an IP3-dependent rise in Ca2+ (see below) known as ‘Ca2+-pulses’, which are suggested to trigger activation of IKCa, and thus a hyperpolarization, which can spread to the underlying smooth muscle through myoendothelial gap junctions (Ledoux et al., 2008).
In addition to the major contribution of IKCa to the vasorelaxation evoked by LPI, the response was also sensitive to blockade of BKCa by iberiotoxin, although the magnitude of inhibition was less than that observed for charybdotoxin (44% for charybdotoxin and 25% for iberiotoxin). In support of this, Bondarenko et al. (2010) demonstrated that LPI caused GPR55-dependent elevation of intracellular Ca2+ in the human endothelial cell line EA.hy296 that was associated with activation of BKCa. They later reported that the lysolipid has a direct action to modulate the open probability of BKCa in endothelial cells (Bondarenko et al., 2011a). Thus, it is very likely that LPI, in addition to its initiation of receptor-mediated activation of IKCa, and probably BKCa, has a direct action on the BKCa in our preparations.
The CB1 cannabinoid receptor antagonist, AM 251, had no significant effect on the relaxation to LPI, showing that the response was not mediated by this receptor. However, it should be noted that AM 251 has been reported to be an agonist at GPR55 (Brown and Wise, 2001; Lauckner et al., 2008) but we have not observed relaxation to this drug in any of our studies on the rat mesenteric artery (data not shown). One reason for this apparent discrepancy may lie in the observation that the studies in which AM 251 has been reported to be an agonist were carried out with human receptors in expression systems (see Brown and Hiley, 2009). This contrasts with the current study of the actions of LPI at native rat receptors. That the receptor mediating relaxation in our preparations is GPR55 is supported by our observation that the newly discovered antagonist at GPR55, CID 16020046, inhibited the response.
It also has to be considered that two antagonists of the ‘endothelial anandamide receptor’, O-1918 and rimonabant, enhanced the vasorelaxation to LPI rather than either blocking it or having no effect. These agents were used here at concentrations that antagonized vasorelaxation to abnormal cannabidiol and anandamide in rat small mesenteric arteries, under conditions in which other CB1 and CB2 receptor antagonists had no effect on the relaxation to these two cannabinoids (White and Hiley, 1998; Begg et al., 2003; Offertaler et al., 2003). This also may indicate a dimerization, or other interaction, between the lysolipid site and the ‘endothelial anandamide receptor’. In this respect, Balenga et al. (2011) reported strong modulation of CB2 receptor-mediated responses in human blood neutrophils through pathways involving the small GTPases, Rac2 and Cdc42. It should be noted here that LPI has no direct activity at the two cannabinoid receptors, CB1 and CB2 (Yin et al., 2009).
The data in this study show that vasorelaxation to LPI was entirely endothelium-dependent and mainly involved activation of IKCa. In endothelial cells, activation of IKCa requires elevation of intracellular Ca2+ evoked by IP3 (Ledoux et al., 2008) and we therefore investigated the involvement of this pathway in the vasorelaxation to LPI. In fact, pretreatment with the PLC inhibitor, U73122 (Bleasdale et al., 1989; 1990,; Shi et al., 2008), significantly reduced the relaxation to the lipid, suggesting an intermediary role for the PLC-IP3 pathway. Indeed, the IP3 receptor antagonist, 2-APB, at a concentration sufficient to block methoxamine-induced vasoconstriction, abolished the response. Thus, LPI activated a receptor that was linked to PLC-IP3 to cause a rise in cytosolic Ca2+ with subsequent activation of IKCa. LPI-initiated Ca2+ signals mediated by GPR55 have been reported in several studies. Interestingly, inhibition of ROCKs, the immediate downstream targets of RhoA, by Y-27632, also reduced the vasorelaxation. However, the level of inhibition was less than that observed with the IP3 receptor antagonist, but the same as that when the PLC inhibitor was used, suggesting, perhaps, that more than one pathway is involved in the response. To test this, an attempt was made to use a combination of U73122 and Y-27632 in order to block both pathways; but it was not possible to constrict the vessels with U46619 or methoxamine, alone or in combination, under these conditions (data not shown). Nevertheless, it is clear that both the PLC and the RhoA-ROCK pathways are involved in the process of raising endothelial Ca2+. Previous studies have shown that Rho family GTPases can regulate and activate PLC (Seifert et al., 2004; Hains et al., 2006; Henstridge et al., 2009). In addition, RhoA-dependent pathways have been reported to be linked through Gα12/13 (Hart et al., 1998; Suzuki et al., 2003; Dutt et al., 2004), Gαq (Booden et al., 2002) and Gαi/o (Stahle et al., 2003). Moreover, in GPR55-expressing cells, LPI evokes RhoA-dependent Ca2+ signals (Henstridge et al., 2009).
