Abstract
The burden of squamous cell carcinoma of the head and neck (SCCHN) is greater for blacks than for whites, especially in oropharyngeal cases. We previously showed retrospectively that disease-free survival was significantly greater in white than in black SCCHN patients treated with chemoradiation, the greatest difference occurring in the oropharyngeal subgroup. Oropharyngeal cancer is increasing in incidence and in its association with human papillomavirus (HPV) infection; HPV-positive oropharyngeal cancer patients have significantly better outcomes (versus HPV-negative). These collective data led to the present analyses of overall survival (OS) in our retrospective cohort and of OS and HPV status (tested prospectively in pretreatment biopsy specimens) in the phase 3, multicenter TAX 324 trial of induction chemotherapy followed by concurrent chemoradiation in SCCHN patients. Median OS in the retrospective cohort of 106 white and 95 black SCCHN patients was 52.1 months (white) versus only 23.7 months (black; P = 0.009), due entirely to OS in the subgroup of patients with oropharyngeal cancer—69.4 months (whites) versus 25.2 months (blacks; P = 0.0006); no significant difference by race occurred in survival of non-oropharyngeal SCCHN (P = 0.58). In TAX 324, 196 white patients and 28 black patients could be assessed for HPV status. Median OS was significantly worse for black patients (20.9 months) than for white patients (70.6 months; P = 0.03) and dramatically improved in HPV-positive (not reached) versus HPV-negative (26.6 months, 5.1 hazard ratio) oropharyngeal patients (P < 0.0001), 49% of whom were HPV-16 positive. Overall, HPV positivity was 34% in white versus 4% in black patients (P = 0.0004). Survival was similar for black and white HPV-negative patients (P = 0.56). This is the first prospective assessment of confirmed HPV status in black versus white SCCHN patients. Worse OS for black SCCHN patients was driven by oropharyngeal cancer outcomes, and that for black oropharyngeal cancer patients by a lower prevalence of HPV infection. These findings have important implications for the etiology, prevention, prognosis, and treatment of SCCHN.
Cancer incidence and death rates differ among racial and ethnic groups. Data from numerous sources indicate that the incidence of and the mortality from squamous cell carcinoma of the head and neck (SCCHN) are higher in blacks than in whites (1). There is no consensus on the causes of these differences, but they may include differences in access to care, stage at diagnosis, insurance status, and attitudes of health providers. Whether significant biological rather than socioeconomic differences account for some of the disparities in outcomes for blacks and whites remains largely undefined, especially in SCCHN, and the literature on this issue offers different views. The National Cancer Institute Black/White Cancer Survival Study showed poorer survival for black (versus white) patients with colon, breast, uterine, and bladder tumors after adjustment for both clinical and socioeconomic characteristics (2, 3). These findings gave credence to the concept that some cancers may be biologically more aggressive in blacks compared with whites. A more recent meta-analysis of clinical studies involving 14 cancer sites including the head and neck found that decreased survival for blacks occurred only for breast, uterine, and bladder cancers, differences that the study design suggested were related to biological racial differences (4). The authors of this study concluded that the generally accepted disparities in survival for other tumors (including head and neck tumors) are principally due to differences in treatment, stage at presentation, and mortality from other conditions.
Several studies recently analyzed national data from the U.S. Surveillance, Epidemiology, and End Results database. The oral and pharyngeal cancer analyses within these studies consistently showed that blacks present with more advanced disease and have twice the age-adjusted mortality rate compared with whites (5, 6). A recent analysis of the same Surveillance, Epidemiology, and End Results data suggested that even after correcting for stage at diagnosis, blacks had a significantly worse survival than did whites (7). A recent analysis of racial disparities in 35 trials from the Southwest Oncology Group found worse survival for black patients with sex-specific cancers (breast, ovarian, and prostate), but not for black patients with lung cancer, colon cancer, lymphoma, myeloma, or leukemia (8). No head and neck cancer trials were included in this analysis.
