Abstract
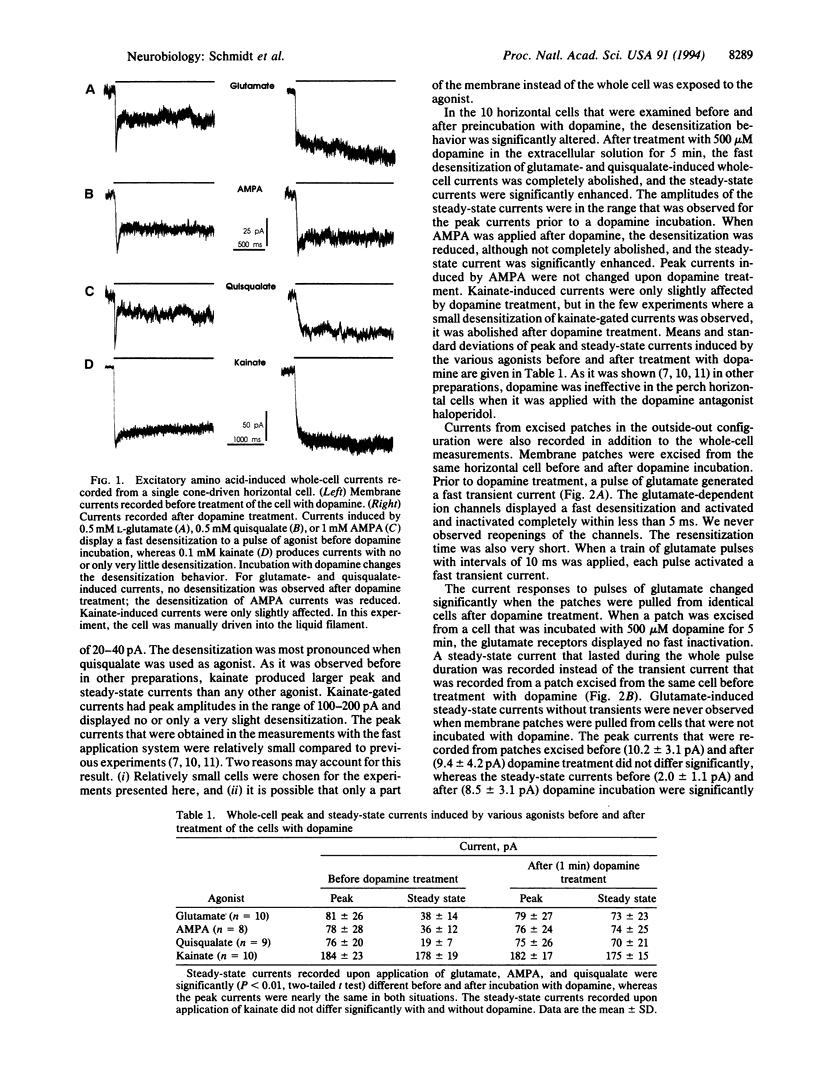
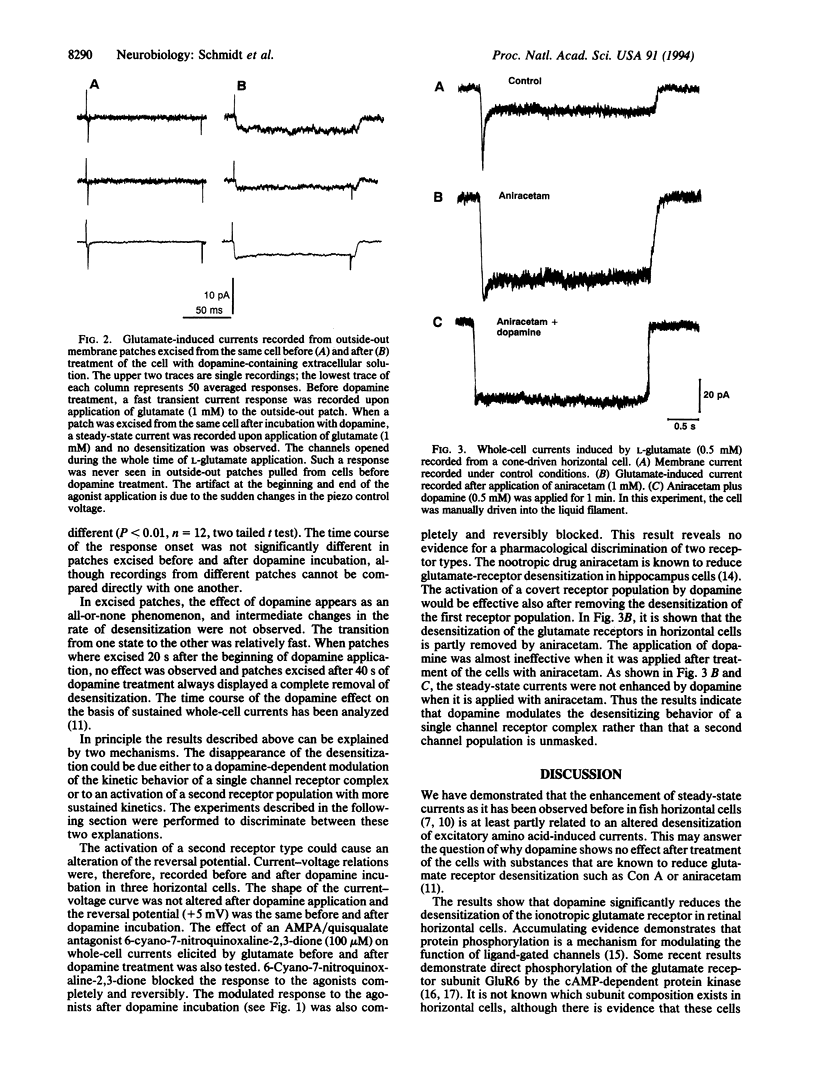
The patch-clamp technique in combination with a fast liquid filament application system was used to study the effect of dopamine on the glutamate receptor desensitization in horizontal cells of the perch (Perca fluviatilis). Kinetics of ligand-gated ion channels in fish horizontal cells are modulated by dopamine. This modulation is presumably mediated by a cAMP-dependent protein phosphorylation. Before incubation with dopamine, the glutamate receptors of horizontal cells activate and desensitize with fast time constants. In the whole-cell recording mode, fast application of the agonists L-glutamate, quisqualate, or alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid prior to the dopamine incubation gives rise to fast transient currents with peak values of about 200 pA that desensitize within 100 ms. Kainate as agonist produced higher steady-state currents but no transient currents. After incubation of the cells with dopamine for 3 min, the desensitization was significantly reduced and the agonists L-glutamate, quisqualate, or alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid induced steady-state currents with amplitudes that were similar to the previously observed transient currents. Kainate-induced currents were only slightly affected. Fast desensitizing currents upon fast application of L-glutamate were also recorded from outside-out patches that were excised from horizontal cells before incubation with dopamine. The currents from excised patches desensitized to a steady-state level of about 0.2 of the peak amplitude with time constants of less than 2 ms. When the outside-out patches were excised from cells after dopamine incubation, steady-state currents were enhanced and no transient currents were observed. The results may indicate that the dopamine-dependent modulation of glutamate-induced currents, which is presumably mediated by a protein phosphorylation, is due to an alteration of the desensitization of the glutamate receptors.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Besharse J. C., Iuvone P. M. Is dopamine a light-adaptive or a dark-adaptive modulator in retina? Neurochem Int. 1992 Feb;20(2):193–199. doi: 10.1016/0197-0186(92)90167-p. [DOI] [PubMed] [Google Scholar]
