Abstract
Introduction
A combination of cetuximab and sorafenib in patients with recurrent and/or metastatic (R/M) head and neck squamous cell carcinoma (HNSCC) were assessed for potential benefit.
Material and Methods
In a randomized phase II study, R/M HNSCC patients were treated with cetuximab 400mg/m2 IV on day 1 followed by 250mg/m2 IV weekly (Arm A), or cetuximab at the same dose/schedule plus sorafenib 400mg PO twice-a-day (Arm B). Each cycle was 21 days. Tumor p16 and HPV status, and plasma immunomodulatory cytokine levels were assessed.
Results
Of 55 patients enrolled (Arm A-27, Arm B-28), 52 patients received assigned treatments and 43 were evaluable for response. Overall response rate was 8% for both arms. Median overall survival (OS) and progression-free survival (PFS) were 9.0 and 3.0 months in Arm A, and 5.7 and 3.2 months in Arm B, respectively. Forty-four patients had tumors available for p16 staining (35-negative, 9-positive). Three of nine p16-positive tumors were also HPV positive. The p16-negative patients had significantly better PFS compared to the p16-positive patients (3.7 vs. 1.6 months; p-value: 0.03), regardless of study arms. Twenty-four plasma samples were tested for 12 cytokine levels and patients with higher TGFβ1 levels had inferior PFS compared to lower levels (1.9 vs. 4.7 months; adjusted p-value: 0.015), regardless of study arms.
Conclusions
A subset of R/M patients with p16-negative tumors or lower plasma TGFβ1 levels had longer PFS given the cetuximab-based therapy. However, both arms showed only modest response and sorafenib given with cetuximab did not demonstrate clinical benefit.
Keywords: cetuximab, sorafenib, head and neck squamous cell carcinoma, recurrence, metastasis, p16, TGFβ1, response, toxicity, phase II trial
INTRODUCTION
While the use of multimodality therapy has significantly improved survival of locally advanced head and neck squamous cell carcinoma (HNSCC) over the past decade, minimal survival improvement has been realized once recurrent, refractory or metastatic disease develops. In addition, the incidence of human papillomavirus (HPV)-positive HNSCC has been rising since early 1990’s and the prognosis of HPV-positive patients is more favorable compared to HPV-negative patients; however, the incidence of distant metastasis does not differ significantly between the two groups occurring in approximately 9–15% [1]. Therefore, new regimens should be investigated to meet current clinical needs in the management of patients with recurrent and/or metastatic (R/M) HNSCC.
It is well established that epidermal growth factor receptor (EGFR) is a key tyrosine kinase membrane receptor and overexpressed in greater than 90% of HNSCC. High expression of EGFR is strongly associated with a poor prognosis [2–5]. To date, the only survival benefit was seen with cetuximab, an anti-EGFR monoclonal antibody, which demonstrated modest but significant improvement in median progression-free survival (PFS), median overall survival (OS) and response rate (RR) when combined with chemotherapy in R/M HNSCC patients [6]. Activation of EGFR is also shown to upregulate expression of vascular endothelial growth factor receptor (VEGFR), a receptor tyrosine kinase that stimulates angiogenesis upon activation by several ligands [7]. Inhibition of EGFR results in a dose-dependent inhibition of microvessel density and VEGF expression [7]. Thus, targeting both the EGFR and VEGFR pathways is a rational strategy. In fact, evidence of clinical benefit was noted when bevacizumab, an anti-VEGF monoclonal antibody, was added to either cetuximab or erlotinib in patients with recurrent or metastatic HNSCC [8, 9].
In this study, we evaluated sorafenib, an oral multi-kinase inhibitor of B-raf, PDGFR-beta and VEGFR-2. Thus, sorafenib has a potential to inhibit downstream targets of EGFR pathway as well as angiogenesis. A phase II trial of sorafenib monotherapy in metastatic or refractory HNSCC demonstrated a response rate of only 2%, but median PFS was 4 months and median OS was 9 months suggesting a potential, albeit modest, effect on disease control [10]. We hypothesized that a combination of EGFR and VEGFR-2 inhibitors has a synergistic effect compared to monotherapy of each agent, and we conducted a randomized phase II evaluation of cetuximab alone versus cetuximab plus sorafenib in patients with R/M HNSCC.