A single-institution retrospective study confirmed that disease-specific mortality was significantly higher for black than for white head and neck cancer patients after adjusting for other factors including tumor-node-metastasis stage (9). Intensity of treatment was similar for the two groups and did not account for the differences in survival. In a previously reported retrospective analysis (10), we showed that disease-free survival was significantly greater in white than in black SCCHN patients who were treated with chemoradiation; the greatest black-white difference occurred in the oropharyngeal subgroup. We also recently analyzed race-related outcomes in two Radiation Therapy Oncology Group studies in head and neck cancer, finding in both studies that blacks had a significantly poorer outcome compared with whites, despite receiving identical treatment (11).
Significant data of the last few years show that human papillomavirus (HPV; primarily HPV-16) infection is associated with a significant percentage of oropharyngeal cancers, primarily of the base of the tongue and tonsil (12, 13). Furthermore, the incidence of oropharyngeal cancer is increasing, as is the prevalence of HPV infection in oropharyngeal cancer patients (14, 15). Very recent data indicate that HPV-positive cancers have a significantly better prognosis than do the HPV-negative diseases (16). The striking effect of HPV on cervical screening (17) and neoplasia risk and the U.S. Food and Drug Administration approval of HPV vaccines to reduce this risk (18) highlight the growing focus on HPV in research of head and neck cancer etiology, therapy, and prevention.
These collective data on overall and oropharyngeal SCCHN outcome disparities and on the growing association of HPV with SCCHN development and outcome led us to test the hypothesis in the present study that HPV may play an important role in racial disparity within SCCHN. We first examined overall survival (OS) differences by oropharyngeal and non-oropharyngeal sites in a retrospective group of black and white patients with stage III and IV SCCHN treated at the University of Maryland Marlene and Stewart Greenebaum Cancer Center. We then analyzed patients from the multicenter randomized phase 3 TAX 324 trial for the presence of HPV-16 in pretreatment biopsy specimens. In TAX 324, stage III or IV SCCHN patients with no distant metastases received induction cisplatin and fluorouracil alone (PF) or induction PF plus docetaxel (TPF), followed by radiotherapy with concomitant carboplatin (19). We evaluated the overall effect of HPV positivity, compared the rate of HPV positivity between black and white patients, and evaluated the effect of HPV positivity on survival differences between the two racial groups in TAX 324.
Patients and Methods
Patients
Retrospective patients
From November 1995 through July 2006, roughly 2,000 patients with SCCHN were treated at the University of Maryland Marlene and Stewart Greenebaum Cancer Center. Patients with American Joint Committee on Cancer stage III/IV squamous cell carcinoma of the oral cavity, oropharynx, hypopharynx, and larynx who had long-term follow-up were identified. From these patients, we selected a homogeneous cohort of white and black patients treated curatively with concurrent chemoradiotherapy for previously untreated SCCHN with no evidence of distant metastases. The strongest findings of our previously published and related retrospective analysis [based on a shorter median follow-up (versus the present analysis) of 33 months] involved disease-free survival (10). For the retrospective analysis presented here, we updated survival data with a focus on OS in all and oropharyngeal SCCHN patients and how OS may have differed by race. We also specifically compared OS for oropharyngeal cancer with that in other head and neck sites, which was not part of the original analysis.
Prospective patients
TAX 324 was a phase 3 clinical trial in which patients with stage III or IV SCCHN and no distant metastases were randomly assigned to induction PF or TPF. After this induction chemotherapy, patients received radiotherapy with concomitant carboplatin. As previously reported, after a minimum of 2 years of follow-up for each patient, mortality was 30% lower in the TPF than in the PF arm (death hazard ratio of 0.70; P = 0.006; ref. 19). For the present study, we selected TAX 324 patients for whom pretreatment paraffin-embedded biopsy specimens were available.
