A 73-year-old woman presented with a 4-day history of progressive confusion. Her family reported that she was behaving erratically and had developed paranoia and hallucinations.
The patient had complained of intermittent headaches in the weeks leading up to this episode but had not reported other neurologic or systemic symptoms. There was no history of recent illnesses. There were no witnessed seizures.
She had history of hypertension, hypothyroidism, mild depression, and carotid endarterectomy for occlusive carotid artery disease.
On examination she was afebrile. Vital signs and general medical examination were unremarkable. She was agitated and had paranoid persecutory delusions as well as visual and auditory hallucinations. Her speech was at times incomprehensible and tangential. There were no focal abnormal neurologic signs.
She had mild neutrophilia of 10 × 109/L (2–8 × 109/L) with normal hemoglobin and platelet counts. Thyroid-stimulating hormone was elevated (5.4 mIU/L [0.27–4.2]), but T3 and T4 levels were within normal limits. Normal investigations included liver and renal function tests, antinuclear antibody, anti-neutrophil cytoplasmic antibody, extractable nuclear antigen, complement levels, serum protein electrophoresis, porphyria screen, EKG, chest x-ray, and blood cultures. HIV and Treponema pallidum serology were negative.
Initial CT and MRI brain were normal. CSF analysis was normal, including protein, glucose, cells, oligoclonal band screen, and PCR studies for herpes simplex virus 1 and 2, varicella-zoster virus, enterovirus, meningococcus, and pneumococcus. EEG showed severe bihemispheric slowing.
Because there was no evidence supporting an infectious cause for the patient's symptoms, a paraneoplastic/autoimmune encephalopathy was suspected. Tumor markers including αFPP, HCG, CA125, CA19, and CEA were negative. CT scan of thorax/abdomen/pelvis (with contrast) showed no evidence of malignancy.
Negative antibodies included those targeting thyroid peroxidase antibody, Hu, Ri, Yo, amphiphysin, glycine receptor, NMDA receptor, glutamic acid decarboxylase, Ma/Ma2, GABA receptor a/b, and CV2/CRMP5. A positive serum voltage-gated potassium channel complex-related protein antibody (VGKC-RP Ab) test was confirmed 4 weeks after admission with a low titer of 166 pM (0–100). The antibody was not directed against CASPR2 or LGI1.
The patient was treated with IV immunoglobulin (IVIg; 0.4 g/kg/day × 5 days), with clinical improvement associated with minor improvement in Montreal Cognitive Assessment (MoCA) scores (10/30 to 12/30).
Repeat MRI brain 6 weeks after admission showed symmetrical high signal changes on T2 and fluid-attenuated inversion recovery sequences predominantly affecting the cortex, subcortical, and deep white matter in the posterior temporal and parieto-occipital regions (figure). The limbic cortex was spared. There was no evidence of abnormal enhancement post contrast injection or diffusion restriction.
Figure. MRI brain 6 weeks post admission.
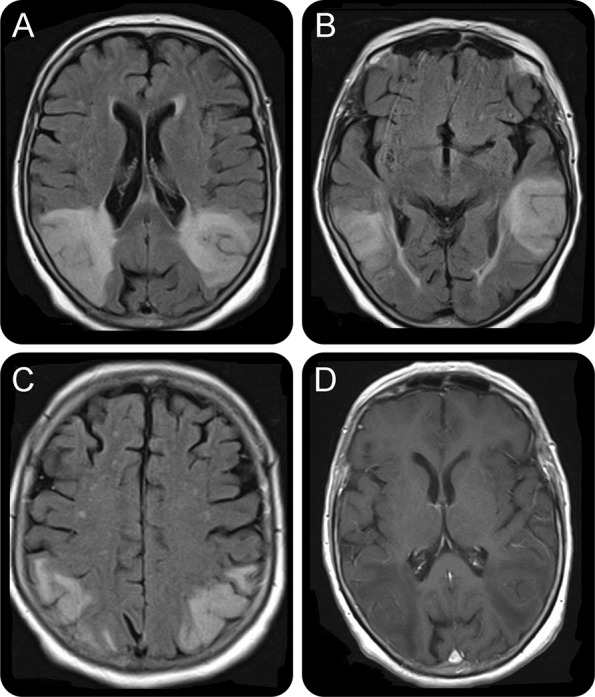
(A–C) Symmetrical high signal changes on fluid-attenuated inversion recovery sequences predominantly affecting the cortex, subcortical, and deep white matter in the posterior temporal and parieto-occipital regions. The areas of abnormal signal are wedge-shaped and extend to the ependymal surface of the lateral ventricles. There is gyral swelling and sulcal effacement. The limbic cortex is spared. (D) T1- weighted sequences plus contrast demonstrating lack of significant contrast enhancement. Of note, there was no diffusion restriction.
Repeat CSF analysis was negative for JC virus PCR and negative for anti-VGKC-RP and anti-NMDA receptor antibodies.
