Abstract
Background and Purpose
Endocannabinoids are a family of lipid mediators involved in the regulation of gastrointestinal (GI) motility. The expression, localization and function of their biosynthetic enzymes in the GI tract are not well understood. Here, we examined the expression, localization and function of the enzyme diacylglycerol lipase-α (DAGLα), which is involved in biosynthesis of the endocannabinoid 2-arachidonoylglycerol (2-AG).
Experimental Approach
Cannabinoid CB1 receptor-deficient, wild-type control and C3H/HeJ mice, a genetically constipated strain, were used. The distribution of DAGLα in the enteric nervous system was examined by immunohistochemistry. Effects of the DAGL inhibitors, orlistat and OMDM-188 on pharmacologically induced GI hypomotility were assessed by measuring intestinal contractility in vitro and whole gut transit or faecal output in vivo. Endocannabinoid levels were measured by mass spectrometry.
Key Results
DAGLα was expressed throughout the GI tract. In the intestine, unlike DAGLβ, DAGLα immunoreactivity was prominently expressed in the enteric nervous system. In the myenteric plexus, it was colocalized with the vesicular acetylcholine transporter in cholinergic nerves. In normal mice, inhibiting DAGL reversed both pharmacologically reduced intestinal contractility and pharmacologically prolonged whole gut transit. Moreover, inhibiting DAGL normalized faecal output in constipated C3H/HeJ mice. In colons incubated with scopolamine, 2-AG was elevated while inhibiting DAGL normalized 2-AG levels.
Conclusions and Implications
DAGLα was expressed in the enteric nervous system of mice and its inhibition reversed slowed GI motility, intestinal contractility and constipation through 2-AG and CB1 receptor-mediated mechanisms. Our data suggest that DAGLα inhibitors may be promising candidates for the treatment of constipation.
Tables of Links
| TARGETS |
|---|
| Enzymesa2012 |
| DAGLα, diacylglycerol lipase α (DGLα) |
| DAGLβ, diacylglycerol lipase β (DGLβ) |
| FAAH, fatty acid amide hydrolase |
| MAGL, monoacylglycerol lipase |
| NOS |
| GPCRsb2012 |
| CB1 receptors |
| CB2 receptors |
| Ion channelsc2012 |
| TRPV1 channels |
| Transportersd2012 |
| VAChT, vesicular acetylcholine transporter (SLC18A3) |
| LIGANDS |
|---|
| 2-AG, 2-arachidonoylglycerol |
| Anandamide, N-arachidonoylethanolamide |
| AM251 |
| Bethanechol |
| JZL184 |
| Loperamide |
| Orlistat |
| Scopolamine |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,b,c,dAlexander et al., 2013a,b,c,d,,,).
Introduction
Endocannabinoids are a family of lipid mediators which are increasingly being recognized for their important intercellular and intracellular signalling functions (Piomelli, 2003; Di Marzo and De Petrocellis, 2012; Galve-Roperh et al., 2013). Classically, endocannabinoids act at the cannabinoid CB1 and CB2 receptors, the receptors for the psychotropic constituent of cannabis, Δ9-tetrahydrocannabinol (Howlett, 2002; Pertwee et al., 2010). The two best-described endocannabinoids are N-arachidonoylethanolamide (anandamide) and 2-arachidonoylglycerol (2-AG). They are synthesized from membrane lipids as they are required. The endocannabinoid system and the enzymes involved in their biosynthesis and degradation have been fully described in recent review articles (Dinh et al., 2002; Izzo and Sharkey, 2010; Ueda et al., 2010; Di Marzo, 2011; Di Marzo and De Petrocellis, 2012; Blankman and Cravatt, 2013).
Here, we have studied 2-AG and its biosynthesis. 2-AG is the most abundant endocannabinoid and its synthesis occurs following activation of phosphoinositide-specific phospholipase C which hydrolyzes inositol phospholipids producing diacylglycerol (DAG). The hydrolysis of DAG by the sn-1-selective DAG lipases α (DAGLα) and DAGLβ leads to the formation of 2-AG (Bisogno et al., 2003; Ueda et al., 2011; Blankman and Cravatt, 2013). DAGLα and DAGLβ are proteins of 1042 and 672 amino acids, respectively, and have extensive homology in their membrane and substrate binding domains. They have similar activities and their encoding genes are highly conserved between mouse and human. DAGLα cleaves equally well most fatty acids from the sn-1 position of DAGs, while DAGLβ has a slight preference for linoleic, oleic, arachidonic and stearic acid. In the adult mouse brain, DAGLα is much more abundant than DAGLβ and both are localized to the postsynaptic dendrite. This localization correlates with the postsynaptic synthesis of 2-AG, as a retrograde regulator of excitatory neurotransmission (Bisogno et al., 2003). In the small and large intestine of rats, mRNA for both DAGLα and DAGLβ is present, although the former was more abundant than the latter (Iannotti et al., 2013).
The distribution of endocannabinoids, their CB receptors, and their degradative enzymes, have been well characterized in the gastrointestinal (GI) tract (Izzo and Camilleri, 2008; Izzo and Sharkey, 2010; Abalo et al., 2012; Alhouayek and Muccioli, 2012). Under physiological conditions, endocannabinoids are produced, giving rise to a ‘tone’ that serves as a mechanism to limit basal GI motility, principally by regulating the release of ACh from enteric nerves (Pertwee, 2001; Izzo and Camilleri, 2008; Izzo and Sharkey, 2010). To date, however, there is no well-defined role for 2-AG in the regulation of GI motility and only little is known about the expression and localization of DAGLs in the enteric nervous system as modulators of GI motility. Because cannabinoid receptors reduce GI contractility and propulsive motility, we considered that by inhibiting the biosynthesis of their endogenous ligands, we might reduce the level of endocannabinoid tone and hence its braking action, thereby enhancing motility. In this study, we focused on the distribution and function of the biosynthetic enzyme DAGL because in contrast to N-acyl phosphatidylethanolamine PLD, inhibitors of DAGL are available and inhibiting DAGLα gives us the opportunity to study 2-AG tone as a potential regulator of GI motility.
Methods
Full details of specific methods are found in the Supporting Information Appendix S1. In general, all assays were conducted in a blinded fashion and the data were generated by an investigator unaware of the treatments administered.
Animals
All animal care and experimental procedures complied with the guidelines of the Canadian Council of Animal Care. and were approved by the University of Calgary Animal Care Committee. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A total of 151 mice were used in the experiments described here.
Male C57BL/6N wild-type (WT) mice were supplied by Charles River (Montreal, QC, Canada) and CB1 receptor-deficient mice (CB1−/−) were bred at the University of Calgary mouse breeding facility as described previously (Storr etal., 2010). C3H/HeJ mice, which have a spontaneous mutation in TLR4 gene (Tlr4lps-d) and are chronically constipated (Anitha et al., 2012), and their control background mice, C3H/HeOuJ, (Jackson Laboratory, Bar Harbor, ME, USA), all at 5–12 weeks of age, were used in these studies.
Immunohistochemistry
Immunohistochemistry was performed as previously described (Duncan et al., 2008; Bashashati et al., 2012). Full details are found in the Supporting Information Appendix S1. Briefly, whole-mount preparations were incubated with the primary rabbit antibody against DAGLα or DAGLβ (1:50–1:500, optimal dilution 1:100) raised by Ken Mackie, Indiana University, Indianapolis, IN, USA (Katona et al., 2006; Jain et al., 2013) alone or in double-labelling experiments with the vesicular acetylcholine transporter (vAChT; Phoenix Pharmaceuticals Inc., Burlingame, CA, USA), substance P (Medicorp Inc., Montreal, QC, Canada) or neuronal NOS (Sigma-Aldrich Ltd., Oakville, ON, Canada). Positive DAGLα labelling was completely abolished by pre-absorption with 100 μg·mL−1 of the immunizing peptide.
Real-time PCR
Real-time PCR was performed as described previously (Bashashati et al., 2012). Full details are found in the Supporting Information Appendix S1. Briefly, TaqMan gene expression assay kit for the DAGLα gene (Mm00813830-m1; Applied Biosystems, Frederick, MD, USA) was used for the real-time PCR. Relative quantification (RQ) value was defined as fold changes in mRNA expression compared with a calibrator sample from the stomach and presented as mean ± SEM of RQ values in each group.
