Abstract
Purpose:
The aims of this study were to determine if there were any statistically significant immediate effects of upper thoracic spinal manipulative therapy (SMT) on cardiovascular physiology in hypertensive individuals.
Introduction:
Preliminary research suggests that SMT to various regions of the spine may be capable of lowering systolic and diastolic blood pressure in hypertensive individuals. Further studies are warranted to corroborate or refute these findings as well as measure how other attributes of cardiovascular physiology are impacted by SMT.
Methods:
Fifty hypertensive participants (age = 45.5±13.9 years, height = 1.69±0.10 m, body mass = 93.9±21.5 kg: mean±standard deviation (SD)) were equally randomized into a single-blind, controlled trial involving two study groups: supine diversified anterior upper thoracic SMT of T1–4, or a ‘no T-spine contact’ control. Outcome measures were electrocardiogram, bilateral pulse oximetry, and bilateral blood pressure measurement performed at baseline, post 1-minute intervention, and post 10-minute intervention. An independent samples t-test was used to compare between-group differences at baseline. A repeated measures ANOVA was used to compare within-group changes over time.
Results:
Within-group changes in PR interval and QRS duration demonstrated that the atria were transiently less active post-SMT and the ventricles were more active post-SMT, however the changes were clinically minimal.
Conclusion:
The results of this study, and the limited existing normotensive, thoracic-specific SMT research in this field, suggest that cardiovascular physiology, short-term, is not affected by upper thoracic spine SMT in hypertensive individuals to a clinically relevant level.
Keywords: Blood pressure, Chiropractic, Manipulation, Heart rate
Introduction
High blood pressure prevalence is on the rise in the United States.1 It has been estimated that 41.9 million men and 27.8 million women have pre-hypertension, 12.8 million men and 12.2 million women have stage-1 hypertension, and 4.1 million men and 6.9 million women have stage-2 hypertension.1 Globally, cardiovascular disease results in 17 million deaths per year, with 9.4 million of these being due to complications from hypertension.2 Thus far, hypertension has been found to be responsible for 45% of deaths from heart disease and 51% of deaths due to stroke.3 Any form of treatment to lower the prevalence and morbidity associated with hypertension should be pursued.
Preliminary research suggests that one possible way to lower blood pressure may be spinal manipulative therapy (SMT). Some researchers have shown that spinal manipulation to various regions of the spine can lower blood pressure.4–13 Unfortunately, some of these studies have suffered from methodological flaws described elsewhere.14
Anatomically, the upper thoracic spine is poised to impact cardiovascular physiology. Sympathetic nervous system preganglionic neurons for the heart originate from the lateral gray columns of T1–5.15,16 Sympathetic nerve endings traveling to the heart ultimately serve to increase the chronotropic and inotropic events of the heart and cause mild vasodilation of the coronary arteries.15–18 Overall these actions chiefly increase heart rate, stroke volume, and cardiac output. Owing, in part, to this anatomic relationship, some chiropractic researchers have claimed that thoracic spine manipulation may impact sympathetic nervous system output.19–21 If upper thoracic spine SMT could modify sympathetic nervous system output, then the degree to which it is able to do so should be clearly investigated as that may impact blood pressure.
Studies focusing on thoracic spine manipulation and cardiovascular response have yielded mixed results. Welch and Boone10 theorized that manipulating the thoracic spine would cause a sympathetic response in patients. However, when they measured normotensive participants who underwent SMT, they found that there were minimal blood pressure changes (−1.8 mmHg systolic blood pressure and +3.0 mmHg diastolic blood pressure). Similarly, Ward et al.14 found minimal change in blood pressure post-manipulation amongst normotensive participants (−3.6 mmHg systolic blood pressure and +0.0 mmHg diastolic blood pressure post 1-minute SMT right arm). Likewise, Holt et al.6 likewise found insignificant changes in blood pressure in response to thoracic spine SMT (−0.3 mmHg systolic blood pressure and −0.6 mmHg diastolic blood pressure). These findings strongly contrast with those demonstrated by Yates et al.11 In their study on hypertensive patients, they observed a drop in systolic blood pressure of 14.7 mmHg and a drop in diastolic blood pressure post-thoracic spine manipulation of 13.0 mmHg.11 Thus, this topic warrants further review.