In addition, we evaluated p16 expression, HPV status and immunomodulatory cytokine levels as correlative studies. Tumor p16 is a well-established surrogate marker for HPV-related squamous cell carcinoma and a favorable prognostic marker in newly diagnosed and locally advanced HNSCC with oropharynx as the primary site [11]. However, its role as a prognostic marker in R/M HNSCC is less clear. Therefore, we evaluated p16 expression and HPV status in tumors and their association with the survival outcomes. Furthermore, cetuximab has shown to induce anti-tumor effects by antibody-dependent cell-mediated cytotoxicity (ADCC) in addition to the inhibition of the EGFR signal transduction. We have previously shown that up-regulation of TGFβ1 expression by the tumor cells can result in cetuximab resistance through suppression of ADCC as well as TGFβ1-mediated activation of AKT providing an EGFR-independent signaling downstream [12]. We measured 12 immunomodulatory cytokines in plasma and evaluated their association with the survival outcomes.
MATERIAL AND METHODS
Patient Selection
Eligible patients included those 18 years of age or older with recurrent, refractory or metastatic, incurable HNSCC of the oral cavity, oropharynx, larynx, hypopharynx, paranasal sinus, unknown primary or nasopharyngeal carcinoma World Health Organization Type 1 (keratinizing, well differentiated). Patients had an Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) of 0–2 and may have received up to one prior palliative chemotherapy regimen for recurrent, refractory or metastatic HNSCC. Chemotherapy given as part of a regimen for curative intent for recurrent disease did not count as “prior chemotherapy.” Patients must not presently be candidates for curative therapy. If primary therapy was given for curative intent, at least 4 weeks must have elapsed after completion of primary therapy prior to enrollment on this clinical trial. However, toxicities from prior treatment must have resolved to grade 1 or less. The study was approved by the institutional review board at all participating institutions. All patients provided signed informed consent for study participation. This trial was conducted by the Southeast Phase II Consortium.
Study Design
The trial was designed as a blinded, randomized phase II, placebo controlled study of cetuximab 400 mg/m2 IV on day 1 and then 250 mg/m2 IV weekly plus placebo twice-a-day (Arm A) or cetuximab at the same dose/schedule plus sorafenib 400 mg PO twice-a-day (Arm B). Each treatment cycle was 21 days. Patients with swallowing difficulties were allowed to dissolve placebo or sorafenib per a defined protocol in water and administer through a gastrostomy tube. However, after nineteen patients were enrolled, the trial was amended to remove the placebo (and blinding) due to issues with difficulties in the placebo tablet solubility. Thus, Arm A consisted of cetuximab alone and Arm B consisted of cetuximab plus sorafenib. Response was evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST). Patients continued on study therapy until progressive disease, unacceptable toxicity or patient desire to withdraw.
Statistics
The primary objective of this trial was to evaluate the PFS of the combination of cetuximab and sorafenib to that of cetuximab plus placebo in patients with recurrent, refractory or metastatic HNSCC. Secondary objectives included evaluations of RR, OS, and toxicities. We anticipated an increase in median PFS from 3 months in the control arm (Arm A) to 5 months in the combination arm (Arm B). A sample size of 84 patients (42 per study arm) yields 83% power to detect such an increase in PFS, or a hazard rate of 1.667 between the two arms, at a significance level (alpha) of 0.10 using a one-sided log rank test. These results assumed that one interim analysis was performed using the O’Brien-Fleming spending function (interim look at alpha = 0.01, final look at alpha = 0.09 for an overall alpha of 0.10) to determine the test boundaries, that the survival times were exponential, and that accrual time was 42 months and follow-up length was 6 months with an accrual rate of about 2 patients per month. We further specified that the hazard ratio at the interim look must be greater than 1.0 in order to proceed to the second stage of the study. Taking into account that there would be about 4 drop-out patients over the course of this study, we planned to enroll a total of 88 patients (44 per arm) into this trial. Interim analysis was to be performed at the midpoint of this study, or about 28 months from study activation when approximately 56 patients were estimated to have been entered into this trial. The intent-to-treat approach was used for data analysis of this trial.
Correlative Studies
Archived formalin-fixed, paraffin-embedded (FFPE) tumor tissue was requested at the time of enrollment. Blood for correlative studies was obtained at pre-treatment on day 1 of each new cycle. Correlative objectives included evaluation of the tumor p16 expression, HPV status and plasma immunomodulatory cytokine levels including TGFβ1, TGFβ2, IFN-gamma, IFN-alpha, TNF-alpha, IL-6, IL-8, IL-10, IL-12-p70, HGF, FGF-basic and VEGF. In brief, immunohistochemistry was performed to determine p16 expression using a p16 mouse monoclonal antibody (predilute, mtm-CINtech, E6H4) as previously described [11]. p16 staining was considered to be positive when diffuse staining was seen in greater than 70% of tumor cells. High-risk HPV status was determined by in situ hybridization (ISH) using a cocktail probe that detects HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58 and 66 (GenPoint HPV Probe Cocktail, Dako) [11]. HPV ISH was interpreted as positive when nuclear specific staining was detected in the tumor cells. The cytokines were detected using multiplex Luminex bead assays as described in previous publications [13]. These laboratory findings were correlated with clinical parameters (RR, PFS and OS) in both arms.