Assessment of intestinal contractility in vitro
Assessment of intestinal contractility was performed as previously described (Storr et al., 2010; Bashashati et al., 2012). Briefly, longitudinal segments of the ileum and distal colon of mice were studied. Electrical field stimulation (EFS) was applied and the effects of cumulative concentrations of the DAGL inhibitors (orlistat or OMDM-188; 1–5 μM; 15 min apart) on contractile responses to EFS were investigated. In additional experiments, the tension generated by bethanechol (10 μM) in the absence of and at a maximum concentration of the DAGL inhibitors used for the EFS experiments were compared.
In separate sets of experiments, cumulative concentrations of the muscarinic antagonist scopolamine (1–100 nM) or the μ-opioid receptor agonist loperamide (10–100 nM) were added to the organ bath. Scopolamine and loperamide concentration-dependently decreased EFS-induced contractility. To see if inhibiting DAGL or CB1 receptor antagonism could reverse the effect of scopolamine or loperamide on EFS contractility, orlistat (5 μM), OMDM-188 (1 μM) or AM251 (100 nM) were added to the organ bath 15 min before the first concentration of scopolamine or loperamide.
Before the addition of drugs, the mean of three successive EFS contractions were used as the internal control (100%). Also, vehicle controls were performed for all experiments. The amplitude of contractions in the presence of the compounds was reported as the percentage of the internal control.
GI transit studies in vivo
Whole gut transit was performed as described (Storr et al., 2010). Briefly, mice were acclimated for 60 min in individual transparent plastic cages without bedding. Orlistat (1 mg·kg−1) or OMDM-188 (1 mg·kg−1) were injected i.p. Twenty minutes later, 200 μL of an Evans blue (5% Evans blue; Sigma-Aldrich, St. Louis, MO, USA; 5% gum arabic; Sigma-Aldrich, USA) marker was gavaged into the stomach. The time taken to detect Evans blue in the faeces (in min) was recorded and presented as the whole gut transit time.
The direct effect of 2-AG on GI motility has not been well investigated. In a previous study, we showed that inhibiting monoacylglycerol lipase (MAGL) attenuates whole gut transit in mice (Duncan et al., 2008). Therefore, in the current study, we tested the effect of 2-AG (10 mg·kg−1, i.p.) on whole gut transit in the presence and absence of the MAGL inhibitor JZL184 (18 mg·kg−1, i.p.) in WT and in CB1 gene-deficient mice.
In preliminary experiments, we established that scopolamine (0.5 mg·kg−1, i.p.) or loperamide (0.5 mg·kg−1, i.p.) significantly delayed whole gut transit in the mouse. To see if the inhibition of DAGL could ameliorate the slowed GI transit in these models, mice were pretreated with scopolamine or loperamide followed 20 min later by the gavage of Evans blue. Twenty minutes before or 2 h after the gavage of marker, orlistat (1 mg·kg−1) or OMDM-188 (1 mg·kg−1) were injected i.p. and subsequently mice were monitored for coloured stools. To see if the potential effects of DAGL inhibitors are mediated through the CB1 receptors, CB1−/− mice were used as control for each set of experiments.
Treatment with the DAGL inhibitors 2 h after the gavage gave us a dynamic range for demonstrating the effects of these compounds because vehicle 2 h after the gavage did not speed up the presence of coloured stool (whole gut transit time: 203.6 ± 14.0 min) compared with animals treated with vehicle before gavage (whole gut transit time: 185 ± 9.4 min) (t-test; P value > 0.05) and loperamide or scopolamine consistently increased the transit time to more than 200 min.
Faecal output studies
C3H/HeJ mice and their background mice, C3H/HeOuJ were injected with orlistat or OMDM-188 (1 mg·kg−1, i.p.) or their vehicle. After 20 min, the animals were transferred to separate cages without any bedding and covered with lids. Faecal pellets were collected every 15 min and weighed immediately for a total duration of 1 h for each mouse. Total stool weight was calculated and the result was presented as the percentage of stool weight in vehicle-treated background mice.
Identification and quantification of endocannabinoids
Ileal or colonic tissues ((distal colon) from WT mice were incubated with orlistat (5 μM), OMDM-188 (1 μM) or vehicle for 15 min. In some experiments, the tissues were collected at this point. In another set of experiments, cumulative concentrations of scopolamine (1, 10 and 100 nM; 15 min intervals between consecutive concentrations) were added to the bath and the tissues were collected at 60 min. This protocol was designed to mimic the organ bath contractility studies described earlier.
Additionally, to see if the DAGL inhibitors alter the levels of endocannabinoids in vivo, we collected and analysed intestinal tissues (as described above) from C3H/HeJ and C3H/HeOuJ mice 45 min after treatment with orlistat, OMDM-188 (1 mg·kg−1, i.p.) or vehicle.
Collected tissues (60 mg of ileum; 50 mg of colon) were homogenized in 0.6 mL of chloroform/methanol/Tris HCl 50 mM (2:1:1) containing 10 pmol of d8-anandamide and 50 pmol of d5-2-AG per sample and extracted with 0.6 ml of chloroform four times. The lipid-containing organic phase was dried down under nitrogen, weighed, and pre-purified by open-bed chromatography on silica gel (pore size 60 Å, 70–230 mesh, Sigma Aldrich) mini-columns. Fractions were obtained by eluting the column with 99:1, 90:10 and 50:50 (v/v) chloroform/methanol. The 90:10 fraction was used for AEA and 2-AG quantification by LC/MS using an HPLC Shimadzu (LC10ADVP; Kyoto, Japan) coupled to a single quadrupole mass spectrometer (LCMS2020) equipped with an APCI interface, as previously described and using selected ion monitoring (SIM) at M + 1 values for the two compounds and their deuterated homologues, as described by Di Marzo et al., (2001). Briefly, AEA and 2-AG were separated using a Discovery C18 column (15 cm × 4.6 mm; I.D. 5 μm; Supelco) and eluted with an isocratic flow of methanol:water (85:15, 0.1% acetic acid). LC-10ADVP HPLC pumps were used to deliver solvent at a flow rate of 1ml/min. Samples were injected in 10 μl using an autosampler (SIL-lOADvp). Ions were generated using a capillary voltage of 4.5 kV and APCI temperature was set at 400°C. The levels of the compounds were expressed as pmol g−1 (anandamide) or nmol g−1 (2-AG) of wet tissue weight.
Data analysis
Data are presented as mean ± SEM of experiments. One or two-way anova followed by appropriate post hoc tests were used for comparison of more than two means.
Materials
For the organ bath intestinal contractility studies and the endocannabinoid measurements, stock solutions (0.01 M) of the DAGL inhibitors OMDM-188 and orlistat (Cayman Chemical, Ann Arbor, MI, USA) and the CB1 receptor antagonist AM251 (Tocris, Ellisville, MO, USA) were prepared in DMSO and the muscarinic antagonist scopolamine hydrobromide, loperamide and bethanechol (all from Sigma-Aldrich) were dissolved in water.
For the in vivo experiments, the animals were treated with 2-AG (Tocris), the MAGL inhibitor JZL184 (Tocris), OMDM-188, orlistat, scopolamine or loperamide dissolved in normal saline containing 2.5% DMSO and 2.5% Tween 80.
The IC50 values of OMDM-188 and orlistat (tetrahydrolipstatin) for inhibiting DAGLα are 0.016 and 1 μM respectively. The concentrations of OMDM-188 (1 μM) and orlistat (5 μM) for inhibiting DAGL in the organ bath, and of JZL184 (16 mg·kg−1) for the in vivo experiments, were selected based published data (Ortar et al., 2008; Min et al., 2010; Zhang et al., 2011).
Results
DAGLα is present in the enteric nervous system of mice
DAGLα immunoreactivity was detected as pericellular labelling surrounding unlabelled enteric neurons in the myenteric plexus of mouse ileum and colon (Figure 1). This pericellular labelling was present around many, but not all, myenteric neurons. Punctate labelling was also observed on muscle cells throughout the circular muscle. No immunoreactivity to DAGLβ was observed in either the ileum or the colon. Double-labelling revealed that DAGLα colocalizes to a large extent with the vAChT (Figure 1A), but only to a minimal extent with either substance P or NOS immunoreactivity in both the ileum (Figure 1A) and colon (Figure 1B).
Figure 1.