Case studies have suggested that SMT may lower medicated hypertensive patients’ blood pressure.13,22 Some researchers have warned practitioners to be careful when manipulating hypertensive patients because they believe that SMT applied to patients on blood pressure medication may cause an unsafe drop in blood pressure.13 In addition to the dramatic results observed by Yates et al.,11 Torns’5 research on pre-hypertensive to stage-2 hypertensive patients demonstrated a drop in blood pressure post-SMT. After receiving SMT, participants’ systolic blood pressured dropped 20.2 mmHg and diastolic blood pressure dropped 6.8 mmHg. As a result, practitioners using manipulative interventions may need to be careful when applying them to the thoracic spine of hypertensive patients.
The aim of this study is to help fill an existing gap in the literature on the immediate impact that upper thoracic spine SMT has on cardiovascular physiology amongst hypertensive patients. Additionally, this study will help determine if there is an unsafe drop in blood pressure amongst hypertensive patients receiving thoracic spine SMT. This study’s original hypothesis was that upper thoracic SMT would transiently lower blood pressure similar to the results of Yates et al.11 with hypertensive patients, yet have minimal impact on other cardiovascular parameters.
Methods
This study was approved by the Texas Chiropractic College (TCC) Institutional Review Board and was registered with the University hospital Medical Information Network Clinical Trials Registry (UMIN-CTR) 000013322.
Experimental design
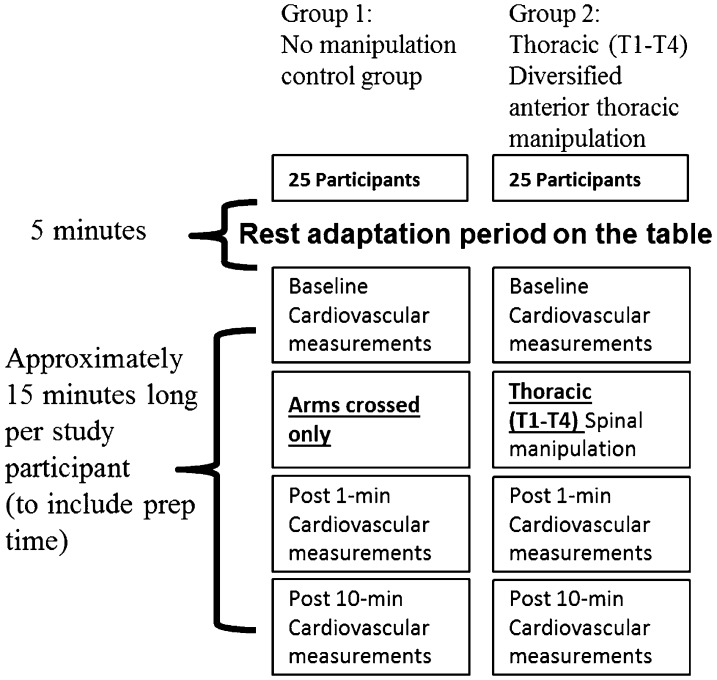
This was a single-blind, randomized, and controlled trial of the immediate effect of supine anterior upper thoracic SMT on cardiovascular response of hypertensive individuals. Fifty participants were randomly assigned to one of two groups: control (age = 45.6±13.8 years, height = 1.68±0.10 m, mass = 89.6±19.9 kg) or thoracic spine SMT of T1–4 (age = 45.3±14.0 years, height = 1.70±0.10 m, mass = 98.2±23.1 kg). Study participants (Table 1) attended one study session that involved baseline, post 1-minute intervention, and post 10-minute intervention assessments of cardiovascular function. These measurements included ECG recording, bilateral pulse oximetry analysis, and bilateral electronic automated blood pressure readings as illustrated in Fig. 1. This study took place in a research lab with a room temperature that was regulated as close as possible to 24°C. A 5′×3′ towel was placed on the adjusting table to ensure uniform temperature against the skin between test sessions. The experiment was performed in a quiet lab with minimal ambient noise. On arrival to the laboratory, study participants would lay supine and engage in a 5-minute rest period prior to undergoing their initial cardiovascular measurements.