- Dong C. J., McReynolds J. S. The relationship between light, dopamine release and horizontal cell coupling in the mudpuppy retina. J Physiol. 1991;440:291–309. doi: 10.1113/jphysiol.1991.sp018709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J. E., Pak M. W., Lasater E. M. White perch horizontal cells in culture: methods, morphology and process growth. Brain Res. 1985 Dec 23;360(1-2):331–338. doi: 10.1016/0006-8993(85)91250-8. [DOI] [PubMed] [Google Scholar]
- Dowling J. E. Retinal neuromodulation: the role of dopamine. Vis Neurosci. 1991 Jul-Aug;7(1-2):87–97. doi: 10.1017/s0952523800010968. [DOI] [PubMed] [Google Scholar]
- Franke C., Hatt H., Dudel J. Liquid filament switch for ultra-fast exchanges of solutions at excised patches of synaptic membrane of crayfish muscle. Neurosci Lett. 1987 Jun 15;77(2):199–204. doi: 10.1016/0304-3940(87)90586-6. [DOI] [PubMed] [Google Scholar]
- Greengard P., Jen J., Nairn A. C., Stevens C. F. Enhancement of the glutamate response by cAMP-dependent protein kinase in hippocampal neurons. Science. 1991 Sep 6;253(5024):1135–1138. doi: 10.1126/science.1716001. [DOI] [PubMed] [Google Scholar]
- Hughes T. E., Hermans-Borgmeyer I., Heinemann S. Differential expression of glutamate receptor genes (GluR1-5) in the rat retina. Vis Neurosci. 1992 Jan;8(1):49–55. doi: 10.1017/s0952523800006489. [DOI] [PubMed] [Google Scholar]
- Isaacson J. S., Nicoll R. A. Aniracetam reduces glutamate receptor desensitization and slows the decay of fast excitatory synaptic currents in the hippocampus. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10936–10940. doi: 10.1073/pnas.88.23.10936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennelly P. J., Krebs E. G. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J Biol Chem. 1991 Aug 25;266(24):15555–15558. [PubMed] [Google Scholar]
- Kirsch M., Wagner H. J., Djamgoz M. B. Dopamine and plasticity of horizontal cell function in the teleost retina: regulation of a spectral mechanism through D1-receptors. Vision Res. 1991;31(3):401–412. doi: 10.1016/0042-6989(91)90093-k. [DOI] [PubMed] [Google Scholar]
- Knapp A. G., Dowling J. E. Dopamine enhances excitatory amino acid-gated conductances in cultured retinal horizontal cells. 1987 Jan 29-Feb 4Nature. 325(6103):437–439. doi: 10.1038/325437a0. [DOI] [PubMed] [Google Scholar]
- Knapp A. G., Schmidt K. F., Dowling J. E. Dopamine modulates the kinetics of ion channels gated by excitatory amino acids in retinal horizontal cells. Proc Natl Acad Sci U S A. 1990 Jan;87(2):767–771. doi: 10.1073/pnas.87.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse M., Schmidt K. F. Studies on the dopamine-dependent modulation of amino acid-gated currents in cone horizontal cells of the perch (Perca fluviatilis). Vision Res. 1993 Oct;33(15):2031–2042. doi: 10.1016/0042-6989(93)90001-d. [DOI] [PubMed] [Google Scholar]