HPV-16 analysis
DNA extraction from paraffin sections
Sections of pretreatment paraffin-embedded biopsy specimens were deparaffinized in xylene. The tissue was scraped with a sterile scalpel into a microcentrifuge tube, rehydrated with graded ethanol washes, and air-dried. DNA extraction was done using the QIAamp DNA Micro Kit (Qiagen) following the instruction manual protocol for laser-microdissected tissue. DNA was quantitated using the Quant-iT dsDNA Assay Kit (Invitro-gen) and stored at −80°C. Working dilutions of 0.5 ng/μL were prepared from stored aliquots.
HPV-16 PCR. PCR was done for the E6 (forward, AAACTAA-GGGCGTAACCGAAA; reverse, TAGTTGTTTGCAGCTCTGTGC) and E7 (forward, ACAAGCAGAACCGGACAGAG; reverse, GATGGGGCACACAATTCCTA) genes of HPV-16. These primers are type specific for HPV-16. Forty cycles of standard three-step PCR (annealing temperature, 55°C) were done on 0.5 ng of DNA. Multiple negative controls (no DNA) were included in every PCR run. Products were visualized on 1.7% agarose gels stained with GelStar nucleic acid stain (Cambrex). Only cases positive for both genes were scored HPV-16 positive. Cases positive for one but negative for the other gene were excluded. In the case of ambiguity in interpreting either gene, both genes were amplified again from a freshly diluted DNA aliquot using a different primer set (overlapping but not nested with the original primer set). Cases that remained ambiguous were excluded. HPV-16 status could be scored for 237 of the 265 cases with sufficient DNA. In a validation series of 49 repeat reactions, the error rate was 0% for the E6 gene and 1% for the E7 gene.
Statistical methods
The effects of HPV status and race on patient OS and the related hazard ratios were assessed using univariate Cox proportional hazards models. The Kaplan-Meier survival distribution functions were estimated for different groups of patients. Patients were grouped by race, site of SCCHN, and HPV status. The survival distributions were compared using the log-rank test at the 0.05 level of significance.
Results
Patient characteristics
Our retrospective cohort contained 201 patients (106 white and 95 black), treated curatively with concurrent chemoradiotherapy for stage III and stage IV SCCHN. All patients were previously untreated and had no evidence of distant metastases. There were 124 oropharyngeal cancer patients (54 black, 70 white) and 77 patients with tumors at other head and neck sites (41 black, 36 white).
In the TAX 324 trial, pretreatment, paraffin-embedded biopsy specimens were available for 270 of 561 patients. Sufficient DNA for PCR analysis was recovered for 265 of these 270 patients. Of the 270 patients, 237 had specimens that could be scored for HPV infection. Ninety-five percent (224 of 237) of the patients self-identified as either white or black. The base- line characteristics of this TAX 324 cohort, including numbers and percentages of oropharyngeal and other head and neck cancer sites, are presented in Table 1.
Table 1.
