Abstract
Background and Purpose
Two of the most relevant unmet needs in epilepsy are represented by the development of disease-modifying drugs able to affect epileptogenesis and/or the study of related neuropsychiatric comorbidities. No systematic study has investigated the effects of chronic treatment with antipsychotics or antidepressants on epileptogenesis. However, such drugs are known to influence seizure threshold.
Experimental Approach
We evaluated the effects of an early long-term treatment (ELTT; 17 weeks), started before seizure onset (P45), with fluoxetine (selective 5-HT-reuptake inhibitor), duloxetine (dual-acting 5-HT-noradrenaline reuptake inhibitor), haloperidol (typical antipsychotic drug), risperidone and quetiapine (atypical antipsychotic drugs) on the development of absence seizures and comorbid depressive-like behaviour in the WAG/Rij rat model. Furthermore, we studied the effects of these drugs on established absence seizures in adult (6-month-old) rats after a chronic 7 weeks treatment.
Key Results
ELTT with all antipsychotics did not affect the development of seizures, whereas, both ELTT haloperidol (1 mg·kg−1 day−1) and risperidone (0.5 mg·kg−1 day−1) increased immobility time in the forced swimming test and increased absence seizures only in adult rats (7 weeks treatment). In contrast, both fluoxetine (30 mg·kg−1 day−1) and duloxetine (10–30 mg·kg−1 day−1) exhibited clear antiepileptogenic effects. Duloxetine decreased and fluoxetine increased absence seizures in adult rats. Duloxetine did not affect immobility time; fluoxetine 30 mg·kg−1 day−1 reduced immobility time while at 10 mg·kg−1 day−1 an increase was observed.
Conclusions and Implications
In this animal model, antipsychotics had no antiepileptogenic effects and might worsen depressive-like comorbidity, while antidepressants have potential antiepileptogenic effects even though they have limited effects on comorbid depressive-like behaviour.
Tables of Links
| TARGETS |
|---|
| 5-HT receptors |
| D1 receptor |
| D2 receptor |
| D3 receptor |
| LIGANDS | ||
|---|---|---|
| 5-HT | Duloxetine | Noradrenaline |
| Aripiprazole | Fluoxetine | Quetiapine |
| Dopamine | Haloperidol | Risperidone |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013).
Introduction
The relationship between epilepsy and psychiatric disorders has been known since ancient times, and the most frequent psychiatric comorbidities in epilepsy are represented by depressive disorders and anxiety (Kanner et al., 2012; Sankar and Mazarati, 2012).
Absence epilepsy is a generalized non-convulsive form of epilepsy, characterized by a paroxysmal loss/decrease in consciousness level associated with bilateral synchronous spike-wave discharges (SWDs) on the EEG. It represents a common form of epilepsy accounting for 10% of all epilepsies in children 15 years and younger (Berg et al., 2014), and is generally responsive to currently available medical treatment although 10% of patients do not attain complete seizure suppression with ethosuximide or valproic acid (Perucca, 2001). Furthermore, absence epilepsy has been recently associated with behavioural, affective and cognitive disturbances that may persist in adulthood (Caplan et al., 2008; Vega et al., 2010).
Rats of the Wistar Albino Glaxo/Rijswijk rats (WAG/Rij) strain display spontaneous absence-type seizures that are accompanied by generalized SWDs morphologically similar to human absence seizures (van Luijtelaar and Zobeiri, 2014). WAG/Rij rats, further than being validated as an animal model of absence epilepsy, have been more recently recognized as an animal model of mild depression (dysthymia) with decreased investigative activity in the open field test, increased immobility in the forced swimming test (FST), and decreased sucrose consumption/preference (anhedonia); therefore, this strain is generally considered a good model of genetic absence epilepsy with comorbid depressive-like symptoms (Sarkisova et al., 2010; Sarkisova and van Luijtelaar, 2011; Russo et al., 2013a). Finally, WAG/Rij rats have been recently indicated as a relevant animal model of genetically determined epileptogenesis (Blumenfeld et al., 2008; Russo et al., 2011a; 2013b,). More specifically, WAG/Rij rats, as well as genetic absence epilepsy rats from Strasbourg (GAERS), are genetically prone to develop spontaneous absence seizures during their lifespan with only few early immature SWDs appearing on the EEG (WAG/Rij rats after P50 and GAERS probably earlier), which increase in number and duration with ageing also changing their morphology to become fully matured and expressed in all rats of the strain only after 2–3 months of age (van Luijtelaar et al., 2011; 2014,; Dezsi et al., 2013). In this light, both strains of rats can be considered models of epileptogenesis where an early intervention can modify the underlying process and the future development of the genetically determined phenotype (Blumenfeld et al., 2008; Giblin and Blumenfeld, 2010; Pitkanen and Engel, 2014; White and Loscher, 2014).
Clinical and experimental studies suggest that imbalances in neurotransmitter systems (i.e. GABA, glutamate, noradrenaline, 5-HT and dopamine) commonly observed in people with epilepsy may contribute to the development of comorbid psychiatric disorders in these patients (Kanner et al., 2012; Sankar and Mazarati, 2012).
Previous behavioural and electrophysiological studies have demonstrated functional deficits in the brain dopaminergic system in WAG/Rij rats responsible for depressive-like behaviour (Sarkisova et al., 2008; 2013,). A dopaminergic pathway is also associated to the pathophysiology of epilepsies; namely, a decrease in inhibitory dopaminergic activity predisposes to hyperexcitability and epilepsy (Ciumas et al., 2008). Furthermore, earlier clinical data showed that antipsychotic drugs may aggravate absence seizures (Itil and Soldatos, 1980). As the brain dopaminergic system is one of the main targets for neuroleptic drugs, an unknown dysfunction of the brain dopaminergic system was proposed to exist in absence epilepsy patients; it was found that the dopamine transporter gene is specifically altered in human patients with idiopathic generalized absence epilepsy. Thus, hypothetically, altered reuptake of dopamine would contribute to enhanced network excitability, and consequently, to a lower threshold for SWDs to occur (Sander et al., 2000). Finally, systemic administration of dopamine antagonists such as haloperidol aggravate SWDs while apomorphine (dopamine agonist) and aripiprazole (dopamine partial agonist) reduce SWDs (de Bruin et al., 2000; Deransart et al., 2000; Russo et al., 2013a).
5-HT, another monoamine implicated in depression, also modulates seizure susceptibility and may be involved in at least some aspects of depression and epilepsy comorbidity (Richerson and Buchanan, 2011; Cardamone et al., 2013; Hamid and Kanner, 2013). Epidemiological evidence supports the idea that depression and other psychopathologies represent a risk factor for the development of seizures and epilepsy (Hesdorffer et al., 2012), and that antidepressant treatment may influence epilepsy onset (Hesdorffer et al., 2012); new emerging theories propose that depression and epilepsy may share common pathogenesis mechanisms (Kanner, 2012; Kanner et al., 2012; Sankar and Mazarati, 2012). There is growing evidence that 5-hydroxytryptaminergic neurotransmission modulates experimentally-induced seizures and is involved in the enhanced seizure susceptibility observed in some genetically epilepsy-prone rodent models (Gerber et al., 1998; Filakovszky et al., 1999; Citraro et al., 2011; Sankar and Mazarati, 2012; Cardamone et al., 2013; Hamid and Kanner, 2013).
Generally, agents that elevate extracellular 5-HT levels, such as 5-hydroxytryptophan and 5-HT reuptake blockers, inhibit both limbic and generalized seizures (Prendiville and Gale, 1993; Yan et al., 1994b). Conversely, depletion of brain 5-HT lowers the threshold to audiogenically, chemically and electrically evoked seizures (Lazarova et al., 1983; Statnick et al., 1996a). Notably, high doses of pro-5-hydroxytryptaminergic agents alone, or more commonly, in combination, can cause 5-HT syndrome, which is mainly characterized by altered mental status, autonomic stimulation and neuromuscular excitation including seizures (Boyer and Shannon, 2005).
5-Hydroxytryptaminergic neurotransmission also seems to play an important role in the generation and maintenance of epileptic activity in WAG/Rij rats (Filakovszky et al., 1999; Jakus et al., 2003; Graf et al., 2004). 5-HT regulates (either increase or decrease depending on receptor subtype) SWDs in absence epilepsy through various different 5-HT receptors (Jakus et al., 2003; Graf et al., 2004) acting on the components of the thalamocortical loop generating absence seizures in this strain of rats.
In WAG/Rij rats, chronic injection (15 days) of the tricyclic antidepressant imipramine induced a therapeutic (antidepressant) effect on depressive-like behaviour with a decrease in the duration of immobility and an increase in the duration of swimming in the FST and increase in sucrose consumption/preference (Sarkisova et al., 2008). WAG/Rij rats also respond to chronic (15 days) fluoxetine treatment; however, in WAG/Rij rats, fluoxetine is less effective than imipramine in the FST and in the sucrose consumption/preference test (Sarkisova and Folomkina, 2010).