To further clarify this at the cellular level, endothelial cells isolated from rat mesenteric arteries were subjected to single-cell Ca2+ imaging. In these cells, LPI induced a biphasic elevation of intracellular Ca2+. The initial transient rise was abolished by inhibition of the PLC-IP3 pathway and significantly reduced by inhibition of ROCKs. The late sustained phase was attenuated by the IP3 receptor antagonist and the ROCK inhibitor. Interestingly, this late phase was not affected by the PLC inhibitor; instead, the time to peak was significantly shortened. Since the compound U73122, which inhibits PLCβ and PLCγ isoforms (Heemskerk et al., 1997; Lockhart and McNicol, 1999), did not inhibit the later phase of the Ca2+ response, this may suggest the involvement of another isoform of PLC. In fact, there are six major families of PLC enzymes (PLCβ, PLCγ, PLCδ, PLCε, PLCζ and PLCη), which consist of at least 13 PLC isoforms, that have been found to be regulated by heterotrimeric G proteins coupled to GPCRs (Katan, 2005). Specifically, PLCβ isozymes, which regulate inositol lipid signalling, are activated by Gαq (Waldo et al., 2010). More importantly, PLCε is the only known isoform that is directly activated by Rho, the downstream target of Gα12/13 (Wing et al., 2003; Katan, 2005). As the late phase was also abolished by Y-27632, the inhibitor of the kinase p160ROCK that is the direct downstream target of RhoA (Ishizaki et al., 1997; Tominaga et al., 1998), this may suggest that PLCε (which is regulated by RhoA) is the possible target in this phase. Further studies by using specific inhibitors of this PLC isoform are required to confirm this. Moreover, whether or not p160ROCK has direct regulatory activity on PLC, or indirectly through RhoA, is open to investigation. A similar pattern of responses was also observed in human brain microvascular endothelial cells (results not shown). Thus, it is very likely that elevation of cytosolic Ca2+ in endothelial cells is responsible for the observed vasorelaxation induced by LPI.
Collectively, these findings provide evidence for a novel endothelial site through which LPI induced vasorelaxation. It is interesting to consider if this site is the G protein-coupled receptor, GPR55. mRNA transcripts for GPR55 are present in these resistance arteries. Moreover, the presence of the receptor in the mesenteric vasculature has recently been confirmed using fluorescent ligand binding (Daly et al., 2010). In addition, expression of GPR55 has been reported in cultured human endothelial cells (Waldeck-Weiermair et al., 2008). The physiological role of GPR55 in the vasculature has been reported only in a few studies. In an unpublished talk to the Oxford Meeting of the British Pharmacological Society in December 2006, P.J. Greasley from AstraZeneca reported that GPR55 knockout mice were hypertensive. In addition, results from a later study also indicated that, in GPR55-deficient mice, basal mean arterial pressure was higher than that of wild-type group (Johns et al., 2007).
With reference to LPI, in nearly all the currently published studies on GPR55, the lipid always activates the receptor. Oka et al. (2007) suggested that LPI is the endogenous ligand for GPR55, as it induced rapid phosphorylation of ERK, elicited Ca2+ transients and stimulated GTPγS binding in GPR55-expressing cells. Lauckner et al. (2008) similarly reported that it elicited Ca2+ transients upon binding to GPR55 in large dorsal root ganglion neurons. In human endothelial cells, LPI activates GPR55 and elicits Ca2+ transients (Waldeck-Weiermair et al., 2008). Thus, these studies support the contention that LPI is the ligand for GPR55.
Conclusions
Overall, all these findings lead to the conclusion that LPI may target GPR55 in the rat small mesenteric artery and the consequent vasodilatation may be the basis of the decrease in mean arterial BP observed on systemic administration of the lipid. The relaxation is endothelium-dependent and the signalling cascade involves both PLC and RhoA and activation of IKCa channels. Such a complex process might explain why the relaxant response is slow to develop, certainly when compared to that for ACh.