Baseline characteristics of the TAX 324 intent-to-treat population and a subset with biopsies informative for HPV status
| Intent-to-treat population | HPV-tested population | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| TPF (n = 280) | PF (n = 259) | All (n = 539) | HPV− (n = 169) | HPV+ (n = 68) | All (n = 237) | |
| Sex, n (%) | ||||||
| Male | 238(85.0) | 213(82.2) | 451(83.7) | 141(83.4) | 54(79.44) | 195(82.3) |
| Female | 42(15.0) | 46(17.8) | 88(16.3) | 28(16.6) | 14(20.6) | 42(17.7) |
| Race, n (%) | ||||||
| Black | 26(9.3) | 26(10.0) | 52(9.6) | 27(16.0) | 1(1.5) | 28(11.8) |
| White | 236(84.3) | 220(84.9) | 456(84.6) | 130(76.9) | 66(97.1) | 196(82.7) |
| Other | 18(6.4) | 13(5.0) | 31(5.8) | 12(7.1) | 1(1.5) | 13(5.5) |
| Age, y | ||||||
| Median | 56 | 56 | 56 | 57 | 55 | 56 |
| Minimum | 38 | 33 | 33 | 34 | 39 | 34 |
| Maximum | 82 | 80 | 82 | 80 | 71 | 80 |
| Anatomic site, n (%) | ||||||
| Hypopharynx | 45(16.1) | 35(13.5) | 80(14.8) | 33(19.5) | 2(2.9) | 35(14.8) |
| Larynx | 153(18.9) | 45(17.4) | 98(18.2) | 51(30.2) | 4(5.9) | 55(23.2) |
| Oral cavity | 36(12.9) | 38(14.7) | 74(13.7) | 25(14.8) | 3(4.4) | 28(11.8) |
| Oropharynx | 146(52.1) | 140(54.0) | 286(53.1) | 60(35.5) | 59(86.8) | 119(50.2) |
| Clinical stage, n (%) | ||||||
| III | 45(16.1) | 51(19.7) | 96(17.8) | 35(20.7) | 14(20.6) | 49(20.7) |
| IV | 195(69.6) | 171(66.0) | 366(67.9) | 106(62.7) | 48(70.6) | 154(65.0) |
| IVA | 34(12.1) | 27(10.4) | 61(11.3) | 21(12.4) | 6(8.8) | 27(11.4) |
| IVB | 6(2.1) | 9(3.5) | 15(2.8) | 7(4.1) | 0(0) | |
| Missing | 1(0.4) | 1(0.2) | ||||
primer set). Cases that remained ambiguous were excluded
Racial outcome disparities by tumor site
Median follow-up of our retrospective cohort was 67 months. Median OS for white patients was 52.1 months, compared with only 23.7 months for black patients (P = 0.009; Fig. 1A). Similar black-white comparative results occurred in the TAX 324 trial (Fig. 3A). Analysis of the retrospective cohort by disease site indicated that the OS disparity between whites and blacks was driven entirely by a striking survival difference in patients with oropharyngeal cancer (OS of 69.4 months for whites versus 25.2 months for blacks; P = 0.0006; Fig. 1B). In non-oropharyngeal cancer, there was no OS difference between black and white patients (P = 0.58; Fig. 1C).
Fig. 1.
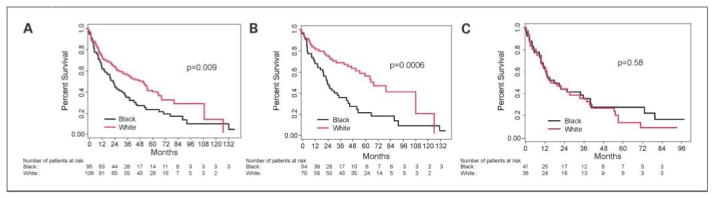
Racial outcome disparity in the University of Maryland retrospective cohort. A, median OS within the entire cohort (stage III and IV SCCHN treated with concomitant chemotherapy and radiation): 52.1 mo (95% CI, 32.9–65.8) for white patients and 23.7 mo (95% CI, 15.9–33.2) for black patients (log-rank test P = 0.009). B, median OS within the oropharyngeal subset: 69.4 mo (95% CI, 52.1–127.3) for white patients and 25.2 mo (95% CI, 18.4–36.0) for black patients (log-rank test P = 0.0006). C, median OS for patients within the non-oropharyngeal subset: 17.1 mo (95% CI, 10.8–37.0) for white patients and 17.5 mo (95% CI, 11.4–39.0) for black patients (log-rank test P = 0.58). The racial outcome disparity in the overall patient group was entirely due to the disparity in oropharyngeal patients; no disparity was seen in other sites.
Fig. 3.