Previous clinical and experimental studies have examined the effects of antidepressant and antipsychotic drugs on seizure frequency, but only few have considered the effects of these drugs on epileptogenesis, the neurobiological process underlying the development of chronic spontaneous seizures (Cardamone et al., 2013; Hamid and Kanner, 2013; Pitkanen et al., 2013).
The current study was designed to answer to the following questions: (i) does early long-term treatment (ELTT) with some antipsychotics (haloperidol, risperidone, quetiapine) and antidepressants (fluoxetine and duloxetine) affect epileptogenesis (absence seizures development) in WAG/Rij rats? (ii) Do these drugs have an effect on absence seizures (SWDs parameters) in WAG/Rij rats? (iii) Would these drugs affect the development of comorbid depressive-like behaviour?
Methods
Animals
Male WAG/Rij rats were used (n = 160). Rat progenitors (60 male and 20 female) were originally purchased from Charles River Laboratories s.r.l. (Calco, Lecco, Italy) at a body weight of ∼60 g (4 weeks old). Animals were housed three/four per cage and kept under controlled environmental conditions (60 ± 5% humidity; 22 ± 2°C; 12/12 h reversed light/dark cycle; lights on at 20:00 h). Female rats (n = 20) at 10 weeks of age were placed with same-age group males for mating, as previously described (Citraro et al., 2014). Dams of all strains were housed two per cage, whereas, all offspring after weaning (P21) were housed three/four per cage. Animals were allowed free access to food and water until the time of the experiments. Procedures involving animals and their care were conducted in conformity with international and national law and policies (EU Directive 2010/63/EU for animal experiments and the Basel declaration including the 3R concept). The experimental protocols and procedures described in this paper were approved by the local ethical committee of the University of Catanzaro. All efforts were made to minimize animal suffering and to reduce the number of animals used. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
Drug administration protocol
Antipsychotic drugs used were: haloperidol (Sigma-Aldrich, Milan, Italy), risperidone (Janssen Cilag, Cologno Monzese, Milan, Italy) and quetiapine (Astra Zeneca, Basilio, Milan, Italy). Antidepressant drugs used were: fluoxetine (fluoxetine DOC 20MG 28 CPS; DOC Generici S.r.l., Milan, Italy) and duloxetine (Cymbalta; 60MG 28 CPS, Eli Lilly SpA, Sesto Fiorentino, Florence, Italy).
Haloperidol (1 mg·kg−1 day−1), risperidone (0.5 mg·kg−1 day−1), quetiapine (10 mg·kg−1 day−1), fluoxetine (10 and 30 mg·kg−1 day−1) and duloxetine (10 and 30 mg·kg−1 day−1) were administered p.o. Final solutions of all drugs used were obtained by adding the desired dose of the drug to 120 mL of drinking water, as previously reported (Russo et al., 2011a). The dose was calculated on the evidence that rats drink on average 12 mL 100 g−1 day−1; this was further confirmed by checking the volume drunk by rats, as previously described (Russo et al., 2013b). Water bottles were wrapped in silver foil to exclude light and solutions were freshly prepared and replaced three times a week.
Drug doses were chosen according to previous publications; with chronic (long term) treatment no obvious side effects were reported and effects on seizures were reported (de Bruin et al., 2001; Jakus et al., 2003; Dobryakova et al., 2011; Citraro et al., 2015); furthermore, such doses are representative of the doses used in patients (see Discussion and conclusions).
In the present study, we tested (i) the effects of antipsychotics and antidepressants treatment on both the epileptogenic process underlying the development of absence seizures and depressive-like behaviour (FST) in WAG/Rij rats and (ii) the effects of a chronic adult treatment (7 weeks) on established absence seizures in adult (started at 6 months of age) WAG/Rij rats.
To evaluate the possible antiepileptogenic effects (ELTT), WAG/Rij rats were randomly divided into seven groups (n = 10 for each drug and dose) and drug administration was started at P45 (before seizure onset, which occurs around P60) up to the age of ∼5 months (17 weeks of treatment), then drug treatment was suspended and animals were normally housed (see housing conditions reported earlier) up to the age of 6 months when they were experimentally evaluated (see section Surgery and EEG recordings). Age-matched male control rats (n = 10 WAG/Rij rats) were kept under the same housing conditions over the same period of time (Citraro et al., 2013b).
To evaluate the effects of chronic treatment on adult (previously untreated) WAG/Rij rats, different groups (n = 10 for each drug and dose) of rats were administered drugs p.o. (see earlier) for 7 consecutive weeks and tested the last day of administration and after 2 weeks of treatment discontinuation. Figure 1 depicts a simplified version of the experimental protocol.
Figure 1.
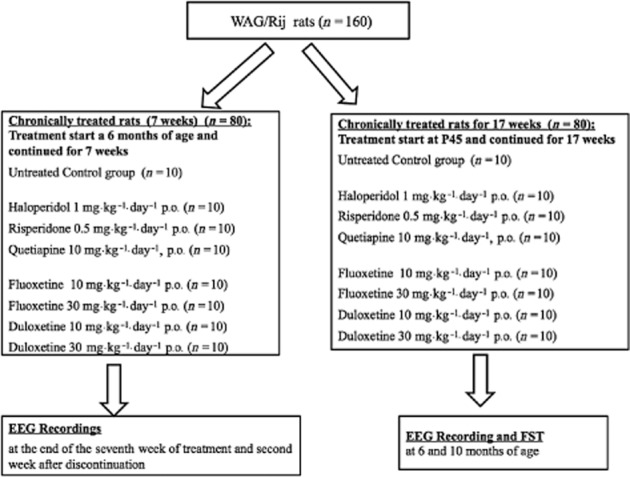
Schematic representation of experimental protocol. P45, 45 days of age.
Surgery and EEG recordings
All WAG/Rij rats, at the age of 6 months, were chronically implanted, under anaesthesia obtained by administration of a mixture of tiletamine/zolazepam (1:1; Zoletil 100®; 50 mg·kg−1 i.p.; VIRBAC Srl, Milan, Italy), using a Kopf stereotaxic instrument, with five cortical electrodes for EEG recordings. The level of anaesthesia was assessed by loss of righting reflex. Stainless-steel screw electrodes were implanted on the dura mater over the cortex: two in the frontal region (AP = −2; L = ±2.5) and two in the parietal region (AP = −6; L = ±2.5), as previously described (Citraro et al., 2013a). The ground electrode was placed over the cerebellum. All animals were allowed at least 1 week to recover and handled twice a day.
Rats were attached to a multichannel amplifier (Stellate Harmonie Electroencephalograph; Montreal, QC, Canada) by a flexible recording cable and an electric swivel, fixed above the cages, permitting free movements for the animals. Rats in the ELTT groups underwent three recording periods for 3 consecutive days. In rats in the chronic (7 weeks, treatment started at 6 months of age) treatment groups, EEG s were recorded only at the end of the seventh week of treatment and 2 weeks after discontinuation. All recordings started at 09:00 h and lasted 3 h without further administration of any drug for every group.
All EEG signals were amplified and conditioned by analogue filters (filtering: below 1 Hz and above 30 Hz at 6 dB per octave) and subjected to an analogue-to-digital conversion with a sampling rate of 300 Hz. The blinded quantification of absence seizures was based on the number and the duration of EEG SWDs, as previously described (Russo et al., 2011b; 2012,). In order to assess the long-term treatment effects of the drugs, every recording session was divided into 30 min epochs; the cumulative SWD duration and number per epoch were calculated and presented as means ± SEM in Table 1.
Table 1.
Effects of ELTT with antipsychotic and antidepressant drugs on SWD parameters recorded in WAG/Rij rats at 6 months of age
| Animal group (n = 10) | nSWDs | dSWDs (s) | sSWD (s) |
|---|---|---|---|
| Recordings at 6 months of age (1 month after the end of drugs long-term treatment) | |||
| Control group | 6.04 ± 1.09 | 55.75 ± 15.71 | 7.19 ± 2.57 |
| QTP-treated group (10 mg·kg−1 day−1) | 5.83 ± 0.77 | 53.51 ± 11.67 | 6.72 ± 0.73 |
| HAL-treated group (1 mg·kg−1 day−1) | 6.51 ± 1.35 | 47.99 ± 9.93 | 6.31 ± 0.70 |
| RISP-treated group (0.5 mg·kg−1 day−1) | 6.5 ± 1.16 | 51.19 ± 10.51 | 6.3 ± 0.51 |
| FLX-treated group (10 mg·kg−1 day−1) | 5.95 ± 1.81 | 49.46 ± 12.3 | 7.31 ± 1.36 |
| FLX-treated group (30 mg·kg−1 day−1) | 3.25 ± 0.71* | 32.98 ± 9.44* | 7.73 ± 2.95 |
| DLX-treated group (10 mg·kg−1 day−1) | 4.91 ± 0.89* | 25.79 ± 4.35* | 5.25 ± 0.87* |
| DLX-treated group (30 mg·kg−1 day−1) | 3.78 ± 1.24* | 24.72 ± 6.42* | 4.58 ± 0.88* |
Data marked with
are significantly different (P < 0.05) from control group.