Acknowledgments
The authors thank Professor Colin Taylor (Department of Pharmacology, University of Cambridge) and his laboratory members for their great help with Ca2+ imaging. We are grateful to Mr Ahmed Al-Mahrouqi (Department of Pharmacology and Clinical Pharmacy, Sultan Qaboos University) for his help with the in vivo experiments and Dr Vanessa Ho (Division of Basic Medical Sciences, St George's University of London, London, UK) for providing LPI for in vivo experiments. We are also very grateful to Sultan Qaboos University for the financial support.
Glossary
- 2-APB
2-aminoethoxydiphenyl borate
- BKCa
large conductance Ca2+-activated K+ channels
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HBS
HEPES-buffered saline
- IKCa
intermediate conductance Ca2+-activated K+ channels
- KCa
Ca2+-activated K+ channels
- LPI
lysophosphatidylinositol
- MAECs
mesenteric arterial bed endothelial cells;
- ODQ
1H–[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- R10 μM
mean vessel relaxation of induced tone achieved at the highest concentration of agonist used
- ROCKs
Rho-associated kinases
- SKCa
small conductance Ca2+-activated K+ channels
Author contributions
Y. M. A. did the experiments and wrote the original manuscript. C. R. H. supervised the study, contributed to the experimental design. and corrected and revised the manuscript both grammatically and scientifically.
Conflicts of interest
The authors declare no conflicts of interest.
Supporting Information
Figure S1 (A) LPI (1 mg·kg−1) was given i.v. to rats anaesthetized with pentobarbital (60 mg·kg−1). The effect of the vehicle (saline; 0.2 mL) is also shown and it was tested in same rat as LPI. (B) Maximum decrease in mean arterial pressure observed after LPI administration. Values are shown as means and vertical lines represent the SEM (n = 7). **Significantly different from the control value (P < 0.01).
Figure S2 Characteristics of isolated endothelial cells from rat mesenteric artery. (A) Confocal images (top panel 63×) of: (1) Arterial endothelial cells, from the rat mesenteric bed, positively staining for von Willebrand factor (vWF) and a negative control field where only secondary antibody was used (2). (3) Primary human umbilical vein endothelial cells immunostained for vWF, used as a positive control. Bottom panel (40×): (4) Rat mesenteric artery smooth muscle cells immunostained for α-actin, whereas the endothelial cells isolated from the same vessel did not stain (5). Corresponding bright-field images are also shown. (B) Representative recording of carbachol-evoked cytosolic Ca2+ signals in endothelial cells isolated from rat mesenteric arteries. The trace also shows that treatment with atropine for 5 min abolished the response to carbachol. Responses were recorded in Ca2+-free HBS. Fluorescence readings (F340 and F380) were calibrated to [Ca2+]i.
Table S1 Methoxamine ± U46619-induced tone in the absence and presence of 2-APB, U73122 or Y-27632.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G Protein-Coupled Receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013b;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ion Channels. Br J Pharmacol. 2013c;170:1607–1651. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AlSuleimani YM. 2011. PhD thesis, University of Cambridge, Cambridge, UK An investigation into the actions of novel lipid signalling molecules in the vasculature.