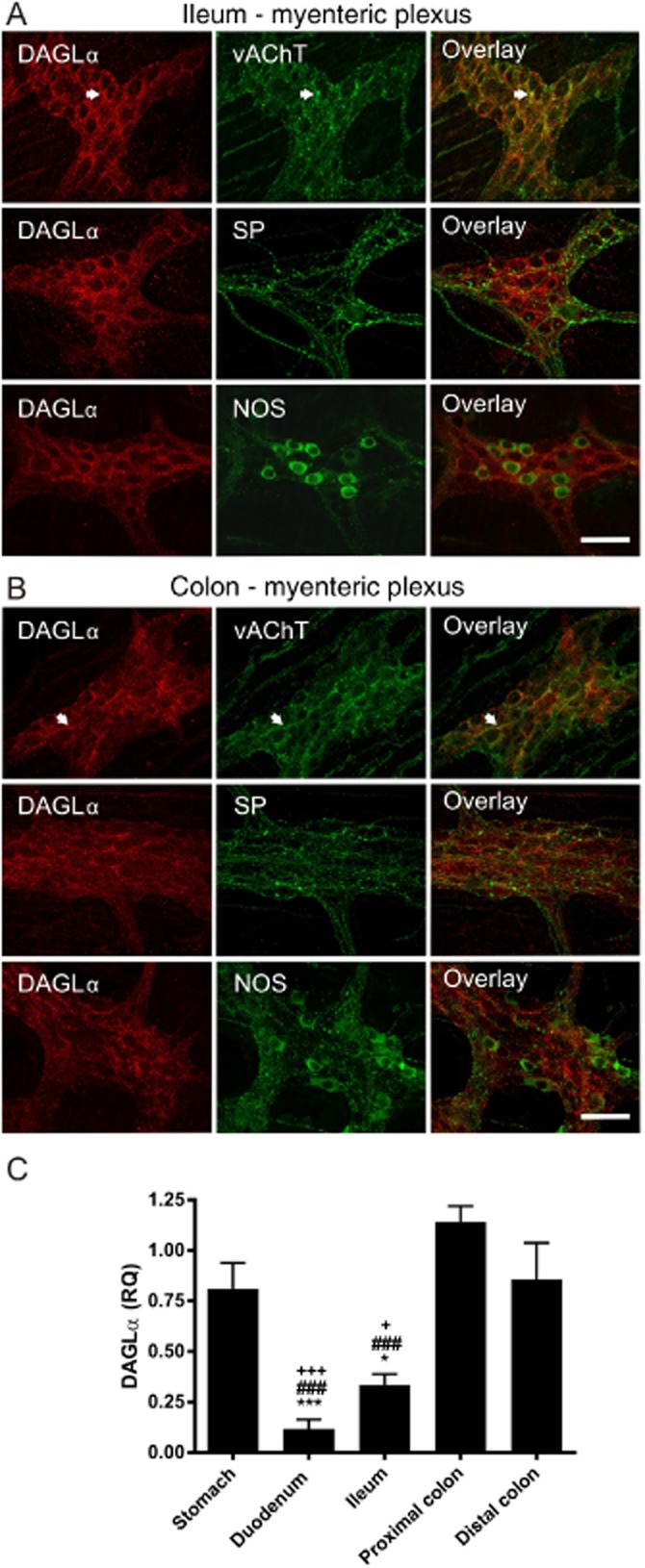
DAGLα immunoreactvity and mRNA expression in the mouse GI tract. DAGLα immunoreactvity is present in punctate terminals and nerve processes surrounding enteric neurons in the myenteric plexus of the ileum (A) and colon (B). DAGLα colocalizes with the vAChT in the myenteric plexus (arrowheads) but only to a minimal extent with either substance P (SP) or NOS immunoreactivity in both ileum and colon. Scale bars: 50 μM. DAGLα mRNA is expressed throughout the GI tract (C). The relative expression is significantly lower in the duodenum and ileum compared with the colon and stomach. n = 3–5 mice per group. F (degrees of freedom): F (4,15) = 20.34, P < 0.001; one-way anova: Bonferroni post hoc test. *P < 0.05, ***P < 0.001, compared with stomach; ###P < 0.001, compared with proximal colon; +P < 0.05, +++P < 0.001, compared with distal colon.
To confirm that the DAGLα gene was expressed in the GI tract, we performed real-time PCR. Real-time PCR showed that DAGLα mRNA was expressed throughout the length of the mouse GI tract, with the highest levels of expression in the stomach and colon (Figure 1C).
2-AG regulates GI motility in vivo
To examine if 2-AG has any effect on GI transit, we tested the effects of exogenous 2-AG in vivo. 2-AG had no effects on whole gut transit when administered alone, but in the presence of JZL184, which inhibits its metabolic inactivation, transit was dramatically slowed (Figure 2). The effects of JZL184 either alone or together with 2-AG were absent in CB1−/− mice, suggesting that they are mediated by CB1 receptors.
Figure 2.
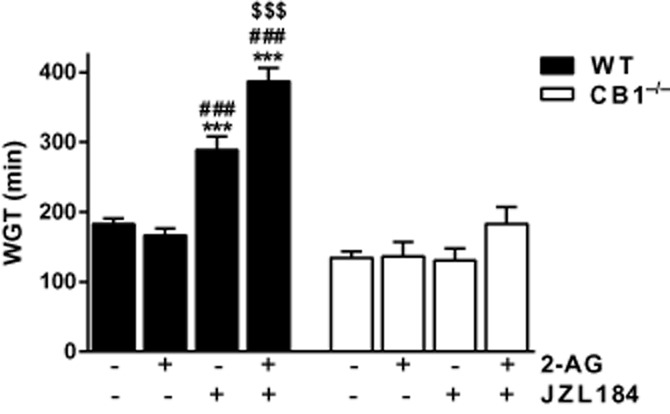
Effects of 2-AG and the MAGL inhibitor JZL184 on whole gut transit (WGT) in mice. 2-AG (10 mg·kg−1, i.p.) did not change WGT in WT or CB1−/− mice. However, JZL184 (18 mg·kg−1, i.p.) prolonged WGT and enhanced the effect of 2-AG in the WT but not the CB1−/− mice. n = 4–14 mice per group; F (degrees of freedom) for interaction: F (3,56) = 13.62, P < 0.001; two-way anova; Bonferroni post hoc test. ***P < 0.001, compared with WT vehicle; ###P < 0.001, compared with WT 2-AG alone; $$$P < 0.001, compared with WT JZL184 alone.
DAGL inhibitors do not change baseline intestinal contractility or motility
We next examined whether inhibiting DAGL activity would alter ileal and colonic contractility in vitro or whole gut transit in vivo under basal conditions. Incubation of the ileal and colonic preparations with either orlistat or OMDM-188 did not have any effect on EFS-induced contractility (EFS: 100.1 ± 0.6% and 95.3 ± 2.1% of initial contraction in the ileum and colon with orlistat and 97.9 ± 2.6% and 96.2 ± 8.5% of initial contraction in the ileum and colon with OMDM-188 up to 5 μM respectively). Similarly, these drugs had no effect on bethanechol-induced contractility (99.2 ± 3.4% and 106.1 ± 8.3% of initial bethanechol contraction in the ileum and colon with orlistat and 103.6 ± 6.9% and 97.7 ± 17.9% of initial bethanechol contraction in the ileum and colon with OMDM-188 up to 5 μM respectively). Consistent with these data, i.p. injection of orlistat (1 mg·kg−1) or OMDM-188 (1 mg·kg−1) did not alter whole gut transit in WT mice (188.0 ± 32.5 min after orlistat and 191.7 ± 15.9 min after OMDM-188 treatment vs. 203.6 ± 14.0 min in controls, P > 0.05).
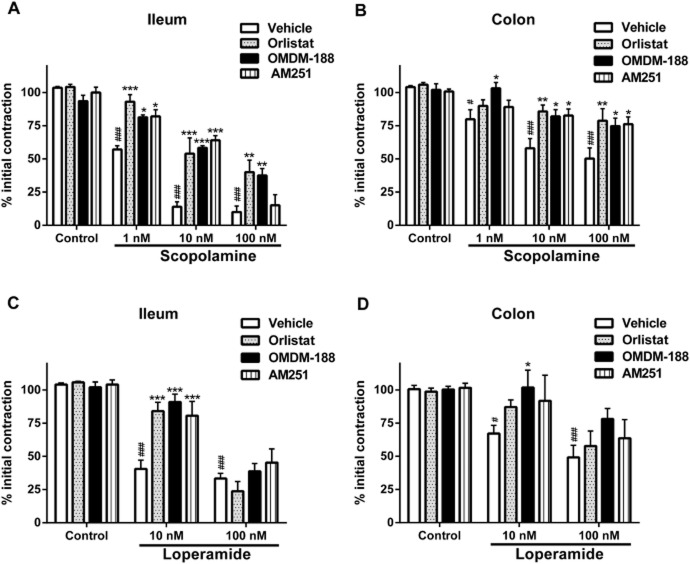
Inhibiting DAGL partly reverses the effects of scopolamine and loperamide on ileal and colonic contractility in vitro
It has become clear that the endocannabinoid system plays an important role in conditions where motility is disturbed (Izzo and Camilleri, 2008; Izzo and Sharkey, 2010). We therefore wondered whether blocking the synthesis of 2-AG when motility was reduced would reveal an action of this ligand. We first reduced contractility in the organ bath using scopolamine (1–100 nM) or loperamide (10–100 nM), both of which inhibited EFS-induced contractility in the ileum and colon of WT mice. The DAGL inhibitors (OMDM-188 1 μM and orlistat 5 μM) significantly reversed the effects of both scopolamine (10–100 nM) and loperamide (10 nM) on the ileal and colonic EFS-induced contractility (Figure 3). Furthermore, incubating tissues with the CB1 receptor antagonist AM251 (100 nM) reversed the effects of both scopolamine and loperamide to about the same extent as the DAGL inhibitors (Figure 3), suggesting that an endocannabinoid ligand acting at CB1 receptors was acting to limit contractility. Importantly, AM251, similar to the two DAGL inhibitors, was inactive in unstimulated preparations (Figure 3) despite it being an inverse agonist, suggesting that only after stimulation by scopolamine or loperamide, are the endogenous 2-AG levels sufficiently increased to inhibit contractility so that the inhibition of its synthesis/action can result in an effect.