FST
The FST is one of the most widely used tests for evaluating depressive-like behaviour in animals and for screening antidepressant drug action in rodents, even though many limitations are known (Nestler and Hyman, 2010). All tests were carried out with the support of EthoVision software and equipment from Noldus (Wageningen, The Netherlands), as previously described (Russo et al., 2013a). Briefly, rats were placed individually for 6 min into glass cylinders (height: 26.5 cm; diameter: 16.5 cm) containing 15 cm of water, maintained at 22–23°C. The total duration of immobility was recorded during the last 4 min of a 6 min testing period. The total duration of immobility, including passive swimming, was measured by EthoVision software for all groups. The criterion for passive swimming was floating vertically in the water while making only those movements necessary to keep the head above the water. After the FST, animals were removed and dried with a towel before being placed in their home cages. In every experimental animal group evaluated, the test was started at 09:00 h and finished before 11:00 h in order to avoid possible circadian alteration of test results (Russo et al., 2013b). Mean swimming velocity was also statistically analysed for every experimental group in order to check for any obvious locomotor impairment, which could influence the test.
Statistical analysis
All statistical procedures were performed using the Statistical Package for the Social Sciences (SPSS) 15.0.0 software (SPSS, Inc., Chicago, IL, USA). EEG recordings were subdivided into 30 min epochs, and the duration and number of SWDs were treated separately for each epoch. Such values were averaged and data obtained are expressed as mean ± SEM for each compound. Animal groups were compared by one-way anova, the treatment being the only variable, followed by a post hoc Bonferroni test for the ELTT groups and Tukey's post hoc test for chronic treatment. Immobility times in the FST were compared by one-way anova followed by Bonferroni's post hoc test. All tests used were two-sided and P ≤ 0.05 was considered significant.
Results
Effects of ELTT with antipsychotic and antidepressant drugs on the development of SWDs in WAG/Rij rats
The EEG recordings' analysis of control WAG/Rij rats, at 6 months of age, revealed that in this group, the mean number of SWDs (nSWDs) for a 30 min epoch was 6.04 seizures with a mean total duration (dSWDs) of 55.75 s and a mean single duration (sSWD) of 7.19 s (Table 1
ELTT with haloperidol (1 mg·kg−1 day−1), risperidone (0.5 mg·kg−1 day−1) or quetiapine (10 mg·kg−1 day−1), for 17 consecutive weeks, had no effect on the development of spontaneous absence seizures in WAG/Rij rats; therefore, these drugs were not able to modify the epileptogenic process (Table 1).
In contrast, ELTT with fluoxetine (30 mg·kg−1 day−1) and duloxetine (10 and 30 mg·kg−1 day−1) significantly reduced the development of absence seizures in adult WAG/Rij rats (one-way anova followed by Bonferroni's post hoc test; P < 0.05: Table 1).
Chronic treatment with fluoxetine at a dose of 10 mg·kg−1 day−1 was not able to significantly reduce the development of absence seizures (Table 1).
Effects of chronic (7 weeks) treatment with antipsychotic and antidepressant drugs on absence seizures in adult WAG/Rij rats
Analysis of EEG recordings from adult WAG/Rij rats on the last day of chronic treatment (7 weeks) revealed that haloperidol (1 mg·kg−1 day−1) and risperidone (0.5 mg·kg−1 day−1) significantly (P < 0.05) increased the number and duration of SWDs in WAG/Rij rats; whereas, quetiapine (10 mg·kg−1 day−1) had no significant effects on SWDs parameters in comparison with the control group (Table 2).
Table 2.
Effects of chronic (7 weeks) treatment with antipsychotic and antidepressant drugs on SWD parameters recorded in WAG/Rij rats both on the last day of administration and after 2 weeks of treatment discontinuation
| Animal group (n = 10) | nSWDs | dSWDs (s) | sSWD (s) |
|---|---|---|---|
| Recordings on last day of chronic (7 weeks) treatment | |||
| Control group | 6.89 ± 0.76 | 28.53 ± 8.32 | 4.06 ± 1.75 |
| QTP-treated group (10 mg·kg−1 day−1) | 5.89 ± 1.11 | 25.51 ± 9.93 | 3.53 ± 0.47 |
| HAL-treated group (1 mg·kg−1 day−1) | 11.89 ± 1.11* | 73.51 ± 9.13* | 6.23 ± 0.75* |
| RISP-treated group (0.5 mg·kg−1 day−1) | 10.73 ± 1.16* | 65.88 ± 6.78* | 6.53 ± 0.47* |
| FLX-treated group (10 mg·kg−1 day−1) | 9.56 ± 1.72* | 67.33 ± 4.35* | 7.35 ± 1.43* |
| FLX-treated group (30 mg·kg−1 day−1) | 8.35 ± 0.59* | 79.23 ± 7.43* | 9.35 ± 0.94* |
| DLX-treated group (10 mg·kg−1 day−1) | 5.98 ± 0.84 | 26.73 ± 6.45 | 3.98 ± 0.56 |
| DLX-treated group (30 mg·kg−1 day−1) | 2.98 ± 0.21* | 9.23 ± 3.6* | 2.1 ± 0.5* |
| Recordings at 2 weeks after termination of chronic treatment | |||
|---|---|---|---|
| Control group | 6.36 ± 0.42 | 32.53 ± 7.93 | 5.21 ± 0.97 |
| QTP-treated group (10 mg·kg−1 day−1) | 5.4 ± 0.69 | 26.07 ± 6.5 | 4.56 ± 0.6 |
| HAL-treated group (1 mg·kg−1 day−1) | 5.89 ± 1.11 | 29.51 ± 9.93 | 5.08 ± 0.75 |
| RISP-treated group (0.5 mg·kg−1 day−1) | 5.73 ± 1.16 | 25.88 ± 6.78 | 4.53 ± 0.47 |
| FLX-treated group (10 mg·kg−1 day−1) | 4.98 ± 2.12 | 27.96 ± 5.05 | 4.9 ± 0.51 |
| FLX-treated group (30 mg·kg−1 day−1) | 5.82 ± 0.79 | 26.54 ± 2.62 | 4.45 ± 2.34 |
| DLX-treated group (10 mg·kg−1 day−1) | 6.33 ± 0.59 | 30.02 ± 7.01 | 5.02 ± 0.64 |
| DLX-treated group (30 mg·kg−1 day−1) | 4.99 ± 1.46 | 25.31 ± 7.48 | 4.31 ± 0.89 |
Data marked with
are significantly different (P < 0.05) from control group.
On the last day of treatment, chronic oral administration of fluoxetine, at both doses (10 and 30 mg·kg−1 day−1), showed a significant (P < 0.05) proabsence effect. Duloxetine (30 mg·kg−1 day−1) significantly (P < 0.05) reduced absence seizure, decreasing every SWD parameter in the EEG recordings; whereas, duloxetine at the dose of 10 mg·kg−1 day−1 had no effects (Table 2).
Two weeks after the suspension of all drug treatments used, all SWDs parameters returned to baseline levels with no enduring effects (Table 2).
Effects of antipsychotic and antidepressant drugs on depressive-like behaviour in WAG/Rij rats
ELTT with either haloperidol (1 mg·kg−1 day−1) or risperidone (0.5 mg·kg−1 day−1) significantly increased immobility time in the FST. This increase was accompanied by a significant (P < 0.05) decrease in total distance moved, but not mean velocity in comparison with their respective control group (data not shown). In contrast, quetiapine treatment failed to influence the immobility time in FST (Figure 2).
Figure 2.
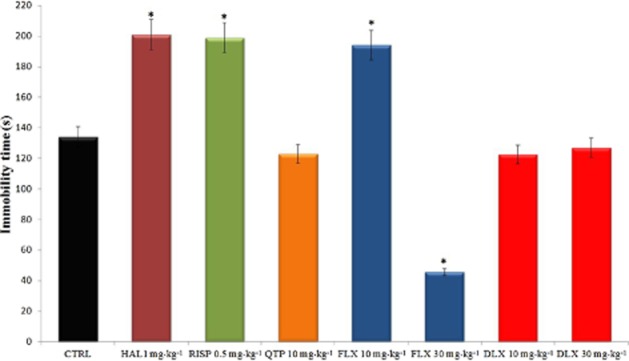
Effects of ELTT groups with antipsychotic and antidepressant drugs on immobility time in the FST in WAG/Rij rats at 6 months of age (1 month after drug suspension). Immobility time expressed in s. Values are means ± SEM; data marked with * are significantly different (P < 0.05) from control group. CTRL, control group.
In contrast, fluoxetine ELTT, at the dose of 30 mg·kg−1 day−1, significantly decreased immobility time in comparison with control WAG/Rij rats, in agreement with a previous report (Sarkisova et al., 2010). At odds with this, fluoxetine at the dose of 10 mg·kg−1 day−1 increased the immobility time exacerbating depressive-like behaviour. Finally, duloxetine did not influence immobility time (Figure 2).