RESULTS
Patient Characteristics
From July 20, 2009 to October 12, 2011, 59 patients were consented on the trial from 7 participating institutions. Four patients were deemed ineligible based on entry criteria for the study. Three patients were deemed eligible but not treated due to consent withdrawal (two patients) and disease progression prior to treatment (one patient). Characteristics of 55 eligible patients are listed in Table 1. Twenty-eight patients (14 in each arm) had received prior chemotherapy for metastatic/recurrent disease. Notably, patients in Arm B were more likely to have an ECOG PS of 2 but it did not differ with a statistical significance [PS 0–1 vs. 2; 9 (33%) vs 19 (67.9%), p=0.35, Fisher exact test]; however, the power of comparison is limited due to the small sample size. Of the 52 treated patients, 43 patients could be evaluated for response (received at least two cycles of therapy and had pre- and post-treatment tumor measurements). Nine of the 52 patients had infusion reactions to the first dose of cetuximab and did not continue on the study. Toxicity was considered evaluable if a patient received any therapy on the study.
Table 1.
Patient Demographic and Clinical Characteristics
| Variable | Category | Cetuximab (Arm A) | Cetuximab+Sorafenib (Arm B) | Overall |
|---|---|---|---|---|
| Age | n | 27 | 28 | 55 |
| Mean | 57.9 | 58.2 | 58.1 | |
| Median (range) | 59 | 60 | 59 (26–74) | |
| Gender, n (%) | F | 2 (7.4) | 5 (17.9) | 7 (12.7) |
| M | 25 (92.6) | 23 (82.1) | 48 (87.3) | |
| Ethnicity n (%) | Hispanic or Latino | 4 (14.8) | 3 (10.7) | 7 (12.7) |
| Non-Hispanic | 22 (81.5) | 25 (89.3) | 47 (85.5) | |
| Unknown | 1 (3.7) | 0 (0) | 1 (1.8) | |
| Race, n (%) | White | 24 (88.9) | 23 (82.1) | 47 (85.5) |
| Black or African American | 1 (3.7) | 1 (3.6) | 2 (3.6) | |
| American Indian or Alaska Native | 2 (7.1) | 2 (3.6) | ||
| Unknown | 2 (7.4) | 2 (7.1) | 4 (7.3) | |
| Performance Status (ECOG) | Ambulatory (0) | 3 (11.1) | 1 (3.6) | 4 (7.3) |
| Fully active (1) | 14 (51.9) | 6 (21.4) | 20 (36.4) | |
| Restricted (2) | 9 (33.3) | 19 (67.9) | 28 (50.9) | |
| Oral Cavity | 3 (11) | 8 (28.5) | 11 (20) | |
| Oropharynx | 12 (44) | 6 (22) | 18 (32.7) | |
| Disease Primary Site | Larynx | 3 (11) | 4 (14.2) | 7 (12.7) |
| Hypopharynx | 0 (0) | 1 (3.5) | 1 (1.8) | |
| Nasopharynx | 2 (7.4) | 0 (0) | 2 (3.6) | |
| Unknown Primary | 3 (11) | 3 (10.7) | 6 (10.9) | |
| Other (i.e. paranasal sinus) | 4 (14.8) | 6 (21.4) | 10 (18.1) |
The protocol called for an interim analysis to be performed at the midpoint of the study. Unless terminated early, 84 evaluable patients were to be enrolled, of which 80 were expected to have events (defined as progression or death). Thus, the midway point of the study would be at 40 events. When the number of events was close to 40, additional study enrollment was suspended pending the interim analysis. At the interim analysis, 43 events had occurred and the study was declared to be futile because the hazard ratio for the cetuximab plus sorafenib arm was 1.038 and thus greater than 1, which was the cutpoint for futility established in the protocol prior to any enrollment. An external review committee consisting of three physicians in the N01 consortium group convened twice; first, they approved the operational timeline for the interim analysis, and later they reviewed the interim analysis report developed by the study statisticians (MJS and XZ) and confirmed the appropriateness of the early termination given the protocol-defined rules.
