Virologic analysis of tissues from a patient with giant cell arteritis (GCA) who was treated with corticosteroids and died of extensive necrotizing granulomatous arteritis revealed widespread varicella-zoster virus (VZV) antigen in multiple large arteries. Long-term treatment with corticosteroids likely potentiated VZV infection.
In 1997, a clinicopathologic case report1 described a 75-year-old woman with fatal aggressive steroid-refractory GCA that manifested as bilateral vision loss and myelopathy while on treatment with corticosteroids. The woman was not otherwise immunocompromised before becoming ill, and no cutaneous signs of herpesvirus infection developed during her 5-month illness. Bilateral temporal artery (TA) biopsies revealed GCA. Postmortem examination revealed spinal cord infarction secondary to extensive necrotizing granulomatous arteritis of spinal arteries.
Based on detection of VZV in GCA-positive TAs2 and documented involvement of other large arteries in most patients with GCA,3 we revisited this case and searched for VZV in the archived TAs and in other large arteries, the spinal cord, and brain. Immunohistochemical examination detected VZV antigen (figure) in both TAs, in the aorta, in the left carotid artery, and in an unidentified artery from the Circle of Willis but not in renal or mesenteric arteries. Viral antigen was not seen in the brain or spinal cord. DNA extracted from every section of each VZV antigen–positive artery was analyzed by PCR with primers specific for VZV and herpes simplex virus (HSV)-1 as described2 and revealed VZV DNA, but not HSV-1 DNA, in the unidentified cerebral artery from the Circle of Willis despite formalin fixation.
Figure. Varicella-zoster virus antigen in the temporal artery, aorta, and carotid artery of a patient with refractory giant cell arteritis.
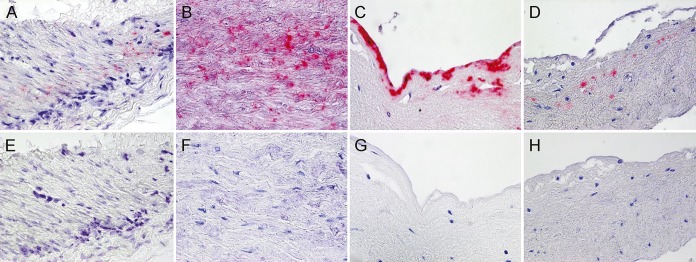
Immunohistochemical staining with mouse anti–varicella-zoster virus (VZV) gE IgG1 antibody2 revealed VZV antigen in the media of the temporal artery (A), the media (B) and intima (C) of the aorta, and the intima of the carotid artery (D) that was not seen when mouse isotype control antibody was substituted for anti-VZV gE IgG1 antibody (E–H).
Discussion.
The pathologic diagnosis of VZV arteritis comes nearly 20 years after this patient's death. Reexamination of this case was prompted by the recognition that VZV is commonly found in the TAs of patients with GCA. VZV and extensive granulomatous arteritis in multiple large arteries was most likely due to reactivation of VZV in an elderly woman followed by potentiation of virus infection by several months of high-dose corticosteroids. Widespread vasculopathy occurs in patients with GCA treated with long-term corticosteroids.4 Recently, virologic studies in a man with thoracic-distribution zoster and a history of corticosteroid abuse who died suddenly revealed extensive VZV infection in multiple organs and arteries, particularly the coronary arteries and aorta, along with subclinical VZV vasculopathy.5 Large artery involvement in GCA has been increasingly documented.3,6 Finally, our findings confirm detection of VZV antigen and VZV DNA in GCA-positive TAs2,7 and indicate that productive VZV infection in the TAs of patients with GCA parallels productive VZV infection in intracerebral arteries of patients with VZV vasculopathy. In fact, GCA is likely to be a form of VZV vasculopathy that predominantly, but not exclusively, affects the TA. Because VZV is triggering the immunopathology of GCA, it is likely that treatment of GCA patients with antiviral agents will not only shorten the course of corticosteroids needed to reduce the immunopathology that produces disease but also prevent spread of VZV and the development of disseminated granulomatous arteritis.
Acknowledgments
Acknowledgment: The authors thank Marina Hoffman for editorial review and Cathy Allen for word processing and formatting.
Footnotes
Author contributions: Dr. Gilden: drafted and revised the manuscript for content, designed and supervised the study, collected, analyzed, and interpreted data. Ms. White: collected, analyzed, and interpreted data, revised the manuscript for content. Dr. Galetta: supplied clinical material necessary for conducting the study, revised the manuscript for content. Dr. Fogt: supplied clinical material necessary for conducting the study, revised the manuscript for content. Dr. Nagel: drafted and revised the manuscript for content, designed and supervised the study, collected, analyzed, and interpreted data.
Study funding: This work was supported in part by NIH grants AG032958 to D.G. and M.A.N.
Disclosure: D. Gilden is a senior associate editor for Journal of NeuroVirology; is on the editorial board for In Vivio, Journal of Virology, Scientific American Medicine, Virus Genes, Neurology, and Journal of the Neurological Sciences; and received research support from NIH. T. White reports no disclosures. S.L. Galetta has received travel funding and/or speaker honoraria from Biogen and Genzyme; is on the editorial board for Neurology and Journal of Neuro-ophthalmology; and has consulted for Genzyme and Biogen. F. Fogt reports no disclosures. M.A. Nagel received research support from NIH. Go to Neurology.org/nn for full disclosure forms. The Article Processing Charge was paid by the authors.
References
- 1.Galetta SL, Balcer LJ, Lieberman AP, Syed NA, Lee JM, Oberholtzer JC. Refractory giant cell arteritis with spinal cord infarction. Neurology 1997;49:1720–1723. [DOI] [PubMed] [Google Scholar]
- 2.Gilden D, White T, Khmeleva N, et al. Prevalence and distribution of VZV in temporal arteries of patients with giant cell arteritis. Neurology 2015;84:1948–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prieto-González S, Arguis P, García-Martínez A, et al. Large vessel involvement in biopsy-proven giant cell arteritis: prospective study in 40 newly diagnosed patients using CT angiography. Ann Rheum Dis 2012;71:1170–1176. [DOI] [PubMed] [Google Scholar]
- 4.Parra J, Domingues J, Sargento-Freitas J, Santana I. Extensive intracranial involvement with multiple dissections in a case of giant cell arteritis. BMJ Case Rep 2014;2014. 10.1136/bcr-2014–204130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagel MA, Lenggenhager D, White T, et al. Disseminated VZV infection and asymptomatic VZV vasculopathy after steroid abuse. J Clin Virol 2015;66:72–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nuenninghoff DM, Hunder GG, Christianson TJH, McClelland RL, Matteson EL. Incidence and predictors of large-artery complication (aortic aneurysm, aortic dissection, and/or large-artery stenosis) in patients with giant cell arteritis. Arthritis Rheum 2003;48:3522–2531. [DOI] [PubMed] [Google Scholar]
- 7.Nagel MA, Khmeleva N, Boyer PJ, Choe A, Bert R, Gilden D. Varicella zoster virus in the temporal artery of a patient with giant cell arteritis. J Neurol Sci 2013;335:228–230. [DOI] [PMC free article] [PubMed] [Google Scholar]


