Abstract
Aim
To study associations of dermatoglyphic features with malocclusion in Indian children.
Materials and methods
A total of 237 children aged 12–16 years, who attended our outpatient clinic in a government medical college, were selected. Finger and palm prints were collected, and fingertip pattern frequencies, total ridge counts (TRCs), and atd angles (formed by the triradii below the first and last digits and that in the hypothenar region of the palm) were calculated. These parameters were analyzed with their Angle’s class of malocclusion using appropriate statistical tests. Dermatoglyphic parameters were examined and asymmetry analysis was conducted in subjects with different occlusion patterns.
Results
Although no fingerprint pattern was found to be specific for a particular class of occlusion, increased tendencies toward high frequencies of whorls in subjects with class II malocclusion and plain arches in those with class III malocclusion were observed. Significant differences in atd angle and TRC were observed among malocclusion types (p = 0.0001). Asymmetry scores did not differ significantly.
Conclusion
Dermatoglyphic analysis can be used as an indicator of malocclusion at an early age, thereby aiding the development of treatments aiming to establish favorable occlusion. Inheritance and twin studies, as well as those conducted in different ethnic groups, are required to examine these relationships further.
Keywords: Dermatoglyphics, Malocclusion, Asymmetry
1. Introduction
Dermatoglyphics is the study of dermal ridge counts and figures on the fingers, palms, and soles (Galton, 1965). The inheritance of dermal traits is considered to follow a classical polygenic model (Holt, 1968). Associations of such traits with orofacial malformations have been studied. Holt (1968) and Verbov (1970) strengthened the predictive validity of dermatoglyphics in medical biology, suggesting that it can aid the diagnosis of genetically and non-genetically determined diseases.
Adams and Niswander (1967) postulated that asymmetry in dermatoglyphic and dental patterns was the manifestation of developmental instability in patients with cleft lip and palate, a condition proposed to have a polygenic basis. In dental research, there has been recent trend toward the investigation of genetic factors related to common oral diseases, including congenital hypodontia (Atasu and Akyuz, 1995), microdontia (Atasu et al., 1996), molar relation (Reddy et al., 1997), bruxism (Polat et al., 2000), and oral clefts (Mathew et al., 2005; Neiswanger et al., 2002).
Cummins (1939) first reported association of specific dermatoglyphic patterns in patients with Down’s syndrome which is a genetic disorder. In recent decades, considerable improvement has been achieved in the establishing the relationships between dermatoglyphic patterns and some medical disorders.
Fingerprints have three basic patterns: arches, loops, and whorls. Loops may be ulnar or radial. These patterns are characterized by the presence or absence of triradii—confluences of three ridge systems. An arch has no triradius, a loop has one, and a whorl has two or more triradii. The axial triradius, located at the base of the palm, may be displaced distally in patients with certain conditions. The atd angle is formed by drawing lines between the triradii below the first and last digits and that in the hypothenar region of the palm (Cummins and Midlo, 1961).
The present study was conducted to explore associations between dermatoglyphic patterns and malocclusion. Dermatoglyphic parameters (fingertip patterns, atd angle, total ridge count [TRC] were examined and asymmetry analysis was conducted in subjects with different occlusion patterns.
2. Materials and methods
2.1. Sample
The present study was conducted using a convenience sample of 237, 12–16-year-old North Indian children attending our institution’s outpatient Department of Pedodontics and Preventive Dentistry between 1 September, 2013 and 28 February, 2014. The institute’s ethics committee approved the study and parents or guardians accompanying the children provided written informed consent. Only children with fully erupted permanent second molars were included in the study, and those undergoing or with histories of orthodontic treatment were excluded. Post-hoc power analysis using G Power® 3.0.10, [Faul et al. (2007), Bonn, Germany] indicated that a standard deviation of 1 would be detected with a power of 0.8 in the present sample.
Three examiners independently classified malocclusion in each subject using Angle’s criteria (Angle, 1899) and dental models. The type of malocclusion was determined by agreement of at least two examiners.
2.2. Dermatoglyphic analysis
Handprints were obtained using the ink and roller method described by Cummins and Midlo (1961) and studied as per the guidelines of Reed and Meier, 1990. In the present study, asymmetry in three dermatoglyphic features was examined (Table 1, Fig. 1 and 2). Two trained investigators independently evaluated handprints. First, fingerprint patterns were classified as arches, loops, or whorls, with loops classified further as ulnar or radial, depending on the side of the finger on which they originated (Galton, 1965). Next, the TRC – a quantitative measure of fingerprint size summed over all fingers – was calculated. The atd angle for each palm was calculated as depicted in Table 1.
Table 1.