Efficacy
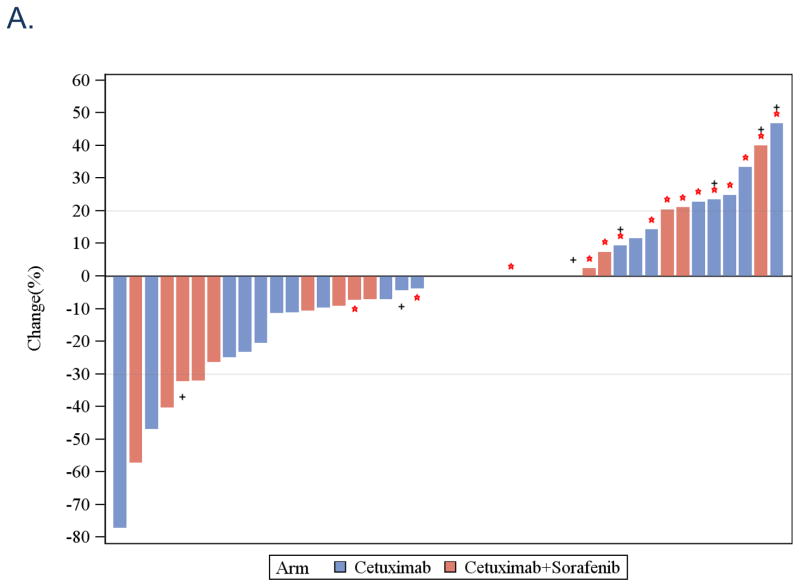
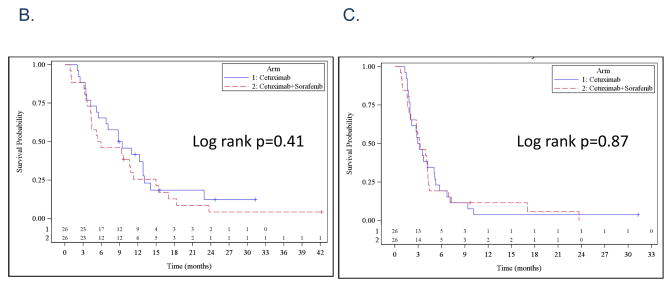
The overall RR was 8% for both arms counting the nine patients not directly evaluated for response as non-responders. A waterfall plot of response for the 43 evaluated patients is noted in Figure 1A. The observed clinical benefit, defined as sum of stable disease, minimal response and partial response, was 12% in each arm. The median OS was 9 months in the cetuximab arm and 5.7 months in the combination arm [p=0.41, 95% Confidence Interval (CI) 5.2–12.9 months and 4.2–10.8 months, respectively; Figure 1B]. The median PFS was 3 months in the cetuximab arm and 3.2 months in the combination arm (p=0.87, 95% CI 1.9–5.0 months and 1.8–4.2 months, respectively; Figure 1C). In terms of “delivered doses”, the total number of cycles delivered in Arm A was 111 while the average number of cycles per patient was 4.1 (range 1–16). The total number of cycles of Arm B delivered was 88 while the average number of cycles per patients was 3.1 (range 1–11). These differences were not statistically significant (p=0.4, 2-sided Wilcoxon Rank-sum test). The mean delivered dose of sorafenib for patients on Arm B was 15,238 mg per cycle.
Figure 1.
A. Waterfall plots of response rates after cetuximab (Arm A, blue bar) and cetuximab+sorafenib (Arm B, red bar). Red star *: progressive disease, Black +: p16 immunohistochemical staining positive. B. Overall survival: Kaplan-Meier plot comparing cetuximab (Arm A, blue line) and cetuximab+sorafenib (Arm B, red line). C. Progression-free survival: Kaplan-Meier plot comparing cetuximab (Arm A, blue line) and cetuximab+sorafenib (Arm B, red line).
Toxicity/Safety
Overall, the regimen was well tolerated. Maculopapular rash was the most common toxicity in both arms; however, only one patient in each arm experienced grade 3 rash while the remaining rash grades were 1 or 2. Fatigue was the second most common toxicity in both arms, with 8 patients in Arm A (7 of which were grade 1 and 2) and 24 patients in Arm B (22 of which were grade 1 and 2). Overall, more grade 3 and 4 toxicities occurred in Arm B than in Arm A, though these were still relatively infrequent on both arms (Table 2). One patient on Arm B required two sorafenib dose reductions due to toxicity. Interestingly, anaphylaxis or infusion-related reaction with the first dose of cetuximab occurred in 8 patients in Arm B but in only 1 patient in Arm A. Three patients (two patients in Arm A and one patient in Arm B) had dose interruptions and did not resume therapy per patient/physician discretion even though attributable dose limiting toxicity was not documented.