Figure 3.
Effects of DAGL inhibitors on pharmacologically inhibited EFS contractility of the mouse ileum (A and C) and colon (B and D). Scopolamine (A and B) or loperamide (C and D) were used at the doses indicated to reduce contractility. Inhibiting DAGL with either orlistat (5 μM) or OMDM-188 (1 μM) reversed the effects of scopolamine or loperamide on the EFS contractility. The CB1 inverse agonist, AM251 (100 nM), also reversed the inhibitory effect of scopolamine and loperamide on the EFS contractility. n = 3–8 mice per group; # and ###P < 0.05 and P < 0.001, respectively, control vehicle compared with scopolamine vehicle or loperamide vehicle; F (degrees of freedom) for interaction of panel A: F (9,40) = 4.63, P < 0.001; panel B: F (9,48) = 1.61, P > 0.05 [scopolamine treatment: F (3,48) = 25.16, P < 0.001, DAGL inhibitors F(3,48) = 7.81, P < 0.001]; panel C: F (6,33) = 3.88, P < 0.01 and panel D: F(6,30) = 0.74, P > 0.05 [loperamide treatment: F (2,30) = 17.32, P < 0.001, DAGL inhibitors: F (3,30) = 2.70, P = 0.06). two-way anova; Bonferroni post hoc test. *P < 0.05, **P < 0.01, ***P < 0.001, compared with respective vehicle at each concentration.
Inhibiting DAGL normalizes drug-induced GI transit in vivo
These intriguing data led us to conduct an in vivo transit study. Scopolamine (0.5 mg·kg−1, i.p.) and loperamide (0.5 mg·kg−1, i.p.) substantially slowed whole gut transit in WT and CB1−/− mice. Basal whole gut transit was slightly, but not statistically significantly, faster in the CB1−/− mice compared with WT controls in agreement with the in vitro AM251 data obtained above. Orlistat (1 mg·kg−1) and OMDM-188 (1 mg·kg−1) given 2 h after Evans blue reversed the effects of both scopolamine and loperamide on whole gut transit in WT mice, but had no effects in CB1−/− mice (Figure 4). When the DAGL inhibitors were given 20 min before loperamide or scopolamine, they were minimally effective (data not shown).
Figure 4.
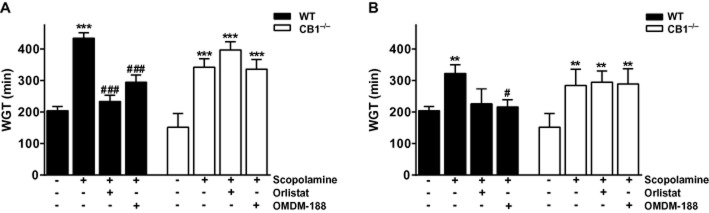
Effects of DAGL inhibitors on whole gut transit (WGT). Mice were treated with scopolamine (A, 0.5 mg·kg−1, i.p.) or loperamide (B, 0.5 mg·kg−1, i.p.) that significantly reduced WGT (increasing the transit time) in the WT or the CB1−/− mice. Orlistat (1 mg·kg−1, i.p.) or OMDM-188 (1 mg·kg−1, i.p.) reversed the effect of scopolamine and loperamide on WGT in the WT but not the CB1−/− mice. Note that neither orlistat nor OMDM-188 had any effect on transit when given alone in untreated animals. n = 4–12 mice per group; F (degrees of freedom) for interaction of panel A: F (3,55) = 11.15, P < 0.001 and panel B: F (3,36) = 4.32, P < 0.05); two-way anova; Bonferroni post hoc test. **P < 0.01, ***P < 0.001 compared with vehicle in WT or CB1−/− mice; #P < 0.05, ###P < 0.001, compared with scopolamine or loperamide in WT mice.
Inhibiting DAGL increases faecal output in C3H/HeJ mice
As described earlier (Anitha et al., 2012), C3H/HeJ mice had significantly lower faecal output compared with the background strain C3H/HeOuJ mice. Inhibiting DAGL, particularly with OMDM-188, increased faecal output in the C3H/HeJ mice, but had no significant effects in the C3H/HeOuJ mice (Figure 5).
Figure 5.
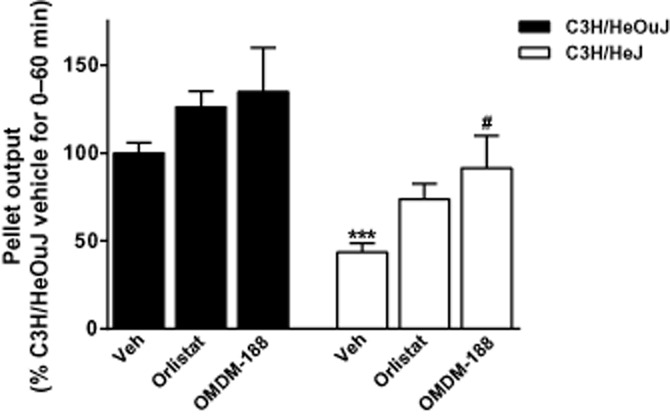
Effects of DAGL inhibitors on faecal output of C3H/HeJ and the background C3H/HeOuJ mice. Monitoring the faecal output for 1 h revealed that C3H/HeJ mice had significantly less output compared with the background strain mice. ***P < 0.001; two-way anova; Bonferroni post hoc test. OMDM-188 (1 mg·kg−1, i.p.) but not orlistat (1 mg·kg−1, i.p.) significantly enhanced faecal output of the constipated C3H/HeJ mice; n = 8–19 mice per group. #P < 0.05, compared with faecal output of vehicle-treated C3H/HeJ mice; two-way anova; Bonferroni post hoc test; (F (degrees of freedom) for interaction: F (2,86) = 0.19, P > 0.05 [genotype: F (1,86) = 33.79, P < 0.001, treatment: F (2,86) = 7.83, P < 0.001]).
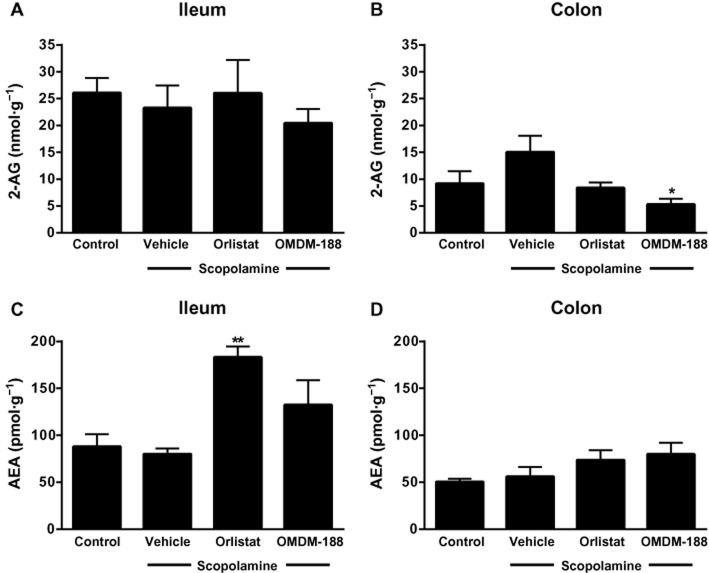
Inhibiting DAGL differentially reduces 2-AG levels in the mouse ileum and colon
We measured the levels of 2-AG and anandamide in the ileum and colon to assess if we could detect changes caused by DAGL inhibitors in vitro. Orlistat (5 μM) and OMDM-188 (1 μM) given alone did not change either 2-AG or anandamide levels in the ileum or colon under basal conditions, in agreement with their lack of effect on EFS contractility under these conditions (Figure 6). Scopolamine tended to increase 2-AG levels in the colon, but not the ileum (Figure 6). In the scopolamine-treated colon, orlistat tended to decrease, and OMDM-188 clearly reduced, 2-AG levels (Figure 6). Neither compound altered 2-AG levels in the scopolamine-treated ileum. Finally, orlistat produced an unexpected rise in anandamide levels in scopolamine-treated ileum, but not in the colon (Figure 6). However, the anandamide : 2-AG ratio (scopolamine alone: 4.47 ± 0.32) was significantly increased in the colon after both orlistat (8.78 ± 0.31) and OMDM-188 (13.58 ± 1.01) treatments (anova, F (3,7) = 17.08, P < 0.01).