Discussion and conclusions
As recently underlined (Brooks-Kayal et al., 2013; Pitkanen et al., 2013), two of the most relevant unmet needs in epilepsy are represented by the study of disease-modifying drugs able to positively affect both epileptogenesis and related neuropsychiatric comorbidities. In this light, several advances have been achieved in understanding some of the underlying mechanisms; however, much more need to be addressed. In this light, also absence epilepsies still suffer a lack of knowledge on neuropsychiatric comorbidities, which affect more than 20% of these patients; furthermore, the recent publication by Berg et al. (2014) supports the novel hypothesis that ethosuximide might have disease-modifying properties in childhood absence epilepsy, as previously demonstrated in animal models (Blumenfeld et al., 2008; Russo et al., 2010; 2011a,; Dezsi et al., 2013). These latter results justify the need for further research in animal models of absence epilepsy in order to define future development of drugs with disease-modifying properties also including drug effects on the development of neuropsychiatric comorbidity.
WAG/Rij rats have recently gained new interest as a suitable animal model of epileptogenesis with comorbid psychiatric symptomatology, making this model a feasible opportunity to study drug effects on both (Brooks-Kayal et al., 2013); although, the underlying mechanisms have not yet been clarified (van Luijtelaar and Zobeiri, 2014; White and Loscher, 2014).
The primary endpoint of our study was to evaluate the potential effects of some antipsychotics and antidepressants on epileptogenesis contemporarily evaluating their effects on comorbid depressive-like behaviour. This topic finds a great relevance in clinical practice, where, in some cases, it is necessary to pharmacologically treat people with epilepsy for comorbid neuropsychiatric disorders (prevalence rates 30–35%) (Kanner et al., 2012; Rai et al., 2012). Regarding most of the drugs used in this field, above all in the past and still for antipsychotics, it is widely accepted that within these two drug classes, the risk of decreasing seizure threshold and increase excitability is high (Hoppe and Elger, 2011; Cardamone et al., 2013; Lertxundi et al., 2013).
Rationale for drug choice and relevance to clinical practice
Several antipsychotics and antidepressants are currently available for clinical use in a variety of diseases including psychiatric comorbidity in people with epilepsy, which is often unfortunately overlooked (Kanner, 2005). A strong link exists between epilepsy and psychiatric disorders; many epileptic patients have psychiatric disorders and conversely depressed patients have a higher risk of becoming epileptic (Kanner, 2011; 2012,; Kanner et al., 2012). Within the two drug groups, we have tried to select the most representative also considering their mechanisms of action. haloperidol may be considered as a prototype for typical antipsychotics while both risperidone and quetiapine may represent the group of atypical antipsychotics (i.e. clozapine and olanzapine) with some differences between their mechanisms of action (see later). Regarding antidepressant drugs, fluoxetine is the prototype of selective 5-HT reuptake inhibitors (i.e. paroxetine, sertraline and escitalopram) while duloxetine, inhibiting both NA and 5-HT reuptake, was chosen in order to also add relevant information on adrenergic neurotransmission further than being a widely used antidepressant drug.
Drug doses were chosen according to previous publications with chronic (long term) treatment not inducing side effects and with an effect on seizures (de Bruin et al., 2001; Jakus et al., 2003; Dobryakova et al., 2011; Citraro et al., 2015); furthermore, such doses are strongly linked to the doses used in patients. Drug metabolism and elimination is in most cases more efficient (faster) in rodents than in humans and, therefore, in most cases, the final dose used in rats is apparently much higher than the one used in clinical practice for patients. Haloperidol is generally used in clinical practice at daily doses up to 10 mg with plasma levels of about 5–10 nM; we have used a dose of 1 mg·kg−1, which might lead to a final plasma level very similar to that which is attained in patients according to previous studies with different doses (Schmitt et al., 1999; Kapur et al., 2003). This is also in agreement with haloperidol differences in half-life between humans (about 24 h) and rats (about 1.5 h) (Cheng and Paalzow, 1992). Risperidone is generally used in patients at a dose of 4–6 mg day−1 with an expected plasma level of about 20 nM, which is in agreement with the measured plasma level (39.36 nM) in rats after 7 days of risperidone treatment at a dose of 1 mg·kg−1 (we have used half of this dose, 0.5 mg·kg−1) (Kapur et al., 2003). Quetiapine's half-life (about 7 h) is identical between rats and humans (Nemeroff et al., 2002), and the dose used (10 mg·kg−1 day−1) is very similar to the one used in clinical practice (about 600–800 mg day−1) (Cerullo and Strakowski, 2013). Fluoxetine is generally used at 20–60 mg day−1 in patients; in a pharmacokinetic study, a daily dose of 20 mg achieved a mean plasma concentration of about 80 ng·mL−1 (Norman et al., 1993); this is in agreement with the mean plasma concentration found in rats after 3 days of treatment with fluoxetine at a dose of 10 mg·kg−1 (McNamara et al., 2013). Duloxetine is used in patients at range doses of 30–120 mg day−1 and a pharmacokinetic study showed that, in patients, plasma levels ranged between 5 and 135 ng·mL−1 (mean 53.56 ng·mL−1) and plasma levels were significantly correlated with efficacy, but also with signs of anxiety and irritability at the highest concentrations (Volonteri et al., 2010). No chronic treatment in rats, to the best of our knowledge, has been published. In any case, the choice of the doses used is supported by the efficacy of duloxetine and fluoxetine in the FST and other animal models of depression in the range 5–40 mg·kg−1 (Reneric and Lucki, 1998; Wong, 1998; Lopez-Rubalcava and Lucki, 2000; Li et al., 2013).
Effects of antipsychotics on absence seizures and their development
Our results demonstrated that in the WAG/Rij rat model, antipsychotic drugs (haloperidol, risperidone and quetiapine) have no effects on the epileptogenic process when administered early before seizure onset and, therefore, have no antiepileptogenic effects. These data, together with our observation that the risperidone and haloperidol increase absence seizures in adult (6 months old) epileptic WAG/Rij rats (chronic quetiapine has no effects on absence seizures), may suggest that these drugs should be avoided in patients at risk of developing spontaneous seizures or with absence seizures. Notably, the proabsence effects of both haloperidol and risperidone were completely reversed 2 weeks after drug withdrawal.
Antipsychotics mainly act through inhibition of dopamine receptors while atypical antipsychotics also act on 5-HT receptors (Correll and Kane, 2014). The dopamine system has a seizure-modulating effect and antipsychotics lower seizure threshold in people with epilepsy and promote seizures in patients with no previous history of the disease (Pisani et al., 2002; Lertxundi et al., 2013). Our data on the pro-absence effects of haloperidol are in agreement with those obtained by Midzianovskaia et al. (2001) in WAG/Rij rats. Different data provide evidence towards changes in the dopamine system in absence epilepsy models (Warter et al., 1988; Midzianovskaia et al., 2001). While dopamine receptor antagonists, such as haloperidol, enhance SWDs, conversely, injection of the mixed dopamine D1/D2 receptor agonist apomorphine or the partial D2 receptor agonist aripiprazole resulted in a reduction in absence seizures in WAG/Rij rats (Midzianovskaia et al., 2001; Russo et al., 2013a). This pharmacological reactivity to dopamine ligands may result from their effects on the basal ganglia circuits known to modulate SWDs (Deransart et al., 2000; van Luijtelaar and Zobeiri, 2014). Dopamine is thought to exert its influence on SWDs through the interplay between the nigrostriatal and the mesolimbic systems and their output on the substantia nigra and thalamus (Deransart et al., 2000). All together, these data support the hypothesis that a dopamine insufficiency might be involved in the pathogenesis of absence epilepsy; however, antagonists of dopamine receptors might not influence epileptogenesis (de Bruin et al., 2000; Deransart et al., 2000; 2001,).
Effects of antidepressants on absence seizures and their development
Early treatment before seizure onset with both fluoxetine and duloxetine is able to reduce the development of spontaneous absence seizures in adulthood showing, therefore, antiepileptogenic properties. Noteworthy, chronic fluoxetine treatment in adult epileptic WAG/Rij rats is proabsence, while duloxetine reduces the number and duration of absence seizures. Both drugs increase 5-HT brain levels by blocking its synaptic transporter; furthermore, duloxetine also increases NA brain levels acting as a double inhibitor of 5-HT and NA reuptake; this difference might account for their divergent effects.