- O'Dell T. J., Christensen B. N. A voltage-clamp study of isolated stingray horizontal cell non-NMDA excitatory amino acid receptors. J Neurophysiol. 1989 Jan;61(1):162–172. doi: 10.1152/jn.1989.61.1.162. [DOI] [PubMed] [Google Scholar]
- Raymond L. A., Blackstone C. D., Huganir R. L. Phosphorylation and modulation of recombinant GluR6 glutamate receptors by cAMP-dependent protein kinase. Nature. 1993 Feb 18;361(6413):637–641. doi: 10.1038/361637a0. [DOI] [PubMed] [Google Scholar]
- Sommer B., Keinänen K., Verdoorn T. A., Wisden W., Burnashev N., Herb A., Köhler M., Takagi T., Sakmann B., Seeburg P. H. Flip and flop: a cell-specific functional switch in glutamate-operated channels of the CNS. Science. 1990 Sep 28;249(4976):1580–1585. doi: 10.1126/science.1699275. [DOI] [PubMed] [Google Scholar]
- Swope S. L., Moss S. J., Blackstone C. D., Huganir R. L. Phosphorylation of ligand-gated ion channels: a possible mode of synaptic plasticity. FASEB J. 1992 May;6(8):2514–2523. [PubMed] [Google Scholar]
- Teranishi T., Negishi K., Kato S. Regulatory effect of dopamine on spatial properties of horizontal cells in carp retina. J Neurosci. 1984 May;4(5):1271–1280. doi: 10.1523/JNEUROSCI.04-05-01271.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Buskirk R., Dowling J. E. Isolated horizontal cells from carp retina demonstrate dopamine-dependent accumulation of cyclic AMP. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7825–7829. doi: 10.1073/pnas.78.12.7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. Y., Salter M. W., MacDonald J. F. Regulation of kainate receptors by cAMP-dependent protein kinase and phosphatases. Science. 1991 Sep 6;253(5024):1132–1135. doi: 10.1126/science.1653455. [DOI] [PubMed] [Google Scholar]
- Wang L. Y., Taverna F. A., Huang X. P., MacDonald J. F., Hampson D. R. Phosphorylation and modulation of a kainate receptor (GluR6) by cAMP-dependent protein kinase. Science. 1993 Feb 19;259(5098):1173–1175. doi: 10.1126/science.8382377. [DOI] [PubMed] [Google Scholar]
- Yang X. L., Wu S. M. Coexistence and function of glutamate receptor subtypes in the horizontal cells of the tiger salamander retina. Vis Neurosci. 1991 Oct;7(4):377–382. doi: 10.1017/s0952523800004867. [DOI] [PubMed] [Google Scholar]