Following a second course of IVIg administered on the eighth week of admission, the patient improved significantly, with objective evidence of cognitive improvement (MoCA 18/30). Her paranoia and hallucinations also resolved, enabling safe discharge from hospital. EEG showed improvement but repeat MRI showed no change.
Over the following 8 months, the patient had 2 relapses characterized by agitation, confusion, and florid psychotic symptoms with paranoid delusions. On both occasions, her symptoms responded to IVIg therapy.
At the time of the second relapse, the patient's original serum sample was tested for antibodies against α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR). Antibodies against AMPAR GluR1 and GluR2 subunit were detected in the patient's serum. Mycophenolate mofetil (2 g/day) was commenced and she has had no further relapses to date.
AMPAR antibody–related encephalitis has recently been described.1 This patient's clinical presentation is consistent with the limited literature describing this entity, including the predilection for older women, pronounced psychotic features, good response to immunotherapy, and frequent relapses.2,3
The low titers of VGKC-RP Ab are likely to be an epiphenomenon. In a previous study, only 4 of 32 patients with low-titer VGKC-RP Ab were considered to have an autoimmune disorder.4 Our patient emphasizes the importance of continuing the search for neuronal antibodies in these cases to confirm the autoimmune diagnosis and to initiate the appropriate immunotherapy. It is worth noting in this case that mGlu5 and dipeptidyl-peptidase-like protein-6 antibodies were not checked and that a lumbar puncture was not repeated to investigate CSF anti-AMPAR levels.
The patient's imaging revealed posterior cortical and white matter involvement in the absence of limbic involvement reported in these cases.5 The patient had no risk factors for conditions typically associated with these radiologic findings, such as posterior reversible encephalopathy syndrome or progressive multifocal leukoencephalopathy. Moreover, her clinical presentation, including the relapsing course and the response to immunotherapy, would not support these diagnoses. The radiologic features associated with immune-mediated encephalopathies may be more extensive than previously recognized, particularly in the context of coexisting autoimmune conditions.6,7 This case suggests that, in the appropriate clinical context, the differential diagnosis of exclusive posterior cortical and white matter change should be expanded to include anti-AMPAR antibody encephalitis.
Footnotes
Author contributions: Dr. Marwa Elamin prepared and wrote the manuscript. Dr. Roisin Lonergan contributed to the intellectual content of the manuscript. Dr. Ronan P. Killeen critically revised the intellectual content of the manuscript. Dr. Sean O'Riordan critically revised the intellectual content of the manuscript. Professor Niall Tubridy critically revised the intellectual content of the manuscript. Dr. Christopher McGuigan supervised all stages of the manuscript and critically revised the intellectual content of the manuscript.
Study funding: No targeted funding reported.
Disclosure: M. Elamin reports no disclosures. R. Lonergan has received travel funding from Biogen Idec, Schering, Sanofi-Aventis, and Novartis. R.P. Killeen holds stock or stock options in Dublin Cyberknife Financing Limited. S. O'Riordan received travel funding from Novartis and Abbvie, speaker honoraria from Abbvie and Lundbeck, and research support from Novartis and Dystonia Ireland. N. Tubridy received travel funding from Novartis, Sanofi-Aventis and Bayer Schering. C. McGuigan received travel funding and/or speaker honoraria from Biogen Idec and Novartis and received research support from Biogen Idec, Genzyme, and Novartis. Go to Neurology.org/nn for full disclosure forms. The Article Processing Charge was paid by the authors.
References
- 1.Lai M, Hughes EG, Peng X, et al. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann Neurol 2009;65:424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graus F, Boronat A, Xifro X, et al. The expanding clinical profile of anti-AMPA receptor encephalitis. Neurology 2010;74:857–859. [DOI] [PubMed] [Google Scholar]
- 3.Kayser MS, Dalmau J. The emerging link between autoimmune disorders and neuropsychiatric disease. J Neuropsychiatry Clin Neurosci 2011;23:90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paterson RW, Zhandi MS, Armstrong R, Vincent A, Schott JM. Clinical relevance of positive voltage-gated potassium channel (VGKC)-complex antibodies: experience from a tertiary referral centre. J Neurol Neurosurg Psychiatry 2014;85:625–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bataller L, Galiano R, Garcia-Escrig M, et al. Reversible paraneoplastic limbic encephalitis associated with antibodies to the AMPA receptor. Neurology 2010;74:265–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei YC, Liu CH, Lin JJ, et al. Rapid progression and brain atrophy in anti-AMPA receptor encephalitis. J Neuroimmunol 2013;261:129–133. [DOI] [PubMed] [Google Scholar]
- 7.Titulaer MJ, Höftberger R, Iizuka T, et al. Overlapping demyelinating syndromes and anti–N-methyl-D-aspartate receptor encephalitis. Ann Neurol 2014;75:411–428. [DOI] [PMC free article] [PubMed] [Google Scholar]