Table 2.
Number of patients with Grade 3 or 4 Toxicity in Arm A and B by CTCAE version 4.0
| Cetuximab (Arm A) | Cetuximab plus Sorafenib (Arm B) | ||
|---|---|---|---|
| Toxicity | Grade 3 | Grade 3 | Grade 4 |
| Fatigue | 1 | 2 | 0 |
| Infusion Reaction | 1 | 5 | 3 |
| Maculopapular Rash | 1 | 2 | 0 |
| Alkaline phosphatase | 0 | 2 | 0 |
| Anemia | 0 | 1 | 0 |
| Anorexia | 0 | 1 | 0 |
| AST increased | 0 | 1 | 0 |
| Diarrhea | 0 | 2 | 0 |
| Dyspepsia | 0 | 1 | 0 |
| Hypertension | 0 | 1 | 0 |
| Hypokalemia | 0 | 1 | 0 |
| Oral Mucositis | 0 | 2 | 0 |
| Nausea | 0 | 1 | 0 |
| Thromboembolic Event | 0 | 1 | 0 |
| Total | 3 | 23 | 3 |
Grade 5 toxicites were not observed.
Evaluation of p16 expression and HPV status by in situ hybridization
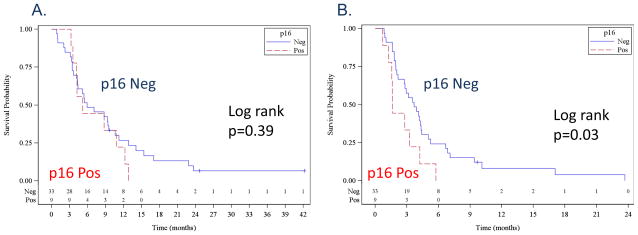
Of the 52 treated patients, 44 (85%) FFPE tumors and 24 (46%) pre-treatment plasma were collected. The FFPE tumors were evaluated for p16 expression as a prognostic marker. Thirty-five (80%) were p16-negative and nine (20%) were p16-positive. All nine p16-positive tumors had a strong and diffuse staining in greater than 70% of the tumors. Thirty four of 35 p16-negative tumors had no staining at all. One tumor had a weak staining in less than 10% of the tumor and was considered negative. There were only 11 tumors from the oropharyngeal primary site (ten p16-negative and one p16-positive) and survival analyses using only oropharyngeal tumors were not feasible. The p16-positive samples were tested for high-risk HPV DNA using ISH, and three of nine (33%) p16-positive tumors were also ISH positive. The HPV positive tumors were from two oral cavity and one oropharyngeal primary sites. Median OS were 5.9 months for p16-negative and 5.2 months for p16-positive patients (Log rank p-value 0.39, Figure 2A). Median PFS were 3.7 months for p16-negative and 1.6 months for p16-positive patients (Log rank p-value 0.03; Figure 2B).
Figure 2.
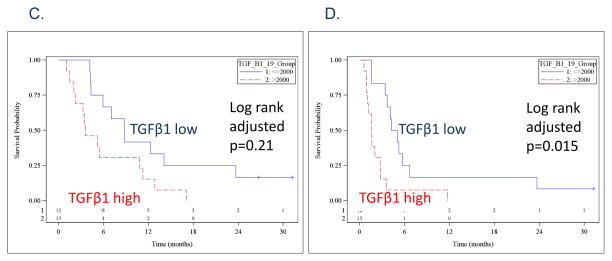
Kaplan-Meier plots comparing p16-negative patients (blue line) and p16-positive patients (red line). A. overall survival, and B. progression-free survival. Kaplan-Meier plots comparing patients with plasma TGFβ1 levels lower than the median (blue line) and higher than the median (red line). C. overall survival, and D. progression-free survival.
Plasma immunomodulatory cytokine evaluation
Twelve immunomodulatory cytokines were determined in 24 pre-treatment plasma samples. These 12 cytokines were selected based on a previous feasibility study for reliable detection in HNSCC patients, but HGF was eliminated from the analyses due to extremely low expression in this dataset [12]. Among the 11 cytokines, we noticed that there were clusters of cytokines with a high correlation in expression levels: 1) TGFβ1 cluster including TGFβ1 and TGFβ2; and 2) IL-8 cluster including IL-8, IFN-alpha, IFN-gamma, TNF-alpha, IL-6, IL-10, IL-12-p70, FGF-basic and VEGF. The two TGFβ cytokines had a 0.84 correlation, and the other 9 cytokines in the IL-8 cluster had a median correlation of 0.67. To limit a multiple variable comparison, we only evaluated TGFβ1, IL-8 and VEGF for their association with survival. Furthermore, the p-values were adjusted for a multiple variable comparison. Median OS durations were 5.3 months in TGFβ1 high group and 8.8 months in low group defined by a higher or lower than a median expression (log rank adjusted p-value 0.21, Figure 2C). Median PFS durations were 1.9 months in TGFβ1 high group and 4.7 months in the low group (log rank adjusted p-value 0.015, Figure 2D).