A growing body of evidence supports the role of 5-HT in the regulation of seizure development, propagation and maintenance (Trindade-Filho et al., 2008; Stefulj et al., 2010). In general, studies on animal models, as well as on humans, demonstrate an inverse correlation between extracellular brain 5-HT levels and susceptibility to seizures, although some exceptions have also been described (Bagdy et al., 2007). Preclinical and clinical studies have shown that reduction of 5-HT concentration in the brain can enhance susceptibility to seizures (Cavalheiro et al., 1994; Savic et al., 2004). Some evidence would indicate that 5-HT deficiency might also be involved in epileptogenesis (Favale et al., 2003; Stefulj et al., 2010). Treatments that enhance 5-HT neurotransmission have been shown to exert anticonvulsant effects in a wide variety of experimental models of generalized epilepsy (Prendiville and Gale, 1993; Yan et al., 1994a; Bagdy et al., 2007). Conversely, treatments that reduce brain 5-HT content have a proconvulsant effect in the same models (Statnick et al., 1996b). However, several controversies are present in this field showing both anti- or pro-convulsant effects for fluoxetine and other antidepressant drugs (Yan et al., 1994a; Favale et al., 2003; Igelstrom, 2012).
Most of the studies done in this field examined the effect of acute treatments, a methodological approach that proved to be useful for analysing the participation of 5-HT in the seizure threshold. However, acute treatments seem not to be the best strategy to predict their effects in clinical practice as they are prescribed on chronic basis. This distinction is really important, considering that many of the mechanisms by which antidepressants exert their therapeutic effects are only seen after a prolonged administration and depend on the induction of plastic changes in the central nervous system (Manji et al., 2003).
The present study provides evidence that treatments designed to increase extracellular 5-HT content might exert antiepileptogenic effects in WAG/Rij rats, as previously suggested (Russo et al., 2012). The proabsence effects after chronic fluoxetine in adult epileptic WAG/Rij rats are in agreement with previous reports (Gerber et al., 1998; Jakus et al., 2003) and 5-HT seems to regulate SWDs in absence epilepsy through various 5-HT receptors (Filakovszky et al., 1999; Graf et al., 2004). Previous extracellular and intracellular recordings revealed that 5-HT and NA induce slow depolarization within the nucleus reticularis thalami neurons and potently inhibit burst firing; decrease in release of 5-HT may promote the occurrence of rhythmic burst oscillations (Pape and McCormick, 1989).
In our study, duloxetine differed from fluoxetine in its effects both on established seizures in adult (7 weeks treatment started in rats at the age of 6 months) WAG/Rij rats and on comorbid depressive-like behaviour (see later). While fluoxetine was proabsence, duloxetine showed some antiabsence effects at the highest dose used. Noteworthy, the effects of both drugs were reversed by treatment withdrawal. The difference likely lies in the different mechanism of action and the ability of duloxetine to also block NA reuptake, and therefore, increase NA neurotransmission. In agreement, acute administration of venlafaxine, another selective 5-HT and NA reuptake inhibitor, partially suppressed seizure susceptibility in mice (Ahern et al., 2006) and acute administration of NA reuptake inhibitors is typically anticonvulsant (Yan et al., 1998; Ahern et al., 2006). Data obtained from animal models of epilepsy showed that damage to NA pathways produces increased seizure susceptibility (Mishra et al., 1994; Giorgi et al., 2004). Therefore, endogenous NA is a critical inhibitor of seizure activity; stimulation of NA signalling powerfully inhibits seizures, whereas depletion of NA increases seizure susceptibility and accelerates epileptogenesis in nearly every animal model tested (Weinshenker and Szot, 2002). No relevant data on the NA system in WAG/Rij rats are currently available to draw conclusions on the different effects of fluoxetine and duloxetine. Based on this knowledge, duloxetine might represent a better choice in case of fluoxetine-dependent increased hyperexcitability or in patients with absence epilepsy.
Effects of antidepressants and antipsychotics on the development of comorbid depressive-like behaviour
We found that ELTT with fluoxetine or duloxetine had completely different effects. Duloxetine did not influence animal behaviour while the highest dose (30 mg·kg−1 day−1) of fluoxetine reduced immobility time in the FST possessing, therefore, antidepressant-like effects. Our results on fluoxetine are partially in agreement with a previous study where it was found that chronic, but not acute, fluoxetine treatment reduced immobility time in the FST similarly to the results observed in our study with the 30 mg·kg−1 dose (Sarkisova and Folomkina, 2010).
Early studies on WAG/Rij rats' depressive-like behaviour demonstrated a relevant role for dopamine and less so for 5-HT. WAG/Rij rats exhibited elevated levels of cFos immediate early gene activation in multiple terminal regions for the dopamine system, including the frontal cortex, nucleus accumbens and striatum. Furthermore, administration of a D2/D3 receptor agonist showed antidepressant-like activity in the WAG/Rij rats, whereas a D2/D3 receptor antagonist exacerbated depressive phenotypes in the FST (Sarkisova et al., 2003; 2008,).
Lower levels of NA and dopamine were found in the nucleus accumbens, prefrontal cortex and striatum and increased density of D2-like dopamine receptors in the nucleus accumbens and ventral tegmental area. Therefore, a hypofunction of the mesolimbic dopamine system (nucleus accumbens) was suggested to be the neurochemical mechanism of depressive-like behaviour in WAG/Rij rats (Sarkisova et al., 2013). More recently, some other papers supported the relationship between absence seizures and depressive-like behaviour in this strain, indicating that SWDs are necessary for the development of depressive-like behaviour (Sarkisova et al., 2010). However, depending on the drug used (i.e. levetiracetam and zonisamide), even when the development of absence seizures was reduced, immobility time was not influenced (higher immobility time in comparison with non-epileptic control rats) and this might be justified by other effects possessed by the drug; in other words, a drug known to induce depressive-like behaviour might have antiepileptogenic effects, but in this specific model where depressive-like behaviour is linked to the development of absence seizures, would also compensate the expected positive effects (reduction in immobility time) on behaviour without inducing significant effects in the FST (Russo et al., 2011a,b; 2013b,,). In this case, the data reported in the present paper supports the idea that neither fluoxetine nor duloxetine are able to prevent comorbid depressive-like behaviour directly and that the observed effects for fluoxetine are only due to its effects on the development of absence seizures. This is also supported by the fact that the lower fluoxetine dose used did not have any effect on the development of absence seizure and induced a pro-depressant-like effect.
Conclusions
The treatment of neuropsychiatric comorbidity in epilepsy is a very actual topic, which has gained much attention in the last decade (Brooks-Kayal et al., 2013). Concomitantly, the use of drugs to treat comorbidity in people with epilepsy is a daily need (Kerr et al., 2011). The role of these drugs in epileptogenesis and their efficacy in people with epilepsy deserves to be further investigated. Our study was both limited by the number of drugs tested (within the groups of antipsychotics and antidepressants there might be differences between single molecules) and the fact that animal studies might not be directly translated into clinical practice. However, our data indicate that antipsychotics, while not seeming to aggravate the epileptogenic process, are pro-absence, which increases the number of seizures and might worsen depressive-like comorbidity. Within the drugs tested (haloperidol, risperidone and quetiapine), quetiapine seemed to be the safest as it did not increase seizure occurrence or aggravate depressive-like behaviour. On the other hand, the two antidepressants, fluoxetine and duloxetine, showed antiepileptogenic effects reducing the development of spontaneous seizures when treatment was started before seizure onset. Between the two drugs, which differ in their mechanism of action, duloxetine seemed to be safer as it was also able to reduce established absence seizures in adult animals while fluoxetine increased their incidence. However, only fluoxetine was able to prevent the development of comorbid depressive-like behaviour.
Author contributions
E. R, P. D. F and G. D. S. wrote the final version of the paper; E. R. and R. C. designed the protocol and wrote the first draft; R. C, E. R and A. L. performed the research and analysed the data.
Conflict of interest
None.