Description of dermatoglyphic parameters recorded in the study.
| Plain arch (Fig. 1) | The plain arch is composed of ridges which pass across the finger with slight bow distally. There are no triradii. Since the pattern has no triradii, the ridge count cannot be done |
| Whorl (Fig. 1) | These are the patterns so constructed that the characteristic ridge courses follow circuits around the core. The shape of the pattern area may be either circular or elliptical. Whorls have two triradii |
| Loop (Fig. 1) | It possesses only one triradius. Twist site of ridges is called head of the loop. From the opposite extremity of the pattern, the ridges flow to the margin of digits. If the loop opens to the ulnar side, it is an ulnar loop and if to the radial margin, it is called a radial loop |
| Finger ridge count (FRC) (Fig. 1) | It was calculated by joining the triradius present in the pattern to the core of the pattern by a straight line. Total finger ridge count (TRC)- it was calculated by addition of the ridge counts of all ten fingers |
| Atd angle (Fig. 2) | It is a feature of the palm that captures the relative position of three triradii – a and d, usually located on the distal palm just inferior to the second and fifth fingers, respectively, and t, whose location can vary on the proximal palm from just distal to the wrist up to the center of the palm. Atd angles were measured for each palm print by drawing two straight lines through the a and t triradii and the d and t triradii, and measuring the resulting angle |
Figure 1.
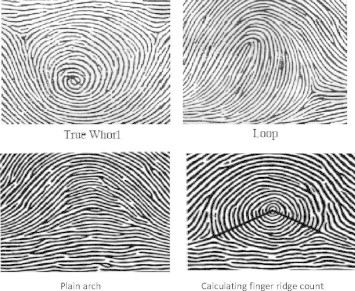
Finger tip dermatoglyphic patterns and calculation of finger ridge count (Galton, 1965).
Figure 2.
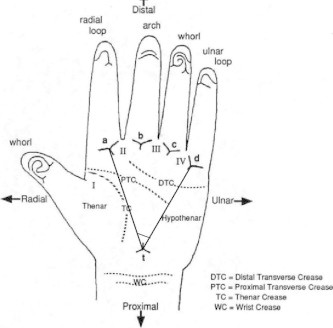
Landmarks and areas on palm and atd angle (Reed and Meier, 1990).
Asymmetry in fingerprint patterns between the right and left hands (range, 0–5) was determined by summing scores of 0 (absent; identical pattern) or 1 (present) for all five digits (Woolf and Gianas, 1977). Following Woolf and Gianas (1977), radial and ulnar loops were scored as identical patterns. Differences in TRC and atd angle were calculated by subtracting the values for the right hand from those for the left hand.
2.3. Statistical procedures
The data obtained were subjected to statistical analysis using SPSS (Statistical package for social sciences, version 16.0) software. The fingerprint patterns for each digit were analyzed and correlated with malocclusion classes using appropriate statistical tests (one way analysis of variance ANOVA, Kruskal–Wallis or Pearson’s chi-square tests, wherever applicable). Mean TRC in each class of malocclusion was analyzed using ANOVA and Mean atd angles in each class were correlated using Kruskal–Wallis test. ANOVA test was carried out for asymmetry analysis between right and left hand for all parameters. (The level of significance was p < 0.05).
3. Results
The study sample comprised 129 boys and 108 girls with class I (96 males, 72 females), class II (18 males, 24 females), and class III (15 males, 12 females) malocclusion. The mean ages of subjects with classes I, II, and III malocclusion were 9.14 ± 2.8, 11.43 ± 2.0, and 12.00 ± 2.4 years, respectively. Interexaminer reproducibility was measured using the kappa coefficient, which was 0.83.
Table 2 shows the distribution of fingertip patterns according to digit and malocclusion type. The ulnar loop pattern was predominant in children with class I malocclusion. The whorl pattern was observed frequently in subjects with class II malocclusion, especially in the thumb. Dermatoglyphic pattern frequencies differed significantly according to malocclusion class (p < 0.01; Table 3).
Table 2.