Table 1. Participant baseline attributes.
| Control | Thoracic SMT | P-value | |
| Sex (M/F) | 14/11 | 14/11 | |
| Age (years) | 45.6±13.8 | 45.3±14.0 | 0.943 |
| Mass (kg) | 89.6±19.9 | 98.2±23.1 | 0.166 |
| Height (m) | 1.68±0.10 | 1.70±0.10 | 0.608 |
| Body mass index (kg/m2) | 31.6±7.0 | 33.8±6.3 | 0.231 |
| On high blood pressure medication/s (yes/no) | 17/8 | 17/8 |
Data listed as mean±standard deviation (SD) unless listed as a ratio.
Between-groups baseline data compared with an independent samples t-test.
SMT: spinal manipulative therapy.
Figure 1.
Experimental design.
Randomization and blinding
A computer-generated randomized intervention list was created before the study began and was used to assign participants as they enrolled in the study to avoid selection bias. By necessity, the chiropractor performing the intervention was aware of the group to which the study participant belonged. The trained graduate research assistant taking all cardiovascular outcome measurements was blinded as to the study participant’s group designation. This was performed by having that assistant leave the lab while the intervention, or lack thereof, took place.
Participants
Participants were recruited with online advertisements and via word-of-mouth.
No a priori power analysis was performed. Owing to funding limitations, the study was capped at 25 participants per group. All study participants provided an informed written consent. They were then screened against inclusion and exclusion criteria. During data collection, men were instructed to not wear a shirt. Females were directed to wear a loose fitting sports bra. Both male and female participants were provided with a cotton gown to cover their chest, at their discretion, for modesty. A trained female research assistant was used to place ECG electrodes on all study participants. Data collection for the participants took place over the course of a 3-month window. This occurred between the hours of 4 PM–7 PM. The intent of these constraints was to decrease the likelihood that seasonal or circadian (e.g., related to cortisol daily cycle) rhythm changes in heart rate and blood pressure could act as covariates. Participants were given a study preparation handout of the exclusion criteria and reminded to avoid the following during the study: caffeine, alcohol, tobacco, and exogenous nutritional supplements that contained omega-3 fatty acids, niacin, magnesium, or potassium. In addition, they were also instructed verbally and in written form to not receive any outside source of SMT or wear skin lotions on their chest during the days that they participated in the study. To reduce any possible anxiety, study participants were clearly instructed about the basic sequence of their portion of the research study.
Inclusion/exclusion criteria
Inclusion criteria were:
no contraindication to thoracic spine SMT (unstable fractures, severe osteoporosis, multiple myeloma, osteomyelitis, inflammatory phase ankylosing spondylitis, spinal cord tumor, Paget’s disease, and similar conditions);
between 18 and 65 years of age;
informed written consent was provided;
patients provided proof of high blood pressure either by showing the researchers their high blood pressure medications with their names written on the prescriptions (which included only the following: angiotensin converting enzyme inhibitors, calcium channel blockers, beta blockers, angiotensin II receptor blockers, or diuretics) or by demonstrating an initial screening blood pressure reading greater than 140/90 mmHg.
Study participants with any of the following were excluded from the study:
torso surgery in the past year;
torso broken bones in the past year;
tobacco product use;
pregnancy;
rheumatoid arthritis;
Down’s syndrome;
fingernail polish on the index fingers;
daily pain rated >3 on a 0–10 numeric rating scale (NRS).