Figure 6.
Effects of DAGL inhibitors on intestinal endocannabinoid levels in WT mice. Pre-incubation of the colon with scopolamine (1–100 nM) induced a tendency towards an increase in 2-AG levels and the DAGL inhibitors orlistat (5 μM) and OMDM-188 (1 μM) reversed this effect. Inhibiting DAGL increased anandamide (AEA) levels in the ileum. n = 3–5 mice per group; F (degrees of freedom) for panel A: F(3,13) = 0.43, P > 0.05; panel B: F (3,9) = 4.23, P < 0.05; panel C: F (3,11) = 9.73, P < 0.01 and panel D: F (3,9) = 1.36, P > 0.05; one-way anova; Bonferroni post hoc test. *P < 0.05. **P < 0.01, compared with vehicle.
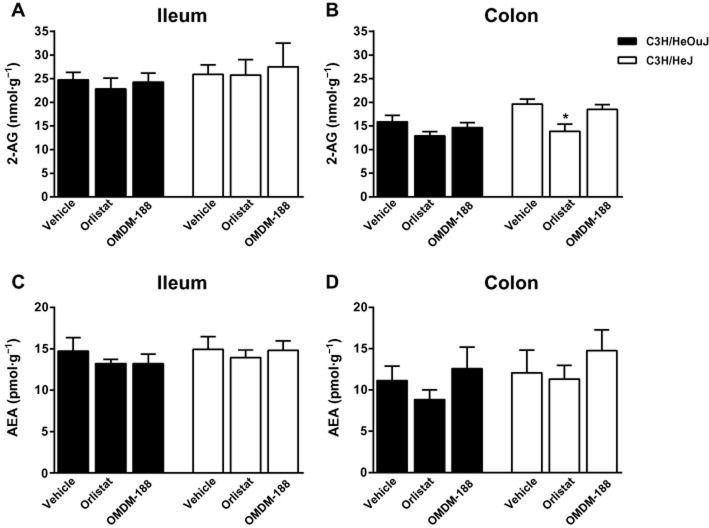
Based on the in vivo experiments, constipated C3H/HeJ mice tended to have a higher colonic levels of 2-AG compared with the background C3H/HeOuJ mice, and orlistat (1 mg·kg−1) significantly decreased 2-AG levels in the former group, suggesting that the enhanced faecal output in these mice after DAGL inhibition was mediated by endocannabinoid down-regulation (Figure 7).
Figure 7.
Effects of DAGL inhibitors on the intestinal endocannabinoid levels in C3H/HeOuJ and C3H/HeJ mice. 2-AG tended to be higher in the colon of C3H/HeJ compared with C3H/HeOuJ mice. P = 0.06. Orlistat significantly decreased 2-AG levels in the colon of C3H/HeJ mice. *P < 0.05 compared with vehicle in the C3H/HeJ group. The dose of orlistat and OMDM-188 was 1 mg·kg−1 i.p. n = 6–7 mice per group. F (degrees of freedom) for treatment (panel B): F(2,34) = 7.55, P < 0.01; two-way anova; Bonferroni post hoc test.
Discussion and conclusions
Complex and sophisticated neural mechanisms are involved in the regulatory control of GI motility and our understanding of these neural mechanisms has advanced considerably over the last 20 years with the discoveries of lipid mediators, including the endocannabinoids (Izzo and Camilleri, 2008; Kasparek et al., 2008; Izzo and Sharkey, 2010). A role for endocannabinoids in the regulation of GI motility has become more relevant with the discovery of (i) single nucleotide polymorphisms such as a functional variant in the fatty acid amide hydrolase (FAAH) gene (C385A) which is associated with irritable bowel syndrome-diarrhoea symptoms and acceleration of colonic transit (Camilleri et al., 2008) and (ii) pharmacogenomic studies such as one indicating that mutation of the CB1 receptor gene (rs 806378) modifies the effect of the non-selective cannabinoid agonist dronabinol on colonic phasic pressure activity in humans (Wong et al., 2011). Despite these findings and while there is considerable evidence for the expression and function of CB receptors in the GI tract, and the enzymic pathways of endocannabinoid degradation (Izzo and Sharkey, 2010), the sites of endocannabinoid biosynthesis remain uncertain. In the present study, we have shown that DAGLα, the principal enzyme for 2-AG biosynthesis, unlike DAGLβ, is localized in the enteric nervous system. We further demonstrated that 2-AG can regulate motility via CB1 receptors and in a manner controlled by MAGL. Finally, by inhibiting DAGL activity with a clinically available DAGL inhibitor, orlistat, and a specific DAGL inhibitor, OMDM-188, and thus potentially reducing 2-AG biosynthesis in the GI tract, we have identified a novel mechanism to enhance contractility in the small and large intestine and propulsive motility in three models of reduced GI transit.
Mechoulam et al. first isolated 2-AG from dog intestine and showed that this endogenous lipid mediator had cannabimimetic effects (Mechoulam et al., 1995). However, the role of 2-AG in the GI tract remains somewhat elusive because of its rapid metabolism. In the current study, when 2-AG hydrolysis was reduced by MAGL inhibition, whole gut transit time was prolonged. Adding exogenous 2-AG with the MAGL inhibitor further increased whole gut transit time through a CB1 receptor-mediated mechanism. These data extend previous reports that showed that MAGL was present in the enteric nervous system and that by inhibiting MAGL, whole gut transit in mice was slowed (Duncan et al., 2008). Using a mouse model of non-steroidal anti-inflammatory drug-induced gastric ulceration, Kinsey et al. demonstrated that blocking MAGL significantly reduced gastric damage and inflammatory cytokines, although exogenous 2-AG alone had no anti-inflammatory effects, very similar to the results we obtained with motility (Kinsey et al., 2013). In this study too, the effects of MAGL inhibition were mediated by CB1 receptors. Along similar lines, JZL184 administration protected mice against the development of acute colitis induced with trinitrobenzene sulphonic acid (Alhouayek et al., 2011). In this model, the actions of JZL184 were mediated by both CB1 and CB2 receptors, reflecting either regional differences or differences that are due to the pathophysiology of the gastric versus colonic inflammation.
In 2003, Bisogno et al. showed that DAGLα mRNA was expressed in the human and mouse GI tract (Bisogno et al., 2003). This has been extended recently by Iannotti et al. who demonstrated that DAGLα and DAGLβ mRNA was expressed in the rat GI tract (Iannotti et al., 2013). Using antibodies to these enzymes, the first evidence for the distribution of DAGLs was shown by Marquez et al. (2009). They showed DAGLα immunoreactivity on the apical surface of epithelial cells, on some plasma cells in the lamina propria and densely distributed in the muscularis mucosae and muscularis externa of the human colon. Numerous DAGLα positive nerve fibres were observed around unlabelled ganglion cells in the myenteric plexus. In this respect, the pattern of labelling was similar to our observations in the mouse myenteric plexus. In contrast to our findings in the mouse, they also demonstrated DAGLβ in the human colonic myenteric plexus, where it was localized in neurons and nerve fibres. The reason for these differences remains to be determined. We have extended these observations and showed that DAGLα is localized on cholinergic nerves and to a much lesser extent on those expressing NO synthase or substance P. In the case of the NOS, this is perhaps not too surprising, as these nerves do not seem to express any components of the endocannabinoid system (Coutts et al., 2002; MacNaughton et al., 2004). In contrast, because substance P is colocalized in excitatory cholinergic nerves in the enteric nervous system (Kunze and Furness, 1999) then we might have expected to see a greater degree of overlap in the immunoreactivity. This apparent disparity may reflect the fact that the majority of substance P immunoreactivity is in a different cellular domain to the membrane bound nature of the DAGLα and so overlap is not obvious. Further studies are needed to examine the subcellular localization of DAGLα in the enteric nervous system to clarify this matter.