Distribution of dermatoglyphic patterns on each finger tip in each class of malocclusion.
| Type of pattern | Hand | Plain arch |
Radial loop |
Ulnar loop |
Whorl |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Class of occlusion | I | II | III | I | II | III | I | II | III | I | II | III | |
| Digit I | Right⁎⁎ | 15 | 3 | 9 | 6 | 0 | 0 | 87 | 15 | 15 | 60 | 24 | 3 |
| Left⁎⁎ | 15 | 9 | 9 | 12 | 0 | 3 | 87 | 15 | 9 | 54 | 18 | 6 | |
| Digit II | Right | 6 | 3 | 3 | 3 | 0 | 0 | 108 | 24 | 18 | 51 | 15 | 6 |
| Left | 12 | 3 | 6 | 0 | 0 | 0 | 111 | 24 | 12 | 45 | 15 | 9 | |
| Digit III | Right⁎⁎ | 0 | 0 | 0 | 0 | 0 | 0 | 96 | 21 | 24 | 72 | 21 | 3 |
| Left⁎ | 6 | 0 | 0 | 0 | 0 | 0 | 93 | 27 | 24 | 69 | 15 | 3 | |
| Digit IV | Right⁎⁎ | 6 | 0 | 6 | 6 | 0 | 0 | 114 | 36 | 15 | 42 | 6 | 6 |
| Left⁎⁎ | 3 | 3 | 12 | 0 | 0 | 0 | 111 | 30 | 12 | 54 | 9 | 3 | |
| Digit V | Right⁎⁎ | 3 | 0 | 9 | 0 | 24 | 12 | 75 | 18 | 6 | 90 | 42 | 27 |
| Left⁎⁎ | 0 | 0 | 6 | 6 | 0 | 0 | 87 | 18 | 12 | 75 | 24 | 9 | |
p < 0.05.
p < 0.01.
Table 3.
Distribution of dermatoglyphic patterns in each class of occlusion.
| Number of subjects (n) | Number of finger tip patterns studied (n × 10) | No. of whorl patterns | No. of plain arches | No. of ulnar loops | No. of radial loops | |
|---|---|---|---|---|---|---|
| Class I occlusion | 168 | 1680 | 612(36.4%) | 66(3.9%) | 969(57.7%) | 33(2%) |
| Class II occlusion | 42 | 420 | 165(39.3%) | 21(5%) | 234(55.7%) | 0(0) |
| Class III occlusion | 27 | 270 | 54(20%) | 60(22.2%) | 153(56.7%) | 3(1.1%) |
P = <0.01 (chi-square analysis).
TRCs differed significantly among malocclusion groups (p = 0.0001; Table 4). Mean TRCs were lowest in subjects with class III malocclusion, followed by those with classes I and II malocclusion. Atd angles also differed significantly among groups (p = 0.00; Table 4).
Table 4.
Mean total ridge counts and atd angles.
| Number of subjects (n) | Total ridge count | Atd angles’ degrees | |
|---|---|---|---|
| Class I occlusion | 168 | 168.02 ± 47.4 | 89.04 ± 9.6 |
| Class II occlusion | 42 | 172.79 ± 45.2 | 83.21 ± 11.8 |
| Class III occlusion | 27 | 124.56 ± 59.9 | 84.67 ± 10.4 |
| Total | 237 | 163.91 ± 50.5 | 87.51 ± 10.3 |
| p = 0.0001 | p = 0.0000 |
Dermatoglyphic asymmetry results are presented in Table 5. The occurrence of asymmetry in fingertip patterns and TRCs did not differ significantly according to malocclusion type but a significant difference in the asymmetry of atd angles was observed among groups (p = 0.0001) (Table 5).
Table 5.
Asymmetry analysis: mean (SD) pattern dissimilarity, TRC difference, and atd angle difference scores.
| Number of subjects (n) | Pattern dissimilarity score | TRC difference score | Atd angle difference score | |
|---|---|---|---|---|
| Class I occlusion | 168 | 3.80 ± 0.7 | −1.63 ± 12.5 | 0.54 ± 2.5 |
| Class II occlusion | 42 | 3.79 ± 0.8 | −1.50 ± 12.8 | −0.50 ± 2.8 |
| Class III occlusion | 27 | 3.56 ± 1.0 | 0.56 ± 13.9 | 0.67 ± 3.0 |
| Total | 237 | 3.77 ± 0.8 | −1.35 ± 12.7 (0.00) | 0.37 ± 2.6 (0.00) |
| p = 0.31 | p = 0.71 | p = 0.0001 |
4. Discussion
The development of occlusion is a result of the interaction and synergistic effects of genetic and environmental factors. The effect of a particular environmental factor on phenotype varies depending on genetic background, which ultimately determines facial and dental morphology (Mossey, 1999).
The epidermal ridges of the fingers and palm and the facial structures originate from the same embryonic tissue: ectoderm. Dermal ridges originate from volar pads, which appear at 6–7 weeks of gestation. The dermal ridge configuration reaches its maximum at around 13 weeks of gestation and is completely established by the 24th week of gestation. After that they remain constant and the configuration changes only in its size.” (Cummins and Midlo, 1961). Facial development begins as early as the 4th week of gestation. Development of the palate begins in the 6th week and is completed by the 12th week of gestation (Kumar, 2008). Thus, the face and dermal ridges not only have same origins, but also develop concurrently; the genetic message contained in the genome is deciphered during this period and is also reflected in dermatoglyphic patterns.