Intervention
The intervention phase of the study was performed by a chiropractor with 20 years of experience and 15 years of SMT technique teaching experience at Texas Chiropractic College. The intervention consisted of either: ‘no T-spine contact’ control or supine diversified anterior upper thoracic SMT to the T1–4 region. The treating chiropractor exerted every effort to ensure similar patient positioning between study participants in each of the two study groups. Participants in both groups were supine on the adjusting table. Participants in the control group had their arms folded across their chest by the treating chiropractor for a few seconds. Then the chiropractor unfolded their arms. Participants in the thoracic SMT group underwent a supine diversified anterior upper thoracic SMT described by Bergmann and Peterson23 as an opposite-side thenar/transverse drop. This form of supine diversified anterior upper thoracic SMT was chosen because it allowed for the patient to not have to change from a supine position for baseline ECG recording to an alternate body position (e.g., prone) for SMT and then back to a supine position to reconnect ECG electrodes and continue with measurements. This additional movement would likely have acted as a covariate due to participants increasing their heart rate with the added motion. Initially participants in the SMT group had their arms folded across their chest. The treating chiropractor then rolled the participant toward him and made a ‘cupped’ hand contact at the T2 spinous level with slight overlap above and below that vertebra. Following this he asked the participant to breathe out fully as he/she was rolled back to a supine position. This researcher then performed a quick drop of his upper body over the participant’s chest with a high-velocity low-amplitude SMT (Fig. 2). During this SMT, the chiropractor attempted to achieve cavitation of the T1–4 segments of the thoracic spine. No second attempt was made if an audible joint cavitation failed to occur. Following this, the chiropractor uncrossed the study participant’s arms.
Figure 2.
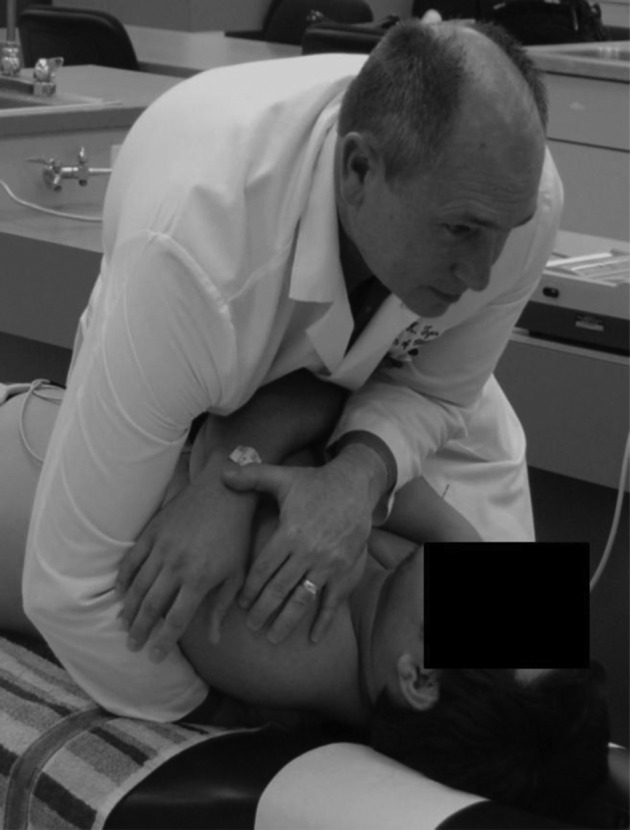
Illustration of supine diversified anterior upper thoracic spinal manipulative therapy (SMT).
Assessments
Cardiovascular measurements were taken at baseline, post 1-minute intervention, and post 10-minute later by one trained female research assistant. Throughout the baseline, post 1-minute intervention, and post 10-minute intervention, the study participant stayed in a supine position on the table. Three measurements were employed for analysis of cardiovascular response: ECG measurement, pulse oximetry readings, and automated blood pressure recording.