- AlSuleimani YM, Hiley CR. Mechanisms of vasorelaxation induced by oleoylethanolamide in the rat small mesenteric artery. Eur J Pharmacol. 2013;702:1–11. doi: 10.1016/j.ejphar.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Balenga NA, Aflaki E, Kargl J, Platzer W, Schroder R, Blattermann S, et al. GPR55 regulates cannabinoid 2 receptor-mediated responses in human neutrophils. Cell Res. 2011;21:1452–1469. doi: 10.1038/cr.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg M, Mo FM, Offertaler L, Batkai S, Pacher P, Razdan RK, et al. G protein-coupled endothelial receptor for atypical cannabinoid ligands modulates a Ca2+-dependent K+ current. J Biol Chem. 2003;278:46188–46194. doi: 10.1074/jbc.M307258200. [DOI] [PubMed] [Google Scholar]
- Bleasdale JE, Bundy GL, Bunting S, Fitzpatrick FA, Huff RM, Sun FF, et al. Inhibition of phospholipase C dependent processes by U-73,122. Adv Prostaglandin Thromboxane Leukot Res. 1989;19:590–593. [PubMed] [Google Scholar]
- Bleasdale JE, Thakur NR, Gremban RS, Bundy GL, Fitzpatrick FA, Smith RJ, et al. Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophils. J Pharmacol Exp Ther. 1990;255:756–768. [PubMed] [Google Scholar]
- Bondarenko AI. Endothelial atypical cannabinoid receptor: do we have enough evidence? Br J Pharmacol. 2014;171:5573–5588. doi: 10.1111/bph.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondarenko A, Waldeck-Weiermair M, Naghdi S, Poteser M, Malli R, Graier WF. GPR55-dependent and -independent ion signalling in response to lysophosphatidylinositol in endothelial cells. Br J Pharmacol. 2010;161:308–320. doi: 10.1111/j.1476-5381.2010.00744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondarenko AI, Malli R, Graier WF. The GPR55 agonist lysophosphatidylinositol acts as an intracellular messenger and bidirectionally modulates Ca2+-activated large-conductance K+ channels in endothelial cells. Pflugers Arch. 2011a;461:177–189. doi: 10.1007/s00424-010-0898-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondarenko AI, Malli R, Graier WF. The GPR55 agonist lysophosphatidylinositol directly activates intermediate-conductance Ca2+-activated K+ channels. Pflugers Arch. 2011b;462:245–255. doi: 10.1007/s00424-011-0977-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booden MA, Siderovski DP, Der CJ. Leukemia-associated Rho guanine nucleotide exchange factor promotes G alpha q-coupled activation of RhoA. Mol Cell Biol. 2002;22:4053–4061. doi: 10.1128/MCB.22.12.4053-4061.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahler S, Kaistha A, Schmidt VJ, Wolfle SE, Busch C, Kaistha BP, et al. Genetic deficit of SK3 and IK1 channels disrupts the endothelium-derived hyperpolarizing factor vasodilator pathway and causes hypertension. Circulation. 2009;119:2323–2332. doi: 10.1161/CIRCULATIONAHA.108.846634. [DOI] [PubMed] [Google Scholar]
- Brown AJ, Hiley CR. Is GPR55 an anandamide receptor? Vitam Horm. 2009;81:111–137. doi: 10.1016/S0083-6729(09)81005-4. [DOI] [PubMed] [Google Scholar]
- Brown AJ, Wise A. 2001. Identification of modulators of GPR55 activity. GlaxoSmithKline Patent No. WO0186305.
- Daly C, Ross R, Whyte J, Henstridge C, Irving A, McGrath J. Fluorescent ligand binding reveals heterogeneous distribution of adrenoceptors and ‘cannabinoid-like’ receptors in small arteries. Br J Pharmacol. 2010;159:787–796. doi: 10.1111/j.1476-5381.2009.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dora KA, Gallagher NT, McNeish A, Garland CJ. Modulation of endothelial cell KCa3.1 channels during endothelium-derived hyperpolarizing factor signaling in mesenteric resistance arteries. Circ Res. 2008;102:1247–1255. doi: 10.1161/CIRCRESAHA.108.172379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutt P, Nguyen N, Toksoz D. Role of Lbc RhoGEF in Gα12/13-induced signals to Rho GTPase. Cell Signal. 2004;16:201–209. doi: 10.1016/s0898-6568(03)00132-3. [DOI] [PubMed] [Google Scholar]
- Falasca M, Silletta MG, Carvelli A, Di Francesco AL, Fusco A, Ramakrishna V, et al. Signalling pathways involved in the mitogenic action of lysophosphatidylinositol. Oncogene. 1995;10:2113–2124. [PubMed] [Google Scholar]