The pericellular localization of DAGLα around enteric neurons is consistent with a postsynaptic source of the endocannabinoid 2-AG in the enteric nervous system. Recently, a detailed electrophysiological analysis was performed on neurons of the mouse myenteric plexus (Hons et al., 2012). This study revealed endocannabinoid-mediated retrograde transmission in the enteric nervous system, indicating that CB1 receptors inhibit transmitter release at enteric synapses and depress synaptic strength basally and in an activity-dependent manner. Although the specific molecular identity of the endocannabinoid was not identified in this study, by analogy with the CNS, the most likely candidate is 2-AG which is released and acts at presynaptic CB1 receptors (Di Marzo, 2011). Our data would support a similar role of 2-AG in the enteric nervous system.
Previous studies have raised the possibility that anandamide tonically controls GI motility in vivo and this was found to be the case in our model system (Izzo et al., 2001; Pinto et al., 2002; Bashashati et al., 2012). Here, we have hypothesized that 2-AG also exerted an endogenous tone controlling intestinal contractility in vitro and GI transit in vivo. An important and novel finding described in the present study is that DAGL inhibitors did not alter evoked contractility or 2-AG levels under basal conditions in either the ileum or colon in vitro and whole gut transit or faecal output in vivo in healthy mice. This observation suggests that basal endocannabinoid tone to inhibit gut contractility is instead largely under the control of anandamide, as previously suggested by Pinto et al. (2002) and Capasso et al. (2005). In fact, unlike anandamide, 2-AG is a product of, and intermediate in, triglyceride digestion in the intestine, which is mediated by lipases other than DAGL (Wood et al., 2010; Cascio, 2013). If such dietary and digestive pathways were spatially and temporally organized in a way to activate CB1 receptors, thereby reducing GI contractility, this might cause constipation after any fatty meal, which is obviously not the case. It is, instead, only the 2-AG that is derived from DAGLα and produced upon stimulation that can activate CB1 receptors on myenteric neurons and inhibit contractility. Another explanation for our findings, however, is that there are alternative pathways for the biosynthesis of the endocannabinoid 2-AG in the gut that maintain 2-AG levels in the face of DAGL inhibition. For example, 2-AG can be synthesized by lysophosphatidylinositol-selective PLC (Muccioli, 2010; Ueda et al., 2011; Murataeva et al., 2014). However, evaluation of the role of alternative pathways needs molecular data and specific inhibitors for this enzyme, which are not available at this time. Our measurements of 2-AG levels in the ileum and colon are not inconsistent with this second possibility. Conversely, it is predictable that inhibiting MAGL, which blocks the degradation of the entire GI 2-AG pool including exogenous 2-AG, represents an artificial (i.e. ‘pharmacological’ rather than ‘physiological’) condition sufficient to strongly and indirectly activate CB1 receptors and hence significantly affect basal motility. Further studies are required to examine the sources and metabolic pathways of 2-AG in the intestine.
Indeed, although we have not detected DAGLβ, lack of evidence should not necessarily be taken as evidence of lack of a role of this isoform in controlling 2-AG biosynthesis in states of reduced motility. In fact, orlistat inhibits with similar efficacy both DAGLα and DAGLβ (Bisogno et al., 2003), whereas OMDM-188 has so far only been tested on DAGLα (Ortar et al., 2008). However, given the similarity between the catalytic triads of the two enzymes, their very similar primary sequence near the binding site, serine lipase, lipase 3 motif (Bisogno et al., 2003; Ortar et al., 2008) and because OMDM-188 was developed from a chemical modification of orlistat, it is expected that this compound is also not preferentially selective for either of the two isoforms of the enzyme. Selective DAGLα and β inhibitors have only just been developed (Baggelaar et al., 2013; Hsu et al., 2013 ) and hence any final conclusion on the role of DAGLβ will have to await the commercial availability of such compounds.
Finding that these compounds had no effect under physiological conditions prompted us to examine whether they may have effects when motility was reduced pharmacologically, a common clinical situation. Two drugs that are known to decrease evoked contractility in the intestine are scopolamine and loperamide (Kachur et al., 1986; Krueger et al., 2013). Interestingly, when we reduced evoked contractility with scopolamine, we observed that OMDM-188 and orlistat could partly or fully reverse the effects in both the ileum and colon depending on the dose of scopolamine employed. Moreover, the two DAGL inhibitors, which had a relatively short effective halflife in vivo also reversed the scopolamine-induced prolongation of whole gut transit, an action of the muscarinic antagonist that mimics slow transit constipation. These data suggest that, following scopolamine administration, 2-AG formation contributes, via CB1 receptor activation, to the reduction of GI motility and that this effect can be attenuated by the DAGL inhibitors. We confirmed that CB1 receptors were responsible for this effect using antagonists and CB1−/− mice. Using a second model that mimics the common problem of opioid-induced constipation, we observed very similar results and that treatment with DAGL inhibitors could partly reverse evoked hypocontractility and restore motility in vivo. These exciting findings suggest that either at the neuromuscular junction and/or in the myenteric plexus, 2-AG biosynthesis can be up-regulated over basal levels via GPCRs (including cholinergic muscarinic and μ-opioid receptors) and contribute to constipation via inhibition of cholinergic transmission following CB1 receptor activation. When 2-AG biosynthesis is inhibited, these mechanisms are also inhibited and motility is increased. It is not known how such up-regulation of 2-AG levels (which we investigated here only in vitro with scopolamine) occurs. It is important to emphasize that the DAGL inhibitors, orlistat and OMDM-188, reduced stimulated, and not the basal or pre-existing, levels of 2-AG also in isolated neurons, brain slices, hypothalamus and liver (Bisogno et al., 2009; 2013,; Alger and Kim, 2011).
As mentioned earlier, in the presence of scopolamine, 2-AG levels in the colon tended to increase and were significantly reduced by the DAGL inhibitor OMDM-188, in agreement with our functional data. Interestingly, and unexpectedly, orlistat increased anandamide levels in the ileum after incubation with scopolamine. Increased anandamide levels obtained by blocking FAAH have been previously shown to reduce 2-AG levels in the brain (Maccarrone et al., 2008). This negative cross-talk between anandamide and 2-AG has been suggested to be mediated by TRPV1 channels, as postsynaptic activation of TRPV1 channels by anandamide subsequently inhibited DAGLα and decreased 2-AG levels (Di Marzo and Cristino, 2008). It is possible that, in turn, 2-AG exerts a negative effect on anandamide levels and that inhibition of 2-AG biosynthesis results in increased anandamide levels. However, DAGLα−/− mice exhibit lower, rather than higher, anandamide levels in some brain regions (Yoshino et al., 2011). To our knowledge, our findings are the first evidence of an effect of blocking DAGLα on anandamide levels. Understanding the mechanism underlying this phenomenon therefore needs further investigation. Unfortunately, inhibitors of anandamide biosynthesis are not currently available to see if reciprocal interactions between these endocannabinoids occur in the GI tract. At any rate, neither of the two DAGL inhibitors tested here affected anandamide levels in the colon, although there was a trend based on the increased anandamide : 2-AG ratio in this organ after treatment with DAGL inhibitors in the presence of scopolamine. The differences between colon and ileum endocannabinoid levels may be attributed to possible differences in the expression of DAGLα in these regions as well as differences in the cholinergic components of these GI organs. Indeed, in a previous publication, we have shown that despite being blocked in the ileum, EFS contractility in the colon is not completely blocked by atropine (Bashashati et al., 2012) and in the present study, we have shown that inhibition of EFS contractility by scopolamine is weaker in the colon than in the ileum, although whether cholinergic-endocannabinoid cross-talk can explain the differential response to DAGL inhibition in the ileum and colon needs further investigation.
When we administered DAGL inhibitors to C3H/HeJ mice which have constipation because of a genetic defect that leads to the loss of a population of NOS immunoreactive neurons (Anitha et al., 2012), we could restore physiological GI motility. Quantification of the endocannabinoids revealed that C3H/HeJ mice tended to have higher colonic 2-AG levels compared with the background mice and orlistat significantly decreased 2-AG levels in the colon of these mice. Therefore, besides the loss of NOS neurons, endocannabinoid imbalance may also contribute to the phenomenon of constipation in these mice. It is possible that the loss of NOS neurons in the myenteric plexus enhances neuronal activity in this region and this increases the local levels of 2-AG such that inhibiting DAGL reveals the role of this mediator in synaptic inhibition. Further detailed electrophysiological studies are required to assess if this is the case. Importantly, although OMDM-188 was more efficacious than orlistat at restoring GI motility in C3H/HeJ mice, it did not significantly reduce 2-AG levels in either the ileum or colon of these animals. This suggests that there might be more than inhibition of 2-AG biosynthesis in the mechanism of the anti-constipation action of OMDM-188 or that its inhibition of DAGL may occur at sites different from the ileum and colon, but still involved in the control of GI motility.