According to the functional matrix theory of Moss and Salentijn (1969), genetic information is located in the neurological, muscular, and neuromuscular fields, which indirectly influence the skeleton. Mastication, facial expression, speech, and swallowing are examples of neuromuscular patterns. The functional matrix is believed to encompass neuromuscular activity, which is influenced by genetics as well as environmentally influenced behavioral and postural adaptations (Moss and Salentijn, 1969).
According to Babler, 1991, epidermal ridges reflect developmental interaction at the epidermal–dermal interface; associations of specific differences in epidermal ridge development with dermatoglyphic differences suggest that ridge configurations may contain developmental information. Dermatoglyphic analysis is an inexpensive and non-invasive method of exploring the genetic associations of malocclusion. Few authors (Reddy et al. (1997), Trehan et al. (2001) and Tikare et al. (2010)) have investigated associations of dermatoglyphic features with malocclusion.
The presence of asymmetry between normally symmetric, bilateral traits has been studied using dermatoglyphic patterns (Palmer and Strobeck, 1986; Parsons, 1992). Excessive asymmetry between the dermatoglyphic patterns of the left and right hands may signify relatively unstable genetic control during embryogenesis (Naugler and Ludman, 1996), which, in turn, may contribute to the development of malformations.
In the present study, the ulnar loop pattern was predominant in subjects with all types of malocclusion. After ulnar loops, high frequencies of plain arches and whorls were found in subjects with classes III and II malocclusion, respectively. Other studies have produced contrasting results. In a study involving 96 subjects, Reddy et al. (1997) observed high frequencies of arches and ulnar loops and a low frequency of whorls in subjects with class II division 2 malocclusion; in subjects with class III malocclusion, they reported high frequencies of arches and radial loops and a low frequency of ulnar loops. In a smaller sample (n = 60), Trehan et al. (2001) observed a high frequency of whorls in subjects with classes I and III malocclusion, and high frequencies of radial loops and arches in those with class I and class II division 1 malocclusion Tikare et al. (2010) observed a trend of high frequencies of whorls in subjects with classes I and III malocclusion. However, reported no statistically significant association between malocclusion and dermatoglyphic features in 696 subjects.
Previous studies did not examine ridge counts or atd angles. We found no significant association of atd angles with malocclusion type, but observed that mean TRCs were highest in subjects with class II malocclusion and lowest in those with class III malocclusion.
We observed no overall asymmetry of dermal traits in the three study groups. These results might be attributed to the lack of examination of parents’ dermatoglyphic patterns. There is an established strong correlation of inheritance in the development of malocclusion (Mossey, 1999). So, the determination of cross inheritance by studying parent’s dermatoglyphic patterns and relating it to asymmetry in children might aid in better analysis. Examination of inheritance and twin studies may be required to establish the types of genetics and inheritance affecting the dental malocclusion.
The present study was performed in North Indian subjects; the associations examined here should be investigated further in samples with diverse demographic and ethnic characteristics and with specific DNA analysis. Prospective studies would be valuable for the establishment of dermatoglyphic markers of malocclusion. Determination of the genetic and environmental origin of malocclusion is important for orthodontic treatment planning and selection of appropriate treatment modalities. Establishment of the genetic component of malocclusion and individual susceptibility to this condition early in life could aid the planning of preventive and interceptive procedures. Dermatoglyphics, in turn, can be immensely helpful for the easy, accessible, noninvasive and economical identification of groups at high risk of developing malocclusion and for timely prevention, especially in developing countries with enormous populations and limited health budgets.
Source of funding
None.
Conflict of interest
None.
Footnotes
Peer review under responsibility of King Saud University.