- Falasca M, Iurisci C, Carvelli A, Sacchetti A, Corda D. Release of the mitogen lysophosphatidylinositol from H-Ras-transformed fibroblasts; a possible mechanism of autocrine control of cell proliferation. Oncogene. 1998;16:2357–2365. doi: 10.1038/sj.onc.1201758. [DOI] [PubMed] [Google Scholar]
- Ford LA, Roelofs AJ, Anavi-Goffer S, Mowat L, Simpson DG, Irving AJ, et al. A role for L-α-lysophosphatidylinositol and GPR55 in the modulation of migration, orientation and polarization of human breast cancer cells. Br J Pharmacol. 2010;160:762–771. doi: 10.1111/j.1476-5381.2010.00743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao M, Hattori Y, Kanno M, Sakuma I, Kitabatake A. Structural differences in the ability of lysophospholipids to inhibit endothelium-dependent hyperpolarization by acetylcholine in rat mesenteric arteries. Biochem Biophys Res Commun. 1996;227:479–483. doi: 10.1006/bbrc.1996.1532. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Southam E, Boulton CL, Nielsen EB, Schmidt K, Mayer B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Mol Pharmacol. 1995;48:184–188. [PubMed] [Google Scholar]
- Hains MD, Wing MR, Maddileti S, Siderovski DP, Harden TK. Gα12/13- and rho-dependent activation of phospholipase C-ε by lysophosphatidic acid and thrombin receptors. Mol Pharmacol. 2006;69:2068–2075. doi: 10.1124/mol.105.017921. [DOI] [PubMed] [Google Scholar]
- Hart MJ, Jiang X, Kozasa T, Roscoe W, Singer WD, Gilman AG, et al. Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Gα13. Science. 1998;280:2112–2114. doi: 10.1126/science.280.5372.2112. [DOI] [PubMed] [Google Scholar]
- Heemskerk JW, Farndale RW, Sage SO. Effects of U73122 and U73343 on human platelet calcium signalling and protein tyrosine phosphorylation. Biochim Biophys Acta. 1997;1355:81–88. doi: 10.1016/s0167-4889(96)00113-9. [DOI] [PubMed] [Google Scholar]
- Henstridge CM, Balenga NA, Ford LA, Ross RA, Waldhoer M, Irving AJ. The GPR55 ligand L-alpha-lysophosphatidylinositol promotes RhoA-dependent Ca2+ signaling and NFAT activation. FASEB J. 2009;23:183–193. doi: 10.1096/fj.08-108670. [DOI] [PubMed] [Google Scholar]
- Henstridge CM, Balenga NA, Schroder R, Kargl JK, Platzer W, Martini L, et al. GPR55 ligands promote receptor coupling to multiple signalling pathways. Br J Pharmacol. 2010;160:604–614. doi: 10.1111/j.1476-5381.2009.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiley CR, Ford WR. Cannabinoid pharmacology in the cardiovascular system: potential protective mechanisms through lipid signalling. Biol Rev. 2004;79:187–205. doi: 10.1017/s1464793103006201. [DOI] [PubMed] [Google Scholar]
- Hiley CR, Kaup SS. GPR55 and the vascular receptors for cannabinoids. Br J Pharmacol. 2007;152:559–561. doi: 10.1038/sj.bjp.0707421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoi PM. 2007. PhD thesis, University of Cambridge, Cambridge, UKCannabinoid receptor pharmacology in the rat small mesenteric artery.
- Hoi PM, Hiley CR. Vasorelaxant effects of oleamide in rat small mesenteric artery indicate action at a novel cannabinoid receptor. Br J Pharmacol. 2006;147:560–568. doi: 10.1038/sj.bjp.0706643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki T, Naito M, Fujisawa K, Maekawa M, Watanabe N, Saito Y, et al. p160ROCK, a Rho-associated coiled-coil forming protein kinase, works downstream of Rho and induces focal adhesions. FEBS Lett. 1997;404:118–124. doi: 10.1016/s0014-5793(97)00107-5. [DOI] [PubMed] [Google Scholar]
- Jensen PE. Calphostin C-sensitive enhancements of force by lysophosphatidylinositol and diacylglycerols in mesenteric arteries from the rat. Br J Pharmacol. 1996;119:15–22. doi: 10.1111/j.1476-5381.1996.tb15671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns DG, Behm DJ, Walker DJ, Ao Z, Shapland EM, Daniels DA, et al. The novel endocannabinoid receptor GPR55 is activated by atypical cannabinoids but does not mediate their vasodilator effects. Br J Pharmacol. 2007;152:825–831. doi: 10.1038/sj.bjp.0707419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katan M. New insights into the families of PLC enzymes: looking back and going forward. Biochem J. 2005;39:7–9. doi: 10.1042/BJ20051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler R, Degenhardt C, Kuhn M, Runkel N, Paul M, Hoyer J. Expression and function of endothelial Ca2+-activated K+ channels in human mesenteric artery: a single-cell reverse transcriptase-polymerase chain reaction and electrophysiological study in situ. Circ Res. 2000;87:496–503. doi: 10.1161/01.res.87.6.496. [DOI] [PubMed] [Google Scholar]