Glossary
- DLX
duloxetine
- dSWDs
mean SWDs total duration for a 30 min epoch
- ELTT
early long-term treatment
- FLX
fluoxetine
- FST
forced swimming test
- GAERS
genetic absence epilepsy rats from Strasbourg
- HAL
haloperidol
- nSWDs
number of SWDs
- QTP
quetiapine
- RISP
risperidone
- sSWD
SWDs mean single duration
- SWDs
spike-wave discharges
- WAG/Rij rats
Wistar Albino Glaxo/Rijswijk rats
References
- Ahern TH, Javors MA, Eagles DA, Martillotti J, Mitchell HA, Liles LC, et al. The effects of chronic norepinephrine transporter inactivation on seizure susceptibility in mice. Neuropsychopharmacology. 2006;31:730–738. doi: 10.1038/sj.npp.1300847. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdy G, Kecskemeti V, Riba P, Jakus R. Serotonin and epilepsy. J Neurochem. 2007;100:857–873. doi: 10.1111/j.1471-4159.2006.04277.x. [DOI] [PubMed] [Google Scholar]
- Berg AT, Levy SR, Testa FM, Blumenfeld H. Long-term seizure remission in childhood absence epilepsy: might initial treatment matter? Epilepsia. 2014;55:551–557. doi: 10.1111/epi.12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld H, Klein JP, Schridde U, Vestal M, Rice T, Khera DS, et al. Early treatment suppresses the development of spike-wave epilepsy in a rat model. Epilepsia. 2008;49:400–409. doi: 10.1111/j.1528-1167.2007.01458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352:1112–1120. doi: 10.1056/NEJMra041867. [DOI] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Bath KG, Berg AT, Galanopoulou AS, Holmes GL, Jensen FE, et al. Issues related to symptomatic and disease-modifying treatments affecting cognitive and neuropsychiatric comorbidities of epilepsy. Epilepsia. 2013;54(Suppl. 4):44–60. doi: 10.1111/epi.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin NM, van Luijtelaar EL, Jansen SJ, Cools AR, Ellenbroek BA. Dopamine characteristics in different rat genotypes: the relation to absence epilepsy. Neurosci Res. 2000;38:165–173. doi: 10.1016/s0168-0102(00)00154-1. [DOI] [PubMed] [Google Scholar]
- de Bruin NM, van Luijtelaar EL, Cools AR, Ellenbroek BA. Dopamine characteristics in rat genotypes with distinct susceptibility to epileptic activity: apomorphine-induced stereotyped gnawing and novelty/amphetamine-induced locomotor stimulation. Behav Pharmacol. 2001;12:517–525. doi: 10.1097/00008877-200111000-00013. [DOI] [PubMed] [Google Scholar]
- Caplan R, Siddarth P, Stahl L, Lanphier E, Vona P, Gurbani S, et al. Childhood absence epilepsy: behavioral, cognitive, and linguistic comorbidities. Epilepsia. 2008;49:1838–1846. doi: 10.1111/j.1528-1167.2008.01680.x. [DOI] [PubMed] [Google Scholar]
- Cardamone L, Salzberg MR, O'Brien TJ, Jones NC. Antidepressant therapy in epilepsy: can treating the comorbidities affect the underlying disorder? Br J Pharmacol. 2013;168:1531–1554. doi: 10.1111/bph.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalheiro EA, Fernandes MJ, Turski L, Naffah-Mazzacoratti MG. Spontaneous recurrent seizures in rats: amino acid and monoamine determination in the hippocampus. Epilepsia. 1994;35:1–11. doi: 10.1111/j.1528-1157.1994.tb02905.x. [DOI] [PubMed] [Google Scholar]
- Cerullo MA, Strakowski SM. A systematic review of the evidence for the treatment of acute depression in bipolar I disorder. CNS Spectr. 2013;18:199–208. doi: 10.1017/S1092852913000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YF, Paalzow LK. Linear pharmacokinetics of haloperidol in the rat. Biopharm Drug Dispos. 1992;13:69–76. doi: 10.1002/bdd.2510130106. [DOI] [PubMed] [Google Scholar]
- Citraro R, Scicchitano F, De Fazio S, Raggio R, Mainardi P, Perucca E, et al. Preclinical activity profile of alpha-lactoalbumin, a whey protein rich in tryptophan, in rodent models of seizures and epilepsy. Epilepsy Res. 2011;95:60–69. doi: 10.1016/j.eplepsyres.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Citraro R, Russo E, Ngomba RT, Nicoletti F, Scicchitano F, Whalley BJ, et al. CB1 agonists, locally applied to the cortico-thalamic circuit of rats with genetic absence epilepsy, reduce epileptic manifestations. Epilepsy Res. 2013a;106:74–82. doi: 10.1016/j.eplepsyres.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Citraro R, Russo E, Scicchitano F, van Rijn CM, Cosco D, Avagliano C, et al. Antiepileptic action of N-palmitoylethanolamine through CB1 and PPAR-alpha receptor activation in a genetic model of absence epilepsy. Neuropharmacology. 2013b;69:115–126. doi: 10.1016/j.neuropharm.2012.11.017. [DOI] [PubMed] [Google Scholar]
- Citraro R, Chimirri S, Aiello R, Gallelli L, Trimboli F, Britti D, et al. Protective effects of some statins on epileptogenesis and depressive-like behavior in WAG/Rij rats, a genetic animal model of absence epilepsy. Epilepsia. 2014;55:1284–1291. doi: 10.1111/epi.12686. [DOI] [PubMed] [Google Scholar]
- Citraro R, Leo A, Aiello R, Pugliese M, Russo E, De Sarro G. Comparative analysis of the treatment of chronic antipsychotic drugs on epileptic susceptibility in genetically epilepsy-prone rats. Neurother. 2015;12:250–262. doi: 10.1007/s13311-014-0318-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciumas C, Wahlin TB, Jucaite A, Lindstrom P, Halldin C, Savic I. Reduced dopamine transporter binding in patients with juvenile myoclonic epilepsy. Neurology. 2008;71:788–794. doi: 10.1212/01.wnl.0000316120.70504.d5. [DOI] [PubMed] [Google Scholar]
- Correll CU, Kane JM. Schizophrenia: mechanism of action of current and novel treatments. J Clin Psychiatry. 2014;75:347–348. doi: 10.4088/JCP.13078co8c. [DOI] [PubMed] [Google Scholar]
- Deransart C, Riban V, Le B, Marescaux C, Depaulis A. Dopamine in the striatum modulates seizures in a genetic model of absence epilepsy in the rat. Neuroscience. 2000;100:335–344. doi: 10.1016/s0306-4522(00)00266-9. [DOI] [PubMed] [Google Scholar]
- Deransart C, Landwehrmeyer GB, Feuerstein TJ, Lucking CH. Up-regulation of D3 dopaminergic receptor mRNA in the core of the nucleus accumbens accompanies the development of seizures in a genetic model of absence-epilepsy in the rat. Brain Res Mol Brain Res. 2001;94:166–177. doi: 10.1016/s0169-328x(01)00240-6. [DOI] [PubMed] [Google Scholar]
- Dezsi G, Ozturk E, Stanic D, Powell KL, Blumenfeld H, O'Brien TJ, et al. Ethosuximide reduces epileptogenesis and behavioral comorbidity in the GAERS model of genetic generalized epilepsy. Epilepsia. 2013;54:635–643. doi: 10.1111/epi.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobryakova YV, Dubynin VA, Luijtelaar G. The effect of haloperidol on maternal behavior in WAG/Rij rats and its consequences in the offspring. Acta Neurobiol Exp (Wars) 2011;71:339–347. doi: 10.55782/ane-2011-1856. [DOI] [PubMed] [Google Scholar]
- Favale E, Audenino D, Cocito L, Albano C. The anticonvulsant effect of citalopram as an indirect evidence of serotonergic impairment in human epileptogenesis. Seizure. 2003;12:316–318. doi: 10.1016/s1059-1311(02)00315-1. [DOI] [PubMed] [Google Scholar]
- Filakovszky J, Gerber K, Bagdy G. A serotonin-1A receptor agonist and an N-methyl-D-aspartate receptor antagonist oppose each others effects in a genetic rat epilepsy model. Neurosci Lett. 1999;261:89–92. doi: 10.1016/s0304-3940(99)00015-4. [DOI] [PubMed] [Google Scholar]
- Gerber K, Filakovszky J, Halasz P, Bagdy G. The 5-HT1A agonist 8-OH-DPAT increases the number of spike-wave discharges in a genetic rat model of absence epilepsy. Brain Res. 1998;807:243–245. doi: 10.1016/s0006-8993(98)00801-4. [DOI] [PubMed] [Google Scholar]
- Giblin KA, Blumenfeld H. Is epilepsy a preventable disorder? New evidence from animal models. Neuroscientist. 2010;16:253–275. doi: 10.1177/1073858409354385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi FS, Pizzanelli C, Biagioni F, Murri L, Fornai F. The role of norepinephrine in epilepsy: from the bench to the bedside. Neurosci Biobehav Rev. 2004;28:507–524. doi: 10.1016/j.neubiorev.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Graf M, Jakus R, Kantor S, Levay G, Bagdy G. Selective 5-HT1A and 5-HT7 antagonists decrease epileptic activity in the WAG/Rij rat model of absence epilepsy. Neurosci Lett. 2004;359:45–48. doi: 10.1016/j.neulet.2004.01.072. [DOI] [PubMed] [Google Scholar]