DISCUSSION
Unresectable R/M HNSCC remains an incurable disease. Cetuximab is the only FDA approved biological agent for treatment in this setting. In combination with a platinum and 5-fluorouracil, this regimen is the only combination of agents that has demonstrated a survival benefit [6]. However, the combination of chemotherapy is relatively toxic. Thus, a more effective and less toxic regimens are in need. In this study, we utilized the combination of cetuximab and sorafenib as an anti-angiogenic agent. Preclinical data supports a favorable effect of anti-angiogenesis when combined with EGFR inhibition in HNSCC [8, 9]. However, our study did not demonstrate clinical benefit given the combination of cetuximab and sorafenib.
We have learned that the combination of cetuximab and sorafenib was overall well-tolerated but was more toxic than cetuximab monotherapy. Secondly, no significant survival difference was detected between cetuximab alone and cetuximab plus sorafenib. While this may be a function of the relatively small sample size, sorafenib did not demonstrate enough activity to warrant further study. The lack of efficacy could be attributed to insufficient anti-angiogenic activity of sorafenib in this disease. Moreover, several patients had to dissolve sorafenib due to dysphagia. While the instructions for dissolution were provided by the pharmaceutical company, the bioavailability of sorafenib may have been less than ideal for maximal anti-tumor effect. Other anti-angiogenic agents with different mechanism of inhibition or modes of administration such as bevacizumab have shown efficacy given with EGFR inhibitors in earlier studies [8, 9]. Further evaluation of anti-angiogenic approach in the management of R/M HNSCC is ongoing through the ECOG-ACRIN phase III trial comparing doublet chemotherapy with and without bevacizumab (E1305; NCT00588770).
Elucidating the molecular underpinnings of inherent or acquired resistance to cetuximab has proven to be challenging; however, the way to rationally develop novel treatment combinations will be through gaining further biological insight. In patients with locally advanced HNSCC from the oropharynx given standard concurrent cisplatin and radiotherapy, positive p16 expression as a surrogate marker of HPV-related tumors is tightly correlated with a favorable survival compared to p16-negative tumors [14–16]. Furthermore, a recent study suggest newly diagnosed patients with p16-positive non-oropharyngeal HNSCC including oral cavity, larynx and hypopharynx sites also have a favorable survival compared to p16-negative tumors although the prognostic effect is less compared to p16-positive oropharyngeal HNSCC [17]. The favorable survival associated with p16 positivity is also observed in patients even after the progression to recurrent and/or metastatic disease [18–20].
In locally advanced disease, cetuximab is presently being evaluated in a strategy of therapeutic “de-intensification” in patients with favorable prognosis. Therefore, it is unexpected that patients with p16-negative tumors had a longer PFS compared to p16- positive patients in our study suggesting that p16-negative patients may derive a more consistent benefit from cetuximab-based therapy. The association between p16 status and EGFR inhibitor response is unclear. In a subset analysis of the randomized phase III SPECTRUM study in which the p16-negative R/M HNSCC patients appeared to gain more benefit in overall survival when combining cisplatin and 5-FU with an EGFR-inhibitor, panitumimab, while p16-positive patients did not benefit from the addition of panitumumab [20]. However, this association was not observed in the EXTREME trial which was the phase III clinical trial comparing cisplatin, 5FU with and without cetuximab in R/M HNSCC patients [19]. These differences thought to be attributable to different p16 IHC staining cutoff points used in these two studies (SPECTRUM: >10% vs. EXTREME: >70% positive staining in the tumors to be considered p16 positive) and potential difference between panitumumab and cetuximab [19, 20]. Our study sample size is small and the data from SPECTRUM and EXTREME studies are from an unplanned subset analysis; hence we cannot draw any firm conclusion regarding lack of p16 expression and cetuximab response. However, the data are intriguing and further studies are indicated.