- Lauckner JE, Jensen JB, Chen HY, Lu HC, Hille B, Mackie K. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc Natl Acad Sci U S A. 2008;105:2699–2704. doi: 10.1073/pnas.0711278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledoux J, Taylor MS, Bonev AD, Hannah RM, Solodushko V, Shui B, et al. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc Natl Acad Sci U S A. 2008;105:9627–9632. doi: 10.1073/pnas.0801963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JC, Kania KD, Wijesuriya H, Chawla S, Sethi JK, Pulaski L, et al. Activation of beta-catenin signalling by GSK-3 inhibition increases p-glycoprotein expression in brain endothelial cells. J Neurochem. 2008;106:1855–1865. doi: 10.1111/j.1471-4159.2008.05537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart LK, McNicol A. The phospholipase C inhibitor U73122 inhibits phorbol ester-induced platelet activation. J Pharmacol Exp Ther. 1999;289:721–728. [PubMed] [Google Scholar]
- Marrelli SP, Eckmann MS, Hunte MS. Role of endothelial intermediate conductance KCa channels in cerebral EDHF-mediated dilations. Am J Physiol Heart Circ Physiol. 2003;285:1590–1599. doi: 10.1152/ajpheart.00376.2003. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton E, Ho V. 2013. Investigating the role of the putative cannabinoid receptor GPR55 in vascular control in mice. Available at: http://www.pa2online.org/abstracts/vol11issue1abst016.pdf (accessed 30/03/2015)
- Offertaler L, Mo FM, Batkai S, Liu J, Begg M, Razdan RK, et al. Selective ligands and cellular effectors of a G protein-coupled endothelial cannabinoid receptor. Mol Pharmacol. 2003;63:699–705. doi: 10.1124/mol.63.3.699. [DOI] [PubMed] [Google Scholar]
- Oka S, Nakajima K, Yamashita A, Kishimoto S, Sugiura T. Identification of GPR55 as a lysophosphatidylinositol receptor. Biochem Biophys Res Commun. 2007;362:928–934. doi: 10.1016/j.bbrc.2007.08.078. [DOI] [PubMed] [Google Scholar]
- Oka S, Toshida T, Maruyama K, Nakajima K, Yamashita A, Sugiura T. 2-Arachidonoyl-sn-glycero-3-phosphoinositol: a possible natural ligand for GPR55. J Biochem. 2009;145:13–20. doi: 10.1093/jb/mvn136. [DOI] [PubMed] [Google Scholar]
- Oka S, Kimura S, Toshida T, Ota R, Yamashita A, Sugiura T. Lysophosphatidylinositol induces rapid phosphorylation of p38 mitogen-activated protein kinase and activating transcription factor 2 in HEK293 cells expressing GPR55 and IM-9 lymphoblastoid cells. J Biochem. 2010;147:671–678. doi: 10.1093/jb/mvp208. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucleic Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosker C, Meur G, Taylor EJ, Taylor CW. Functional ryanodine receptors in the plasma membrane of RINm5F pancreatic beta-cells. J Biol Chem. 2009;284:5186–5194. doi: 10.1074/jbc.M805587200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandow SL, Neylon CB, Chen MX, Garland CJ. Spatial separation of endothelial small- and intermediate-conductance calcium-activated potassium channels (KCa) and connexins: possible relationship to vasodilator function? J Anat. 2006;209:689–698. doi: 10.1111/j.1469-7580.2006.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos CF, Caprio MA, Oliveira EB, Salgado MC, Schippers DN, Munzenmaier DH, et al. Functional role, cellular source, and tissue distribution of rat elastase-2, an angiotensin II-forming enzyme. Am J Physiol Heart Circ Physiol. 2003;285:775–783. doi: 10.1152/ajpheart.00818.2002. [DOI] [PubMed] [Google Scholar]
- Seifert JP, Wing MR, Snyder JT, Gershburg S, Sondek J, Harden TK. RhoA activates purified phospholipase C-epsilon by a guanine nucleotide-dependent mechanism. J Biol Chem. 2004;279:47992–47997. doi: 10.1074/jbc.M407111200. [DOI] [PubMed] [Google Scholar]