In conclusion, here, we have shown that DAGLα, the principal enzyme for 2-AG biosynthesis, is localized in the enteric nervous system. We demonstrated that 2-AG regulates motility via CB1 receptors but that under basal conditions this mechanism is absent. However, in states of reduced motility, we discovered that inhibiting DAGL is a novel mechanism to enhance contractility in the small and large intestine and to propel motility in three models of slow transit. As constipation is a major clinical problem and new approaches to combat this condition are required, our data suggest that DAGL inhibitors may be promising candidate molecules for such conditions. Therefore, besides MAGL inhibitors, such as JZL184, which are potential treatments for diarrhoea (Ramesh et al., 2011), targeting DAGL is a promising treatment strategy for constipation. These findings may provide a new therapeutic approach for patients with chronic, slow transit or drug-induced constipation.
Acknowledgments
This study was supported by the Canadian Institutes of Health Research (K. A. S.), National Institutes of Health (DA011322 and DA021696 to K. M.), Deutsche Forschungsgemeinschaft (DFG-645/9-1, M. A. S.) and grant no. DA009789 (V. D. M.) and the Canadian Consortium for the Investigation of Cannabinoids (M. B.). We thank Anwar Al-Awadhi for help with some of the organ bath experiments and we are grateful to Dr. Giorgio Ortar for supporting the synthesis of the DAGL inhibitor OMDM-188. K. A. S. holds the Crohn's and Colitis Foundation of Canada Chair in Inflammatory Bowel Disease Research at the University of Calgary.
Glossary
- 2-AG
2-arachidonoylglycerol
- DAG
diacylglycerol
- DAGL
diacylglycerol lipase
- EFS
electrical field stimulation
- FAAH
fatty acid amide hydrolase
- GI
gastrointestinal
- MAGL
monoacylglycerol lipase
- RQ
relative quantification
- vAChT
vesicular acetylcholine transporter
Author contributions
M. B., C. M. K., M. A. S., V. D. and K. A. S. designed the studies. M. B., Y. N., C. M. K., W. H. and F. P. conducted the experiments. M. B., Y. N., C. M. K., W. H., F. P., V. D. and K. A. S. analysed data. M. N. and K. M. provided the unique reagents. K. M., M. A. S., V. D. and K. A. S. obtained grant support. All the authors contributed to the writing of the paper, revising the manuscript and approving the final version for submission.
Conflict of interest
The authors have no competing interests.
Supporting Information
Appendix S1 Supplemental Methods.
References
- Abalo R, Vera G, López-Pérez AE, Martinez-Villaluenga M, Martín-Fontelles MI. The gastrointestinal pharmacology of cannabinoids: focus on motility. Pharmacology. 2012;90:1–10. doi: 10.1159/000339072. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013a;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G Protein-Coupled Receptors. Br J Pharmacol. 2013b;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ion Channels. Br J Pharmacol. 2013c;170:1607–1651. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Transporters. Br J Pharmacol. 2013d;170:1706–1796. doi: 10.1111/bph.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger BE, Kim J. Supply and demand for endocannabinoids. Trends Neurosci. 2011;34:304–315. doi: 10.1016/j.tins.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhouayek M, Muccioli GG. The endocannabinoid system in inflammatory bowel diseases: from pathophysiology to therapeutic opportunity. Trends Mol Med. 2012;18:615–625. doi: 10.1016/j.molmed.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Alhouayek M, Lambert DM, Delzenne NM, Cani PD, Muccioli GG. Increasing endogenous 2-arachidonoylglycerol levels counteracts colitis and related systemic inflammation. FASEB J. 2011;25:2711–2721. doi: 10.1096/fj.10-176602. [DOI] [PubMed] [Google Scholar]
- Anitha M, Vijay-Kumar M, Sitaraman SV, Gewirtz AT, Srinivasan S. Gut microbial products regulate murine gastrointestinal motility via toll-like receptor 4 signaling. Gastroenterology. 2012;143:1006–1016. doi: 10.1053/j.gastro.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggelaar MP, Janssen FJ, van Esbroeck AC, den Dulk H, Allarà M, Hoogendoorn S, et al. Development of an activity-based probe and in silico design reveal highly selective inhibitors for diacylglycerol lipase-α in brain. Angew Chem Int Ed Engl. 2013;52:12081–12085. doi: 10.1002/anie.201306295. [DOI] [PubMed] [Google Scholar]
- Bashashati M, Storr MA, Nikas SP, Wood JT, Godlewski G, Liu J, et al. Inhibiting fatty acid amide hydrolase normalizes endotoxin-induced enhanced gastrointestinal motility in mice. Br J Pharmacol. 2012;165:1556–1571. doi: 10.1111/j.1476-5381.2011.01644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Burston JJ, Rai R, Allarà M, Saha B, Mahadevan A, et al. Synthesis and pharmacological activity of a potent inhibitor of the biosynthesis of the endocannabinoid 2-arachidonoylglycerol. ChemMedChem. 2009;4:946–950. doi: 10.1002/cmdc.200800442. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Mahadevan A, Coccurello R, Chang JW, Allarà M, Chen Y, et al. A novel fluorophosphonate inhibitor of the biosynthesis of the endocannabinoid 2-arachidonoylglycerol with potential anti-obesity effects. Br J Pharmacol. 2013;169:784–793. doi: 10.1111/bph.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankman JL, Cravatt BF. Chemical probes of endocannabinoid metabolism. Pharmacol Rev. 2013;65:849–871. doi: 10.1124/pr.112.006387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri M, Carlson P, McKinzie S, Grudell A, Busciglio I, Burton D, et al. Genetic variation in endocannabinoid metabolism, gastrointestinal motility, and sensation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G13–G19. doi: 10.1152/ajpgi.00371.2007. [DOI] [PubMed] [Google Scholar]
- Capasso R, Matias I, Lutz B, Borrelli F, Capasso F, Marsicano G, et al. Fatty acid amide hydrolase controls mouse intestinal motility in vivo. Gastroenterology. 2005;129:941–951. doi: 10.1053/j.gastro.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Cascio MG. PUFA-derived endocannabinoids: an overview. Proc Nutr Soc. 2013;72:451–459. doi: 10.1017/S0029665113003418. [DOI] [PubMed] [Google Scholar]
- Coutts AA, Irving AJ, Mackie K, Pertwee RG, Anavi-Goffer S. Localisation of cannabinoid CB(1) receptor immunoreactivity in the guinea pig and rat myenteric plexus. J Comp Neurol. 2002;448:410–422. doi: 10.1002/cne.10270. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. Endocannabinoid signaling in the brain: biosynthetic mechanisms in the limelight. Nat Neurosci. 2011;14:9–15. doi: 10.1038/nn.2720. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Cristino L. Why endocannabinoids are not all alike. Nat Neurosci. 2008;11:124–126. doi: 10.1038/nn0208-124. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, De Petrocellis L. Why do cannabinoid receptors have more than one endogenous ligand? Philos Trans R Soc Lond B Biol Sci. 2012;367:3216–3228. doi: 10.1098/rstb.2011.0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Goparaju SK, Wang L, Liu J, Bátkai S, Járai Z, et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- Dinh TP, Freund TF, Piomelli D. A role for monoglyceride lipase in 2-arachidonoylglycerol inactivation. Chem Phys Lipids. 2002;121:149–158. doi: 10.1016/s0009-3084(02)00150-0. [DOI] [PubMed] [Google Scholar]
- Duncan M, Thomas AD, Cluny NL, Patel A, Patel KD, Lutz B, et al. Distribution and function of monoacylglycerol lipase in the gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1255–G1265. doi: 10.1152/ajpgi.90500.2008. [DOI] [PubMed] [Google Scholar]
- Galve-Roperh I, Chiurchiu V, Díaz-Alonso J, Bari M, Guzman M, Maccarrone M. Cannabinoid receptor signaling in progenitor/stem cell proliferation and differentiation. Prog Lipid Res. 2013;52:633–650. doi: 10.1016/j.plipres.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Hons IM, Storr MA, Mackie K, Lutz B, Pittman QJ, Mawe GM, et al. Plasticity of mouse enteric synapses mediated through endocannabinoid and purinergic signaling. Neurogastroenterol Motil. 2012;24:e113–e124. doi: 10.1111/j.1365-2982.2011.01860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC. The cannabinoid receptors. Prostaglandins Other Lipid Mediat. 2002;68–69:619–631. doi: 10.1016/s0090-6980(02)00060-6. [DOI] [PubMed] [Google Scholar]