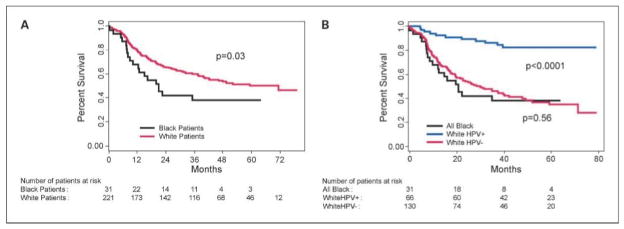
Overall survival by race and HPV-16 status in the TAX 324 trial. A, median OS by race in all patients: 70.6 mo [95% CI, 40.0-not reached (NR)] for white patients versus 20.9 mo (95% CI, 12.4-NR) for black patients (log-rank test P = 0.03). B, median OS by race and HPV status (log-rank test P < 0.0001): NR for white HPV-positive (HPV+) patients; 30.1 mo (95% CI, 19.7–42.0) for white HPV-negative (HPV−) patients; and 20.9 mo (95% CI, 12.4-NR) for black patients. The difference in survival between all black patients and HPV-negative white patients was not significant (P = 0.78). Of 32 black patients with an available biopsy, only one was HPV positive. The difference in survival between HPV-positive white patients and all other patients was highly significant (P < 0.0001).
HPV-16 infection as a predictor of survival in the TAX 324 study
Of the 237 TAX 324 patients with informative HPV results, 68 (29%) had HPV-positive tumors, and 59 (87%) of these 68 tumors were oropharyngeal. For all tumor sites, the median OS was 26.6 months [95% confidence interval (95% CI), 19.9–39.6] for HPV-negative patients versus not reached for HPV-positive patients (P < 0.0001), leaving a HPV-negative hazard ratio of 5.1 (Fig. 2A). Most of this effect was due to differences among the oropharyngeal patients. Fifty-nine (87%) of all 68 HPV-positive tumors were oropharyngeal. Fifty percent (59 of 119) of the analyzed oropharyngeal patients were HPV positive, and 58 (98%) of these HPV-positive oropharyngeal patients were white. Median OS was 20.9 months (95% CI, 12.6–39.6 months) for HPV-negative oropharyngeal cancer patients versus not reached for HPV-positive oropharyngeal patients (P < 0.0001; Fig. 2B).
Fig. 2.
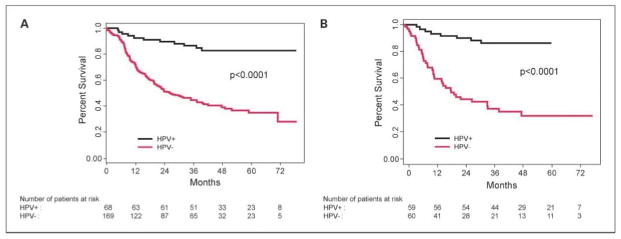
HPV-16 status and OS in the TAX 324 trial. A, median OS by HPV status among all patients: 26.6 mo (95% CI, 19.9–39.6) for HPV-negative (HPV−) patients versus not reached for HPV-positive (HPV+) patients (log-rank test P < 0.0001). B, median OS by HPV status among oropharyngeal patients: 19.7 mo (95% CI, 12.6–34.9) for HPV-negative patients versus not reached for HPV-positive patients (log-rank test P < 0.0001). Fifty-nine of 68 patients with HPV-positive tumors had oropharyngeal cancer.
The distribution of HPV infection by race is shown in Table 2. The proportion of HPV-positive tumors was nearly 9-fold higher in white patients (66 of 196, 34%) than in black patients (1 of 28, 4%; P = 0.0004).
Table 2.
Frequency distribution of HPV by race in TAX 324
| Race | HPV negative, n (%) | HPV positive, n (%) | Total |
|---|---|---|---|
| White | 130 (66) | 66 (34) | 196 |
| Black | 27 (96) | 1 (4) | 28 |
| Total | 157 | 67 | 224 |
Survival by race and HPV-16 status in the TAX 324 trial
Despite the relatively small number of black patients available for survival analysis (31 of 252, 12%), black patients had a statistically significant survival disparity compared with white patients. Median OS was 70.6 months for whites versus 20.9 months for blacks (P = 0.03; Fig. 3A). Median OS for HPV-negative white patients (30.1 months), however, was similar to that for all black patients (20.9 months; P = 0.56; Fig. 3B). The median OS of 30.1 months for white HPV-negative patients is markedly different from the median OS for white HPV-positive patients (not reached; P < 0.0001).