TGFβ1 is a multi-functional cytokine that regulates cell growth, differentiation, and migration by signaling through TGF-β receptors (TGF-βRI, TGF-βRII, and TGF-βRIII) [21]. TGFβ1 has been shown to induce epithelial-to-mesenchymal transition (EMT) in HNSCC [22]. In addition to its effects on intra-tumoral signaling, TGFβ1 can suppress the cytotoxic function of immune effectors [12]. HNSCC cells have been shown to escape treatment effects of cetuximab via suppression of ADCC by up-regulating TGFβ expression [12]. Thus, TGFβ may play a role in both intrinsic and acquired resistance to cetuximab and patients with low expression of TGFβ1 may benefit the most from cetuximab while patients with high expression may be best treated with a combination of cetuximab and an anti-TGFβ agent. There are several TGFβ pathway targeted agents in development, such as GC1008. In addition, immunosuppression induced by TGFβ activation may be mediated by combining cetuximab with novel immune checkpoint inhibitors such as anti-CTLA-4, anti-PD1 or anti-PDL1 antibodies currently in clinical development [23, 24].
In summary, our study demonstrated that sorafenib did not add clinical benefit to cetuximab alone but added toxicities, and was terminated early base on a planned interim analysis. Our correlative studies suggest that patients with p16-negative tumors or low plasma TGFβ1 expression may derive benefits from cetuximab-based therapy. In addition, patients with high plasma TGFβ1 expression may potentially benefit from TGFβ pathway targeted agents or immune checkpoint inhibitors in combination with cetuximab. However, these are very exploratory findings, and further studies are warranted.
Highlights.
Combination of cetuximab+sorafenib is overall well-tolerated in rec/met HNSCC.
Combination of cetuximab+sorafenib is more toxic than cetuximab alone.
No survival difference is detected between cetuximab and cetuximab+sorafenib.
A subset of R/M p16(−) HNSCC has a longer PFS compared to p16(+).
A subset of R/M HNSCC with lower plasma TGFβ1 levels has a longer PFS.
Acknowledgments
Grant Funding: National Cancer Institute NO1 261201100100C, National Institute of Dental & Craniofacial Research, SPORE in Head and Neck Cancer at Johns Hopkins University (P50DE019032).
Footnotes
Conflict of interest: CHC received research funding from Bayer for correlative studies. All other authors have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jill Gilbert, Email: jill.gilbert@vanderbilt.edu.
Michael J. Schell, Email: Michael.Schell@moffitt.org.
Xiuhua Zhao, Email: Xiuhua.Zhao@moffitt.org.
Barbara Murphy, Email: barbara.murphy@Vanderbilt.Edu.
Tawee Tanvetyanon, Email: Tanvetyanon@moffitt.org.
Marino E. Leon, Email: Marino.Leon@Moffitt.org.
D. Neil Hayes, Email: hayes@med.unc.edu.
Missak Haigentz, Jr., Email: mhaigent@montefiore.org.
Nabil Saba, Email: NFSABA@emory.edu.
Jorge Nieva, Email: Jorge.Nieva@med.usc.edu.
Justin Bishop, Email: jbishop@jhmi.edu.
David Sidransky, Email: dsidrans@jhmi.edu.
Rajani Ravi, Email: ravira@jhmi.edu.
Atul Bedi, Email: abedi1@jhmi.edu.
References
- 1.Huang SH, Perez-Ordonez B, Weinreb I, Hope A, Massey C, Waldron JN, et al. Natural course of distant metastases following radiotherapy or chemoradiotherapy in HPV-related oropharyngeal cancer. Oral Oncol. 2013;49:79–85. doi: 10.1016/j.oraloncology.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 2.Grandis J, Melhem M, Gooding W, Day R, Holst V, Wagener M, et al. Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst. 1998;90:824–832. doi: 10.1093/jnci/90.11.824. [DOI] [PubMed] [Google Scholar]
- 3.Ang KK, Berkey BA, Tu X, Zhang HZ, Katz R, Hammond EH, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62:7350–7356. [PubMed] [Google Scholar]
- 4.Chung CH, Ely K, McGavran L, Varella-Garcia M, Parker J, Parker N, et al. Increased epidermal growth factor receptor gene copy number is associated with poor prognosis in head and neck squamous cell carcinomas. J Clin Oncol. 2006;24:4170–4176. doi: 10.1200/JCO.2006.07.2587. [DOI] [PubMed] [Google Scholar]
- 5.Chung CH, Zhang Q, Hammond EM, Trotti AM, 3rd, Wang H, Spencer S, et al. Integrating Epidermal Growth Factor Receptor Assay With Clinical Parameters Improves Risk Classification for Relapse and Survival in Head-and-Neck Squamous Cell Carcinoma. Int J Radiat Oncol Biol Phys. 2011;81:331–338. doi: 10.1016/j.ijrobp.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 7.Yu M, Liu L, Liang C, Li P, Ma X, Zhang Q, et al. Intratumoral vessel density as prognostic factors in head and neck squamous cell carcinoma: A meta-analysis of literature. Head Neck. 2013 doi: 10.1002/hed.23301. [DOI] [PubMed] [Google Scholar]