- Hsu KL, Tsuboi K, Whitby LR, Speers AE, Pugh H, Inloes J, et al. Development and optimization of piperidyl-1,2,3-triazole ureas as selective chemical probes of endocannabinoid biosynthesis. J Med Chem. 2013;56:8257–8269. doi: 10.1021/jm400898x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannotti FA, Piscatelli F, Martella A, Mazzarella E, Allara M, Palmieri V, et al. Analysis of the ‘endocannabinoidome’ in peripheral tissues of obese Zucker rats. Prostaglandins Leukot Essent Fatty Acids. 2013;89:127–135. doi: 10.1016/j.plefa.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Camilleri M. Emerging role of cannabinoids in gastrointestinal and liver diseases: basic and clinical aspects. Gut. 2008;57:1140–1155. doi: 10.1136/gut.2008.148791. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Sharkey KA. Cannabinoids and the gut: new developments and emerging concepts. Pharmacol Ther. 2010;126:21–38. doi: 10.1016/j.pharmthera.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Fezza F, Capasso R, Bisogno T, Pinto L, Iuvone T, et al. Cannabinoid CB1-receptor mediated regulation of gastrointestinal motility in mice in a model of intestinal inflammation. Br J Pharmacol. 2001;134:563–570. doi: 10.1038/sj.bjp.0704293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain T, Wager-Miller J, Mackie K, Straiker A. Diacylglycerol lipaseα (DAGLα) and DAGLβ cooperatively regulate the production of 2-arachidonoyl glycerol in autaptic hippocampal neurons. Mol Pharmacol. 2013;84:296–302. doi: 10.1124/mol.113.085217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachur JF, Morgan DW, Gaginella TS. Effect of dextromethorphan on guinea pig ileal contractility in vitro: comparison with levomethorphan, loperamide and codeine. J Pharmacol Exp Ther. 1986;239:661–667. [PubMed] [Google Scholar]
- Kasparek MS, Linden DR, Kreis ME, Sarr MG. Gasotransmitters in the gastrointestinal tract. Surgery. 2008;143:455–459. doi: 10.1016/j.surg.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Urban GM, Wallace M, Ledent C, Jung KM, Piomelli D, et al. Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci. 2006;26:5628–5637. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: Reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, Wise LE, Ramesh D, Abdullah R, Selley DE, Cravatt BF, et al. Repeated low-dose administration of the monoacylglycerol lipase inhibitor JZL184 retains cannabinoid receptor type 1-mediated antinociceptive and gastroprotective effects. J Pharmacol Exp Ther. 2013;345:492–501. doi: 10.1124/jpet.112.201426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger D, Michel K, Allam S, Weiser T, Demir IE, Ceyhan GO, et al. Effect of hyoscine butylbromide (Buscopan®) on cholinergic pathways in the human intestine. Neurogastroenterol Motil. 2013;25:e530–e539. doi: 10.1111/nmo.12156. [DOI] [PubMed] [Google Scholar]
- Kunze WA, Furness JB. The enteric nervous system and regulation of intestinal motility. Annu Rev Physiol. 1999;61:117–142. doi: 10.1146/annurev.physiol.61.1.117. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Rossi S, Bari M, De Chiara V, Fezza F, Musella A, et al. Anandamide inhibits metabolism and physiological actions of 2-arachidonoylglycerol in the striatum. Nat Neurosci. 2008;11:152–159. doi: 10.1038/nn2042. [DOI] [PubMed] [Google Scholar]
- MacNaughton WK, Van Sickle MD, Keenan CM, Cushing K, Mackie K, Sharkey KA. Distribution and function of the cannabinoid-1 receptor in the modulation of ion transport in the guinea pig ileum: relationship to capsaicin-sensitive nerves. Am J Physiol Gastrointest Liver Physiol. 2004;286:G863–G871. doi: 10.1152/ajpgi.00482.2003. [DOI] [PubMed] [Google Scholar]
- Marquez L, Suarez J, Iglesias M, Bermudez-Silva FJ, Rodriguez de Fonseca F, Andreu M. Ulcerative colitis induces changes on the expression of the endocannabinoid system in the human colonic tissue. PLoS ONE. 2009;4:e6893. doi: 10.1371/journal.pone.0006893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Min R, Testa-Silva G, Heistek TS, Canto CB, Lodder JC, Bisogno T, et al. Diacylglycerol lipase is not involved in depolarization-induced suppression of inhibition at unitary inhibitory connections in mouse hippocampus. J Neurosci. 2010;30:2710–2715. doi: 10.1523/JNEUROSCI.BC-3622-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muccioli GG. Endocannabinoid biosynthesis and inactivation, from simple to complex. Drug Discov Today. 2010;15:474–483. doi: 10.1016/j.drudis.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Murataeva N, Straiker A, Mackie K. Parsing the players: 2-arachidonoylglycerol synthesis and degradation in the CNS. Br J Pharmacol. 2014;171:1379–1391. doi: 10.1111/bph.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortar G, Bisogno T, Ligresti A, Morera E, Nalli M, Di Marzo V. Tetrahydrolipstatin analogues as modulators of endocannabinoid 2-arachidonoylglycerol metabolism. J Med Chem. 2008;51:6970–6979. doi: 10.1021/jm800978m. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucleic Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Cannabinoids and the gastrointestinal tract. Gut. 2001;48:859–867. doi: 10.1136/gut.48.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB(1) and CB(2) Pharmacol Rev. 2010;62:588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto L, Izzo AA, Cascio MG, Bisogno T, Hospodar-Scott K, Brown DR, et al. Endocannabinoids as physiological regulators of colonic propulsion in mice. Gastroenterology. 2002;123:227–234. doi: 10.1053/gast.2002.34242. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Ramesh D, Ross GR, Schlosburg JE, Owens RA, Abdullah RA, Kinsey SG, et al. Blockade of endocannabinoid hydrolytic enzymes attenuates precipitated opioid withdrawal symptoms in mice. J Pharmacol Exp Ther. 2011;339:173–185. doi: 10.1124/jpet.111.181370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storr MA, Bashashati M, Hirota C, Vemuri VK, Keenan CM, Duncan M, et al. Differential effects of CB(1) neutral antagonists and inverse agonists on gastrointestinal motility in mice. Neurogastroenterol Motil. 2010;22:787–796. doi: 10.1111/j.1365-2982.2010.01478.x. , e223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda N, Tsuboi K, Uyama T. Enzymological studies on the biosynthesis of N-acylethanolamines. Biochim Biophys Acta. 2010;1801:1274–1285. doi: 10.1016/j.bbalip.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Ueda N, Tsuboi K, Uyama T, Ohnishi T. Biosynthesis and degradation of the endocannabinoid 2-arachidonoylglycerol. Biofactors. 2011;37:1–7. doi: 10.1002/biof.131. [DOI] [PubMed] [Google Scholar]
- Wong BS, Camilleri M, Busciglio I, Carlson P, Szarka LA, Burton D, et al. Pharmacogenetic trial of a cannabinoid agonist shows reduced fasting colonic motility in patients with nonconstipated irritable bowel syndrome. Gastroenterology. 2011;141:1638–1647. doi: 10.1053/j.gastro.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JT, Williams JS, Pandarinathan L, Janero DR, Lammi-Keefe CJ, Makriyannis A. Dietary docosahexaenoic acid supplementation alters select physiological endocannabinoid-system metabolites in brain and plasma. J Lipid Res. 2010;51:1416–1423. doi: 10.1194/jlr.M002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino H, Miyamae T, Hansen G, Zambrowicz B, Flynn M, Pedicord D, et al. Postsynaptic diacylglycerol lipase mediates retrograde endocannabinoid suppression of inhibition in mouse prefrontal cortex. J Physiol. 2011;589:4857–4884. doi: 10.1113/jphysiol.2011.212225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wang M, Bisogno T, Di Marzo V, Alger BE. Endocannabinoids generated by Ca2+ or by metabotropic glutamate receptors appear to arise from different pools of diacylglycerol lipase. PLoS ONE. 2011;6:e16305. doi: 10.1371/journal.pone.0016305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supplemental Methods.