Discussion
Our new retrospective analysis found a significantly decreased OS in black (versus white) SCCHN patients. This difference was driven entirely by a worse OS for blacks (versus whites) in oropharyngeal cancer because we found no black-white OS difference in non-oropharyngeal patients, the first such demonstration. This retrospective patient set included roughly equal numbers of black and white patients with similar disease stage and treatment, which should have diminished the effect of non-HPV factors such as access to care, stage at diagnosis, treatment bias, and insurance status.
Results from the phase 3 TAX 324 trial—the first prospective, biopsy-confirmed comparison of HPV status in black and white SCCHN patients—help to interpret these retrospective and other SCCHN data. OS in TAX 324 was dramatically better for HPV-positive than for HPV-negative SCCHN patients as a result mainly of improved OS in the oropharyngeal subgroup. HPV positivity was nearly 9-fold higher in white (34% rate of positivity) than in black (4% rate) patients, explaining the greater part of the outcome disparity between black and white patients in the present study and, likely, in other studies over the years.
There was no difference in stage of disease between the HPV-positive and HPV-negative TAX 324 patients, and so the survival difference seems to be directly related to HPV status. HPV-positive patients do well on treatment with combined chemotherapy and radiation, such as that used in the TAX 324 trial. On the other hand, HPV-negative patients do less well with this approach. Because of this substantial positive effect on OS in the subgroup of HPV-positive patients, positive recent studies of combined chemotherapy and radiation in SCCHN should be interpreted with some caution. Biologically, HPV-positive and HPV-negative tumors have markedly different behavior, and our results indicate that the great majority of black patients have HPV-negative diseases that did not respond optimally to chemoradiotherapy.
The reason for the apparent racial difference in HPV prevalence between black and white oropharyngeal patients is not known, nor is the etiologic influence of this difference on oropharyngeal cancer development in the two racial subgroups. Although studies have provided evidence of an association between sexual risk behaviors and HPV-positive oral cancer (20, 21), differences in outcome based on race have not been examined extensively in these studies. Host immunity, viral integration, and oral carcinogenic potential of HPV infection may differ in the two groups. A study of HPV-16 infection in a black South African population showed a strong association between seropositivity for HPV-16 and incidence of cervical, anogenital, and esophageal tumors, but not of oropharyngeal or prostate cancers (22).
The other major neoplasia setting where HPV is an established factor is the cervix, where important differences (besides gender) from the oropharynx include the importance of HPV-16 and HPV-18 (versus just HPV-16 in the oropharynx) and the nearly 100% infection rate (versus ~50% in oropharyngeal cancer; ref. 23). HPV screening in the cervix (which would involve women with and without cervical neoplasia) indicates that the rate of high-risk HPV-16 and HPV-18 may be higher in black than in white women, although the reason for this is not clear and could be confounded by socioeconomic status (24, 25). In high-grade cervical intraepithelial neoplasia, however, HPV infection may be more common in whites than in blacks (26), loosely paralleling our present finding of a greater prevalence of HPV in white than in black oropharyngeal cancer patients. The dual possibility that HPV prevalence is higher in blacks overall but lower in black neoplasia patients suggests that cervical (and oropharyngeal) neoplasia may develop or progress less frequently in HPV-infected blacks than in HPV-infected whites. Black women unquestionably have an increased incidence of and mortality from cervical cancer, but the biological, socioeconomic, or cultural reasons for this are unknown. Cervical cancer is very sensitive to concurrent chemoradiation (27), suggesting a parallel to the increased treatment sensitivity of HPV-related (versus nonrelated) oropharyngeal cancer. Oropharyngeal cancer has a 3:1 ratio of males to females overall and in HPV-related cases (14), suggesting the possibility that gender-related hormonal differences may confound any comparison of oropharyngeal to cervical neoplasia.