- Hamid H, Kanner AM. Should antidepressant drugs of the selective serotonin reuptake inhibitor family be tested as antiepileptic drugs? Epilepsy Behav. 2013;26:261–265. doi: 10.1016/j.yebeh.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Hesdorffer DC, Ishihara L, Mynepalli L, Webb DJ, Weil J, Hauser WA. Epilepsy, suicidality, and psychiatric disorders: a bidirectional association. Ann Neurol. 2012;72:184–191. doi: 10.1002/ana.23601. [DOI] [PubMed] [Google Scholar]
- Hoppe C, Elger CE. Depression in epilepsy: a critical review from a clinical perspective. Nat Rev Neurol. 2011;7:462–472. doi: 10.1038/nrneurol.2011.104. [DOI] [PubMed] [Google Scholar]
- Igelstrom KM. Preclinical antiepileptic actions of selective serotonin reuptake inhibitors – implications for clinical trial design. Epilepsia. 2012;53:596–605. doi: 10.1111/j.1528-1167.2012.03427.x. [DOI] [PubMed] [Google Scholar]
- Itil TM, Soldatos C. Epileptogenic side effects of psychotropic drugs. Practical recommendations. JAMA. 1980;244:1460–1463. [PubMed] [Google Scholar]
- Jakus R, Graf M, Juhasz G, Gerber K, Levay G, Halasz P, et al. 5-HT2C receptors inhibit and 5-HT1A receptors activate the generation of spike-wave discharges in a genetic rat model of absence epilepsy. Exp Neurol. 2003;184:964–972. doi: 10.1016/S0014-4886(03)00352-2. [DOI] [PubMed] [Google Scholar]
- Kanner AM. Should neurologists be trained to recognize and treat comorbid depression of neurologic disorders? Yes. Epilepsy Behav. 2005;6:303–311. doi: 10.1016/j.yebeh.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Kanner AM. Depression and epilepsy: a bidirectional relation? Epilepsia. 2011;52(Suppl. 1):21–27. doi: 10.1111/j.1528-1167.2010.02907.x. [DOI] [PubMed] [Google Scholar]
- Kanner AM. Can neurobiological pathogenic mechanisms of depression facilitate the development of seizure disorders? Lancet Neurol. 2012;11:1093–1102. doi: 10.1016/S1474-4422(12)70201-6. [DOI] [PubMed] [Google Scholar]
- Kanner AM, Schachter SC, Barry JJ, Hesdorffer DC, Mula M, Trimble M, et al. Depression and epilepsy: epidemiologic and neurobiologic perspectives that may explain their high comorbid occurrence. Epilepsy Behav. 2012;24:156–168. doi: 10.1016/j.yebeh.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Kapur S, VanderSpek SC, Brownlee BA, Nobrega JN. Antipsychotic dosing in preclinical models is often unrepresentative of the clinical condition: a suggested solution based on in vivo occupancy. J Pharmacol Exp Ther. 2003;305:625–631. doi: 10.1124/jpet.102.046987. [DOI] [PubMed] [Google Scholar]
- Kerr MP, Mensah S, Besag F, de Toffol B, Ettinger A, Kanemoto K, et al. International consensus clinical practice statements for the treatment of neuropsychiatric conditions associated with epilepsy. Epilepsia. 2011;52:2133–2138. doi: 10.1111/j.1528-1167.2011.03276.x. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarova M, Bendotti C, Samanin R. The role of different types of adrenergic receptors in pentylenetetrazol-induced seizures and the effect of di-n-propylacetate in the rat. Psychopharmacology (Berl) 1983;81:177–182. doi: 10.1007/BF00429015. [DOI] [PubMed] [Google Scholar]
- Lertxundi U, Hernandez R, Medrano J, Domingo-Echaburu S, Garcia M, Aguirre C. Antipsychotics and seizures: higher risk with atypicals? Seizure. 2013;22:141–143. doi: 10.1016/j.seizure.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Li Y, Raaby KF, Sanchez C, Gulinello M. Serotonergic receptor mechanisms underlying antidepressant-like action in the progesterone withdrawal model of hormonally induced depression in rats. Behav Brain Res. 2013;256:520–528. doi: 10.1016/j.bbr.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Lopez-Rubalcava C, Lucki I. Strain differences in the behavioral effects of antidepressant drugs in the rat forced swimming test. Neuropsychopharmacology. 2000;22:191–199. doi: 10.1016/S0893-133X(99)00100-1. [DOI] [PubMed] [Google Scholar]
- van Luijtelaar G, Zobeiri M. Progress and outlooks in a genetic absence epilepsy model (WAG/Rij) Curr Med Chem. 2014;21:704–721. doi: 10.2174/0929867320666131119152913. [DOI] [PubMed] [Google Scholar]
- van Luijtelaar G, Sitnikova E, Littjohann A. On the origin and suddenness of absences in genetic absence models. Clin EEG Neurosci. 2011;42:83–97. doi: 10.1177/155005941104200209. [DOI] [PubMed] [Google Scholar]
- van Luijtelaar G, Onat FY, Gallagher MJ. Animal models of absence epilepsies: what do they model and do sex and sex hormones matter? Neurobiol Dis. 2014;72(Pt B):167–179. doi: 10.1016/j.nbd.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji HK, Quiroz JA, Sporn J, Payne JL, Denicoff K, A Gray N, et al. Enhancing neuronal plasticity and cellular resilience to develop novel, improved therapeutics for difficult-to-treat depression. Biol Psychiatry. 2003;53:707–742. doi: 10.1016/s0006-3223(03)00117-3. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Drummond GB, McLachlan EM, Kilkenny C, Wainwright CL. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Able JA, Liu Y, Jandacek R, Rider T, Tso P, et al. Omega-3 fatty acid deficiency does not alter the effects of chronic fluoxetine treatment on central serotonin turnover or behavior in the forced swim test in female rats. Pharmacol Biochem Behav. 2013;114–115:1–8. doi: 10.1016/j.pbb.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midzianovskaia IS, Kuznetsova GD, Coenen AM, Spiridonov AM, van Luijtelaar EL. Electrophysiological and pharmacological characteristics of two types of spike-wave discharges in WAG/Rij rats. Brain Res. 2001;911:62–70. doi: 10.1016/s0006-8993(01)02705-6. [DOI] [PubMed] [Google Scholar]
- Mishra PK, Burger RL, Bettendorf AF, Browning RA, Jobe PC. Role of norepinephrine in forebrain and brainstem seizures: chemical lesioning of locus ceruleus with DSP4. Exp Neurol. 1994;125:58–64. doi: 10.1006/exnr.1994.1006. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Kinkead B, Goldstein J. Quetiapine: preclinical studies, pharmacokinetics, drug interactions, and dosing. J Clin Psychiatry. 2002;63(Suppl. 13):5–11. [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman TR, Gupta RK, Burrows GD, Parker G, Judd FK. Relationship between antidepressant response and plasma concentrations of fluoxetine and norfluoxetine. Int Clin Psychopharmacol. 1993;8:25–29. doi: 10.1097/00004850-199300810-00004. [DOI] [PubMed] [Google Scholar]
- Pape HC, McCormick DA. Noradrenaline and serotonin selectively modulate thalamic burst firing by enhancing a hyperpolarization-activated cation current. Nature. 1989;340:715–718. doi: 10.1038/340715a0. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucl Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perucca E. The management of refractory idiopathic epilepsies. Epilepsia. 2001;42(Suppl. 3):31–35. doi: 10.1046/j.1528-1157.2001.042suppl.3031.x. [DOI] [PubMed] [Google Scholar]
- Pisani F, Oteri G, Costa C, Di Raimondo G, Di Perri R. Effects of psychotropic drugs on seizure threshold. Drug Saf. 2002;25:91–110. doi: 10.2165/00002018-200225020-00004. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Engel J., Jr Past and present definitions of epileptogenesis and its biomarkers. Neurother. 2014;11:231–241. doi: 10.1007/s13311-014-0257-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen A, Nehlig A, Brooks-Kayal AR, Dudek FE, Friedman D, Galanopoulou AS, et al. Issues related to development of antiepileptogenic therapies. Epilepsia. 2013;54(Suppl. 4):35–43. doi: 10.1111/epi.12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendiville S, Gale K. Anticonvulsant effect of fluoxetine on focally evoked limbic motor seizures in rats. Epilepsia. 1993;34:381–384. doi: 10.1111/j.1528-1157.1993.tb02425.x. [DOI] [PubMed] [Google Scholar]
- Rai D, Kerr MP, McManus S, Jordanova V, Lewis G, Brugha TS. Epilepsy and psychiatric comorbidity: a nationally representative population-based study. Epilepsia. 2012;53:1095–1103. doi: 10.1111/j.1528-1167.2012.03500.x. [DOI] [PubMed] [Google Scholar]
- Reneric JP, Lucki I. Antidepressant behavioral effects by dual inhibition of monoamine reuptake in the rat forced swimming test. Psychopharmacology (Berl) 1998;136:190–197. doi: 10.1007/s002130050555. [DOI] [PubMed] [Google Scholar]