- 8.Cohen EE, Davis DW, Karrison TG, Seiwert TY, Wong SJ, Nattam S, et al. Erlotinib and bevacizumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck: a phase I/II study. Lancet Oncol. 2009;10:247–257. doi: 10.1016/S1470-2045(09)70002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Argiris A, Kotsakis AP, Hoang T, Worden FP, Savvides P, Gibson MK, et al. Cetuximab and bevacizumab: preclinical data and phase II trial in recurrent or metastatic squamous cell carcinoma of the head and neck. Ann Oncol. 2013;24:220–225. doi: 10.1093/annonc/mds245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williamson SK, Moon J, Huang CH, Guaglianone PP, LeBlanc M, Wolf GT, et al. Phase II evaluation of sorafenib in advanced and metastatic squamous cell carcinoma of the head and neck: Southwest Oncology Group Study S0420. J Clin Oncol. 2010;28:3330–3335. doi: 10.1200/JCO.2009.25.6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bedi A, Chang X, Noonan K, Pham V, Bedi R, Fertig EJ, et al. Inhibition of TGF-beta enhances the in vivo antitumor efficacy of EGF receptor-targeted therapy. Mol Cancer Ther. 2012;11:2429–2439. doi: 10.1158/1535-7163.MCT-12-0101-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byers LA, Holsinger FC, Kies MS, William WN, El-Naggar AK, Lee JJ, et al. Serum signature of hypoxia-regulated factors is associated with progression after induction therapy in head and neck squamous cell cancer. Mol Cancer Ther. 2010;9:1755–1763. doi: 10.1158/1535-7163.MCT-09-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ang KK, Zhang Q, Rosenthal DI, Nguyen-Tan PF, Sherman EJ, Weber RS, et al. A randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III–IV head and neck carcinoma: RTOG 0522. 2013. In Preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Posner MR, Lorch JH, Goloubeva O, Tan M, Schumaker LM, Sarlis NJ, et al. Survival and human papillomavirus in oropharynx cancer in TAX 324: a subset analysis from an international phase III trial. Ann Oncol. 2011;22:1071–1077. doi: 10.1093/annonc/mdr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rischin D, Young RJ, Fisher R, Fox SB, Le QT, Peters LJ, et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02. 02 phase III trial. J Clin Oncol. 2010;28:4142–4148. doi: 10.1200/JCO.2010.29.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung CH, Zhang Q, Kong CS, Harris J, Fertig EJ, Harari PM, et al. p16 Protein Expression and Human Papillomavirus Status As Prognostic Biomarkers of Nonoropharyngeal Head and Neck Squamous Cell Carcinoma. J Clin Oncol. 2014 doi: 10.1200/JCO.2013.54.5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fakhry C, Zhang Q, Nguyen-Tan PF, Rosenthal D, El-Naggar A, Garden AS, et al. Human Papillomavirus and Overall Survival After Progression of Oropharyngeal Squamous Cell Carcinoma. J Clin Oncol. 2014 doi: 10.1200/JCO.2014.55.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vermorken JB, Psyrri A, Mesia R, Peyrade F, Beier F, de Blas B, et al. Impact of tumor HPV status on outcome in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck receiving chemotherapy with or without cetuximab: retrospective analysis of the phase III EXTREME trial. Ann Oncol. 2014;25:801–807. doi: 10.1093/annonc/mdt574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vermorken JB, Stohlmacher-Williams J, Davidenko I, Licitra L, Winquist E, Villanueva C, et al. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (SPECTRUM): an open-label phase 3 randomised trial. Lancet Oncol. 2013;14:697–710. doi: 10.1016/S1470-2045(13)70181-5. [DOI] [PubMed] [Google Scholar]
- 21.Massague J. TGFbeta in Cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frederick BA, Helfrich BA, Coldren CD, Zheng D, Chan D, Bunn PA, Jr, et al. Epithelial to mesenchymal transition predicts gefitinib resistance in cell lines of head and neck squamous cell carcinoma and non-small cell lung carcinoma. Mol Cancer Ther. 2007;6:1683–1691. doi: 10.1158/1535-7163.MCT-07-0138. [DOI] [PubMed] [Google Scholar]
- 23.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]