References
- Adams M.S., Niswander J.D. Developmental ‘noise’ and a congenital malformation. Genet. Res. 1967;10:313. doi: 10.1017/s0016672300011071. [DOI] [PubMed] [Google Scholar]
- Angle E.H. Classification of malocclusion. Dent. Cosmos. 1899;4:248–264. [Google Scholar]
- Atasu M., Akyuz S. Congenital hypodontia: a pedigree and dermatoglyphic study. J. Clin. Pediatr. Dent. 1995;19(3):215–224. [PubMed] [Google Scholar]
- Atasu M., Ozbayrak S., Eryilmaz A. Generalized microdontia and associated anomalies: a clinical, genetic, radiologic and dermatoglyphic study. J. Clin. Pediatr. Dent. 1996;20(2):161–172. [PubMed] [Google Scholar]
- Babler W.J. Embryologic development of epidermal ridges and their configurations. Birth Defects Orig. Artic. Ser. 1991;27(2):95–112. [PubMed] [Google Scholar]
- Cummins H. Dermatoglyphic stigmata in mongoloid imbeciles. Anat. Rec. 1939;73:407–415. [Google Scholar]
- Cummins H., Midlo C. Dover Publications Inc.; New York: 1961. Finger Prints Palms and Soles: An Introduction to Dermatoglyphics. [Google Scholar]
- Faul F., Erdfelder E., Lang A.G., Buchner A. G∗Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Galton F Sir. Da Capo Press; New York: 1965. Finger Prints. [Google Scholar]
- Holt S.B. Springfield; Illinois: 1968. The Genetics of Dermal Ridges Charles C Thomas. [Google Scholar]
- Kumar G., editor. Orban’s Oral Histology and Embryology. 12th ed. Reed Elsevier India Private Limited; New Delhi: 2008. [Google Scholar]
- Mathew L., Hegde A.M., Rai K. Dermatoglyphic peculiarities in children with oral clefts. J. Indian Soc. Pedod. Prev. Dent. 2005;23:179–182. doi: 10.4103/0970-4388.19005. [DOI] [PubMed] [Google Scholar]
- Moss M.L., Salentijn L. The primary role of functional matrices in facial growth. Am. J. Orthod. 1969;55:566–575. doi: 10.1016/0002-9416(69)90034-7. [DOI] [PubMed] [Google Scholar]
- Mossey P.A. The heritability of malocclusion: part 2. The influence of genetics in malocclusion. Br. J. Orthod. 1999;26(3):195–203. doi: 10.1093/ortho/26.3.195. [DOI] [PubMed] [Google Scholar]
- Naugler C.T., Ludman M.D. A case–control study of fluctuating dermatoglyphic asymmetry as a risk marker for developmental delay. Am. J. Med. Genet. 1996;66:11–14. doi: 10.1002/(SICI)1096-8628(19961202)66:1<11::AID-AJMG3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Neiswanger K., Cooper M.E., Weinberg S.M., Flodman P., Keglovits A.B., Liu Y., Hu D.N., Melnick M., Spence M.A., Marazita M.L. Cleft lip with or without cleft palate and dermatoglyphic asymmetry: evaluation of a Chinese population. Orthod. Craniofac. Res. 2002;5:140–146. doi: 10.1034/j.1600-0544.2002.02196.x. [DOI] [PubMed] [Google Scholar]
- Palmer A.R., Strobeck C. Fluctuating asymmetry: measurement, analysis, patterns. Annu. Rev. Ecol. Syst. 1986;17:391–421. [Google Scholar]
- Parsons P.A. Fluctuating asymmetry: a biological monitor of environmental and genomic stress. Heredity. 1992;68:361–364. doi: 10.1038/hdy.1992.51. [DOI] [PubMed] [Google Scholar]
- Polat M.H., Azak A., Evlioglu G., Malkondu O.K., Atasu M. The relation of bruxism and dermatoglyphics. J. Clin. Pediatr. Dent. 2000;24(3):191–194. [PubMed] [Google Scholar]
- Reddy S., Prabhakar A.R., Reddy V.V. A dermatoglyphic predictive and comparative study of Class I, Class II, div. 1, div.2 and Class III malocclusions. J. Indian Soc. Pedod. Prev. Dent. 1997;13(1):13–19. [PubMed] [Google Scholar]
- Reed, T., Meier, R, 1990. Taking dermatoglyphic prints – a self instruction manual. Sponsored by the American Association of Dermatoglyphics.
- Tikare S., Rajesh G., Prasad K.W., Thippeswamy V., Javali S.B. Dermatoglyphics–a marker for malocclusion? Int. Dent. J. 2010;60(4):300–304. [PubMed] [Google Scholar]
- Trehan M., Kapoor D.N., Tandon P. Dermatoglyphic study of normal occlusion and malocclusion. J. Indian Orthod. Soc. 2001;34:114–125. [Google Scholar]
- Verbov Julian. Clinical significance and genetics of epidermal ridges-a review of dermatoglyphics. J. Invest. Dermatol. 1970;54:261–271. doi: 10.1111/1523-1747.ep12258550. [DOI] [PubMed] [Google Scholar]
- Woolf C.M., Gianas A.D. A study of fluctuating dermatoglyphic asymmetry in the sibs and parents of cleft lip propositi. Am. J. Hum. Genet. 1977;29:503–507. [PMC free article] [PubMed] [Google Scholar]