- Richerson GB, Buchanan GF. The serotonin axis: shared mechanisms in seizures, depression, and SUDEP. Epilepsia. 2011;52(Suppl. 1):28–38. doi: 10.1111/j.1528-1167.2010.02908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo E, Citraro R, Scicchitano F, De Fazio S, Di Paola ED, Constanti A, et al. Comparison of the antiepileptogenic effects of an early long-term treatment with ethosuximide or levetiracetam in a genetic animal model of absence epilepsy. Epilepsia. 2010;51:1560–1569. doi: 10.1111/j.1528-1167.2009.02400.x. [DOI] [PubMed] [Google Scholar]
- Russo E, Citraro R, Scicchitano F, De Fazio S, Perrotta I, Di Paola ED, et al. Effects of early long-term treatment with antiepileptic drugs on development of seizures and depressive-like behavior in a rat genetic absence epilepsy model. Epilepsia. 2011a;52:1341–1350. doi: 10.1111/j.1528-1167.2011.03112.x. [DOI] [PubMed] [Google Scholar]
- Russo E, Citraro R, Scicchitano F, Urzino A, Marra R, Rispoli V, et al. Vigabatrin has antiepileptogenic and antidepressant effects in an animal model of epilepsy and depression comorbidity. Behav Brain Res. 2011b;225:373–376. doi: 10.1016/j.bbr.2011.07.030. [DOI] [PubMed] [Google Scholar]
- Russo E, Scicchitano F, Citraro R, Aiello R, Camastra C, Mainardi P, et al. Protective activity of alpha-lactoalbumin (ALAC), a whey protein rich in tryptophan, in rodent models of epileptogenesis. Neuroscience. 2012;226:282–288. doi: 10.1016/j.neuroscience.2012.09.021. [DOI] [PubMed] [Google Scholar]
- Russo E, Citraro R, Davoli A, Gallelli L, Di Paola ED, De Sarro G. Ameliorating effects of aripiprazole on cognitive functions and depressive-like behavior in a genetic rat model of absence epilepsy and mild-depression comorbidity. Neuropharmacology. 2013a;64:371–379. doi: 10.1016/j.neuropharm.2012.06.039. [DOI] [PubMed] [Google Scholar]
- Russo E, Citraro R, Donato G, Camastra C, Iuliano R, Cuzzocrea S, et al. mTOR inhibition modulates epileptogenesis, seizures and depressive behavior in a genetic rat model of absence epilepsy. Neuropharmacology. 2013b;69:25–36. doi: 10.1016/j.neuropharm.2012.09.019. [DOI] [PubMed] [Google Scholar]
- Sander T, Berlin W, Ostapowicz A, Samochowiec J, Gscheidel N, Hoehe MR. Variation of the genes encoding the human glutamate EAAT2, serotonin and dopamine transporters and Susceptibility to idiopathic generalized epilepsy. Epilepsy Res. 2000;41:75–81. doi: 10.1016/s0920-1211(00)00120-0. [DOI] [PubMed] [Google Scholar]
- Sankar R, Mazarati A. Neurobiology of depression as a comorbidity of epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 4th edn. Bethesda, MD: Oxford University Press; 2012. [Google Scholar]
- Sarkisova K, Folomkina AA. [Effect of selective serotonin reuptake inhibitor fluoxetine on symptoms of depression-like behavior in WAG/Rij rats] Zh Vyssh Nerv Deiat Im I P Pavlova. 2010;60:98–108. [PubMed] [Google Scholar]
- Sarkisova K, van Luijtelaar G. The WAG/Rij strain: a genetic animal model of absence epilepsy with comorbidity of depression [corrected] Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:854–876. doi: 10.1016/j.pnpbp.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Sarkisova K, Kulikov MA, Kudrin VS, Narkevich VB, Midzianovskaia IS, Biriukova LM, et al. [Neurochemical mechanisms of depression-like behavior in WAG/Rij rats] Zh Vyssh Nerv Deiat Im I P Pavlova. 2013;63:303–315. doi: 10.7868/s0044467713030106. [DOI] [PubMed] [Google Scholar]
- Sarkisova KY, Midzianovskaia IS, Kulikov MA. Depressive-like behavioral alterations and c-fos expression in the dopaminergic brain regions in WAG/Rij rats with genetic absence epilepsy. Behav Brain Res. 2003;144:211–226. doi: 10.1016/s0166-4328(03)00090-1. [DOI] [PubMed] [Google Scholar]
- Sarkisova KY, Kulikov MA, Midzyanovskaya IS, Folomkina AA. Dopamine-dependent nature of depression-like behavior in WAG/Rij rats with genetic absence epilepsy. Neurosci Behav Physiol. 2008;38:119–128. doi: 10.1007/s11055-008-0017-z. [DOI] [PubMed] [Google Scholar]
- Sarkisova KY, Kuznetsova GD, Kulikov MA, van Luijtelaar G. Spike-wave discharges are necessary for the expression of behavioral depression-like symptoms. Epilepsia. 2010;51:146–160. doi: 10.1111/j.1528-1167.2009.02260.x. [DOI] [PubMed] [Google Scholar]
- Savic I, Lindstrom P, Gulyas B, Halldin C, Andree B, Farde L. Limbic reductions of 5-HT1A receptor binding in human temporal lobe epilepsy. Neurology. 2004;62:1343–1351. doi: 10.1212/01.wnl.0000123696.98166.af. [DOI] [PubMed] [Google Scholar]
- Schmitt U, Dahmen N, Fischer V, Weigmann H, Rao ML, Reuss S, et al. Chronic oral haloperidol and clozapine in rats: a behavioral evaluation. Neuropsychobiology. 1999;39:86–91. doi: 10.1159/000026566. [DOI] [PubMed] [Google Scholar]
- Statnick MA, Dailey JW, Jobe PC, Browning RA. Abnormalities in 5-HT1A and 5-HT1B receptor binding in severe-seizure genetically epilepsy-prone rats (GEPR-9s) Neuropharmacology. 1996a;35:111–118. doi: 10.1016/0028-3908(95)00141-7. [DOI] [PubMed] [Google Scholar]
- Statnick MA, Maring-Smith ML, Clough RW, Wang C, Dailey JW, Jobe PC, et al. Effect of 5,7-dihydroxytryptamine on audiogenic seizures in genetically epilepsy-prone rats. Life Sci. 1996b;59:1763–1771. doi: 10.1016/0024-3205(96)00519-x. [DOI] [PubMed] [Google Scholar]
- Stefulj J, Bordukalo-Niksic T, Hecimovic H, Demarin V, Jernej B. Epilepsy and serotonin (5-HT): variations of 5-HT-related genes in temporal lobe epilepsy. Neurosci Lett. 2010;478:29–31. doi: 10.1016/j.neulet.2010.04.060. [DOI] [PubMed] [Google Scholar]
- Trindade-Filho EM, de Castro-Neto EF, de A Carvalho RR, Lima E, Scorza FA, Amado D, et al. Serotonin depletion effects on the pilocarpine model of epilepsy. Epilepsy Res. 2008;82:194–199. doi: 10.1016/j.eplepsyres.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Vega C, Vestal M, DeSalvo M, Berman R, Chung M, Blumenfeld H, et al. Differentiation of attention-related problems in childhood absence epilepsy. Epilepsy Behav. 2010;19:82–85. doi: 10.1016/j.yebeh.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volonteri LS, Colasanti A, Cerveri G, Fiorentini A, De Gaspari IF, Mauri MC, et al. Clinical outcome and tolerability of duloxetine in the treatment of major depressive disorder: a 12-week study with plasma levels. J Psychopharmacol. 2010;24:1193–1199. doi: 10.1177/0269881109104863. [DOI] [PubMed] [Google Scholar]
- Warter JM, Vergnes M, Depaulis A, Tranchant C, Rumbach L, Micheletti G, et al. Effects of drugs affecting dopaminergic neurotransmission in rats with spontaneous petit mal-like seizures. Neuropharmacology. 1988;27:269–274. doi: 10.1016/0028-3908(88)90043-3. [DOI] [PubMed] [Google Scholar]
- Weinshenker D, Szot P. The role of catecholamines in seizure susceptibility: new results using genetically engineered mice. Pharmacol Ther. 2002;94:213–233. doi: 10.1016/s0163-7258(02)00218-8. [DOI] [PubMed] [Google Scholar]
- White HS, Loscher W. Searching for the ideal antiepileptogenic agent in experimental models: single treatment versus combinatorial treatment strategies. Neurother. 2014;11:373–384. doi: 10.1007/s13311-013-0250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DT. Duloxetine (LY 248686): an inhibitor of serotonin and noradrenaline uptake and an antidepressant drug candidate. Expert Opin Investig Drugs. 1998;7:1691–1699. doi: 10.1517/13543784.7.10.1691. [DOI] [PubMed] [Google Scholar]
- Yan QS, Jobe PC, Cheong JH, Ko KH, Dailey JW. Role of serotonin in the anticonvulsant effect of fluoxetine in genetically epilepsy-prone rats. Naunyn Schmiedebergs Arch Pharmacol. 1994a;350:149–152. doi: 10.1007/BF00241089. [DOI] [PubMed] [Google Scholar]
- Yan QS, Jobe PC, Dailey JW. Evidence that a serotonergic mechanism is involved in the anticonvulsant effect of fluoxetine in genetically epilepsy-prone rats. Eur J Pharmacol. 1994b;252:105–112. doi: 10.1016/0014-2999(94)90581-9. [DOI] [PubMed] [Google Scholar]
- Yan QS, Dailey JW, Steenbergen JL, Jobe PC. Anticonvulsant effect of enhancement of noradrenergic transmission in the superior colliculus in genetically epilepsy-prone rats (GEPRs): a microinjection study. Brain Res. 1998;780:199–209. doi: 10.1016/s0006-8993(97)01139-6. [DOI] [PubMed] [Google Scholar]