The great success of HPV vaccines in reducing cervical cancer risk is a model for chemoprevention studies in HPV-related oropharyngeal carcinogenesis (17). These studies would benefit from an understanding of oropharyngeal pre-malignancy, which has yet to be characterized in the literature. Nevertheless, infection clearly precedes cancer development—seropositivity to HPV-16 confers a 14-fold increase in subsequent risk of oropharyngeal cancer (28). Although HPV DNA has been detected in in situ, invasive, and metastatic disease (29), the histopathologic progression from infection to in situ disease is poorly documented. It likely involves HPV infection of the basal cell layer of tonsillar crypts, which seem to be uniquely prone to transformation by the virus (30).
Although the data presented here require confirmation in future analyses, they nevertheless may explain some, but not all, outcome disparities based on race for patients with locally advanced SCCHN. Adding to efforts to eliminate racial socioeconomic disparities, this evidence of a racial biological difference that affected SCCHN survival moves us a step closer, we believe, to realizing the hope expressed by Dr. Otis Brawley: “Perhaps advances in our understanding of biology will lead us away from concerns about race and we will better define high-risk populations using pathologic markers of disease” (31). This approach should allow more intelligent treatment choices for patients using biologically based prognostic information including, but not limited to, HPV status.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest disclosed
References
- 1.Ryerson AB, Peters ES, Coughlin SS, et al. Burden of potentially human papillomavirus-associated cancers of the oropharynx and oral cavity in the US. 1998–2003. Cancer. 2008;113:2901–9. doi: 10.1002/cncr.23745. [DOI] [PubMed] [Google Scholar]
- 2.Hunter CP, Redmond CK, Chen VW, et al. Breast cancer: factors associated with stage at diagnosis in black and white women. Black/White Cancer Survival Study Group. J Natl Cancer Inst. 1993;85:1129–37. doi: 10.1093/jnci/85.14.1129. [DOI] [PubMed] [Google Scholar]
- 3.Eley JW, Hill HA, Chen VW. Racial differences in survival from breast cancer. Results of the National Cancer Institute Black/White Cancer Survival Study. JAMA. 1994;272:947–54. doi: 10.1001/jama.272.12.947. [DOI] [PubMed] [Google Scholar]
- 4.Bach PB, Schrag D, Brawley OW, Galaznik A, Yakren S, Begg CB. Survival of blacks and whites after a cancer diagnosis. JAMA. 2002;287:2106–13. doi: 10.1001/jama.287.16.2106. [DOI] [PubMed] [Google Scholar]
- 5.Goodwin WJ, Thomas GR, Parker DF, et al. Unequal burden of head and neck cancer in the United States. Head Neck. 2008;30:358–71. doi: 10.1002/hed.20710. [DOI] [PubMed] [Google Scholar]
- 6.Shiboski CH, Schmidt BL, Jordan RC. Racial disparity in stage at diagnosis and survival among adults with oral cancer in the US. Community Dent Oral Epidemiol. 2007;35:233–40. doi: 10.1111/j.0301-5661.2007.00334.x. [DOI] [PubMed] [Google Scholar]
- 7.Morse DE, Kerr AR. Disparities in oral and pharyngeal cancer incidence, mortality and survival among black and white Americans. J Am Dent Assoc. 2006;37:203–12. doi: 10.14219/jada.archive.2006.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albain KS, Unger JM, Crowley JJ, Coltman CA, Hershman DL. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst. 2009;101:984–92. doi: 10.1093/jnci/djp175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murdock JM, Gluckman JL. African-American and white head and neck carcinoma patients in a university medical center setting. Are treatments provided and are outcomes similar or disparate? Cancer. 2001;91:279–83. doi: 10.1002/1097-0142(20010101)91:1+<279::aid-cncr19>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 10.Settle K, Taylor R, Wolf J, et al. Race impacts outcome in stage III/IV squamous cell carcinomas of the head and neck after concurrent chemoradiation therapy. Cancer. 2009;115:1744–52. doi: 10.1002/cncr.24168. [DOI] [PubMed] [Google Scholar]