- Shi TJ, Liu SX, Hammarberg H, Watanabe M, Xu ZQ, Hokfelt T. Phospholipase Cβ3 in mouse and human dorsal root ganglia and spinal cord is a possible target for treatment of neuropathic pain. Proc Natl Acad Sci U S A. 2008;105:20004–20008. doi: 10.1073/pnas.0810899105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahle M, Veit C, Bachfischer U, Schierling K, Skripczynski B, Hall A, et al. Mechanisms in LPA-induced tumor cell migration: critical role of phosphorylated ERK. J Cell Sci. 2003;116:3835–3846. doi: 10.1242/jcs.00679. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Nakamura S, Mano H, Kozasa T. Gα12 activates Rho GTPase through tyrosine-phosphorylated leukemia-associated RhoGEF. Proc Natl Acad Sci U S A. 2003;100:733–738. doi: 10.1073/pnas.0234057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga T, Ishizaki T, Narumiya S, Barber DL. p160ROCK mediates RhoA activation of Na-H exchange. EMBO J. 1998;17:4712–4722. doi: 10.1093/emboj/17.16.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JA, Varga K, Jarai Z, Kunos G. Mesenteric vasodilation mediated by endothelial anandamide receptors. Hypertension. 1999;33:429–434. doi: 10.1161/01.hyp.33.1.429. [DOI] [PubMed] [Google Scholar]
- Waldeck-Weiermair M, Zoratti C, Osibow K, Balenga N, Goessnitzer E, Waldhoer M, et al. Integrin clustering enables anandamide-induced Ca2+ signaling in endothelial cells via GPR55 by protection against CB1-receptor-triggered repression. J Cell Sci. 2008;121:1704–1717. doi: 10.1242/jcs.020958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldo GL, Ricks TK, Hicks SN, Cheever ML, Kawano T, Tsuboi K, et al. Kinetic scaffolding mediated by a phospholipase C-β and Gq signaling complex. Science. 2010;330:974–980. doi: 10.1126/science.1193438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R, Hiley CR. The actions of the cannabinoid receptor antagonist, SR 141716A, in the rat isolated mesenteric artery. Br J Pharmacol. 1998;125:689–696. doi: 10.1038/sj.bjp.0702127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing MR, Snyder JT, Sondek J, Harden TK. Direct activation of phospholipase C-ε by Rho. J Biol Chem. 2003;278:41253–41258. doi: 10.1074/jbc.M306904200. [DOI] [PubMed] [Google Scholar]
- Wulff H, Miller MJ, Hansel W, Grissmer S, Cahalan MD, Chandy KG. Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: a potential immunosuppressant. Proc Natl Acad Sci U S A. 2000;97:8151–8156. doi: 10.1073/pnas.97.14.8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Chu A, Li W, Wang B, Shelton F, Otero F, et al. Lipid G-protein-coupled receptor ligand identification Using β-arrestin PathHunter assay. J Biol Chem. 2009;284:12328–12338. doi: 10.1074/jbc.M806516200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Maor Y, Wang JF, Kunos G, Groopman JE. Endocannabinoid-like N-arachidonoyl serine is a novel pro-angiogenic mediator. Br J Pharmacol. 2010;160:1583–1594. doi: 10.1111/j.1476-5381.2010.00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 (A) LPI (1 mg·kg−1) was given i.v. to rats anaesthetized with pentobarbital (60 mg·kg−1). The effect of the vehicle (saline; 0.2 mL) is also shown and it was tested in same rat as LPI. (B) Maximum decrease in mean arterial pressure observed after LPI administration. Values are shown as means and vertical lines represent the SEM (n = 7). **Significantly different from the control value (P < 0.01).
Figure S2 Characteristics of isolated endothelial cells from rat mesenteric artery. (A) Confocal images (top panel 63×) of: (1) Arterial endothelial cells, from the rat mesenteric bed, positively staining for von Willebrand factor (vWF) and a negative control field where only secondary antibody was used (2). (3) Primary human umbilical vein endothelial cells immunostained for vWF, used as a positive control. Bottom panel (40×): (4) Rat mesenteric artery smooth muscle cells immunostained for α-actin, whereas the endothelial cells isolated from the same vessel did not stain (5). Corresponding bright-field images are also shown. (B) Representative recording of carbachol-evoked cytosolic Ca2+ signals in endothelial cells isolated from rat mesenteric arteries. The trace also shows that treatment with atropine for 5 min abolished the response to carbachol. Responses were recorded in Ca2+-free HBS. Fluorescence readings (F340 and F380) were calibrated to [Ca2+]i.
Table S1 Methoxamine ± U46619-induced tone in the absence and presence of 2-APB, U73122 or Y-27632.