- 11.Schumaker L, Nikitakis N, Goloubeva O, Tan M, Taylor R, Cullen KJ. Elevated expression of glutathione S-transferase π and p53 confers poor prognosis in head and neck cancer patients treated with chemoradiotherapy but not radiotherapy alone. Clin Cancer Res. 2008;14:5877–83. doi: 10.1158/1078-0432.CCR-08-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dahlstrom KR, Adler-Storthz K, Etzel CJ, et al. Human papillomavirus type 16 infection and squamous cell carcinoma of the head and neck in never-smokers: a matched pair analysis. Clin Cancer Res. 2003;9:2620–26. [PubMed] [Google Scholar]
- 13.Strome SE, Savva A, Brissett AE, et al. Squamous cell carcinoma of the tonsils: a molecular analysis of HPV associations. Clin Cancer Res. 2002;8:1093–100. [PubMed] [Google Scholar]
- 14.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612–19. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 15.Hammarstedt L, Lindquist D, Dahlstrand H, et al. Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int J Cancer. 2006;119:2620–3. doi: 10.1002/ijc.22177. [DOI] [PubMed] [Google Scholar]
- 16.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–69. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 17.Schiffman M, Wacholder S. From India to the world—a better way to prevent cervical cancer. N Engl J Med. 2009;360:1453–55. doi: 10.1056/NEJMe0901167. [DOI] [PubMed] [Google Scholar]
- 18.FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–27. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 19.Posner MR, Hershock DM, Blajman CR, et al. TAX 324 Study Group. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705–15. doi: 10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 20.D’Souza G, Agrawal Y, Halpern J, Bodison S, Gillison ML. Oral sexual behaviors associated with prevalent oral human papillomavirus infection. J Infect Dis. 2009;199:1263–69. doi: 10.1086/597755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–56. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 22.Sitas F, Urban M, Stein L, et al. The relationship between anti-HPV-16 IgG seropositivity and cancer of the cervix, anogenital organs, oral cavity and pharynx, oesophagus and prostate in a black South African population. Infect Agent Cancer. 2007;2:6. doi: 10.1186/1750-9378-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 24.Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007;297:813–9. doi: 10.1001/jama.297.8.813. [DOI] [PubMed] [Google Scholar]
- 25.Kahn JA, Lan D, Kahn RS. Sociodemographic factors associated with high-risk human papilloma-virus infection. Obstet Gynecol. 2007;110:87–95. doi: 10.1097/01.AOG.0000266984.23445.9c. [DOI] [PubMed] [Google Scholar]
- 26.Khan MJ, Partridge EE, Wang SS, Schiffman M. Socioeconomic status and the risk of cervical intraepithelial neoplasia grade 3 among oncogenic human papillomavirus DNA-positive women with equivocal or mildly abnormal cytology. Cancer. 2005;104:61–70. doi: 10.1002/cncr.21129. [DOI] [PubMed] [Google Scholar]
- 27.Morris M, Eifel PJ, Lu J, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–43. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 28.Mork J, Lie AK, Glattre E, et al. Human papilloma-virus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med. 2001;344:1125–31. doi: 10.1056/NEJM200104123441503. [DOI] [PubMed] [Google Scholar]
- 29.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–20. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 30.Kim SH, Koo BS, Kang S, et al. HPV integration begins in the tonsillar crypt and leads to the alteration of p16, EGFR and c-myc during tumor formation. Int J Cancer. 2007;120:1418–25. doi: 10.1002/ijc.22464. [DOI] [PubMed] [Google Scholar]
- 31.Brawley OW. Is race really a negative prognostic factor for cancer? J Natl Cancer Inst. 2009;101:970–1. doi: 10.1093/jnci/djp185. [DOI] [PubMed] [Google Scholar]