ECG measurements were performed using the 10-lead CardioTouch 3000 ECG monitor (Bionet, Tustin, CA, USA). R41 Skintact (Leonard Lang, Inverness, FL, USA) disposable manufacturer-suggested surface electrodes were utilized. Surface electrodes were placed over the anterior thorax, wrists, and ankles as per the manufacturer’s instruction manual. Study participants were asked to relax just prior to each recording. A 10-second recording was made and analyzed for each study participant at each of the three time points. A printout was produced during each recording. The dependent variables, heart rate, PR interval, QRS duration, QT interval, and QTc interval were extracted from the printouts. Surface electrode stickers were not removed from the body during the baseline, post 1-minute intervention, and post 10-minute intervention data collection steps. The clamps and wires were, however, removed from the sticker electrodes right before SMT and were immediately reconnected to their unmoved surface electrode stickers after SMT. This was done because performing the manipulation with the clamps attached may have harmed the patients.
Pulse oximetry was measured bilaterally using the Pulse Ox 5500™ Finger unit (SPO Medical, Sylmar, CA, USA). The index finger of each hand was used for pulse oximetry recordings. The dependent variable, percent saturation of oxygen on hemoglobin, was determined.
Blood pressure was measured bilaterally using the Omron HEM-705 CP automated blood pressure analyzer (Omron Healthcare Inc., Lake Forest, IL, USA). An automated device was used to reduce possible examiner bias. This monitor was specifically purchased for the study because it had been tested in large epidemiological studies and was shown to be accurate.24 The dependent variables, systolic and diastolic blood pressure, were also recorded. Pulse oximetry and blood pressure were analyzed bilaterally with the intent to determine if any unique unilateral changes occurred in response to upper thoracic spine SMT. All cardiovascular data were transcribed by hand by the blinded research assistants to a form created for this study. Then data were transferred into an Excel spreadsheet (Microsoft Office 2010, Redmond, WA, USA). Following this, pulse pressure, mean arterial pressure, and rate pressure product were determined mathematically in Excel using the previously described dependent variables taken exclusively from the right upper extremity.
Statistical analysis
Data were analyzed in SPSS version 20 (IBM, Armonk, NY, USA). Results were reported as mean±standard deviation (SD) unless otherwise specified. An independent samples t-test was used to compare between-group differences at baseline. Levene’s test for equality of variance was utilized and followed for homogeneity of variance violation. A repeated measures ANOVA was used to compare within-group changes over time. The repeated measure factor was the session of data collection (baseline, post 1-minute, or post 10-minute). Mauchly’s test was used to monitor sphericity and the Greenhouse-Geisser correction was utilized during instances of sphericity violation.25 A Bonferroni post hoc test was utilized on statistically significant data to determine which time intervals were significant. The alpha level of P<0.05 was considered statistically significant for all within-group and between-group variables.
Results
Between-groups comparison at baseline indicated that there were statistically significant differences in left pulse oximetry, t(48) = −1.198, P = 0.020, and PR interval, t(48) = −2.148, P = 0.037 between the groups. The SMT group demonstrated a lower level of oxygenation saturation in their blood as sampled from the left index finger. The SMT group also demonstrated a longer PR interval than the control group, which indicates longer electrical activity in the atria.
There was a statistically significant within-group difference for the experimental group’s PR interval, F(2,48) = 3.275, P = 0.046, and QRS duration, F(2,48) = 4.008, P = 0.025. For the SMT group, the PR interval decreased at post 1-minute transiently and then increased at post 10-minute. Bonferroni post hoc testing indicated there was a significant difference between post 1-minute and post 10-minute for the PR interval. For the SMT group, the QRS duration increased at post 1-minute transiently and then decreased at post 10-minute. Bonferroni post hoc testing demonstrated a statistically significant difference between post 1-minute and post 10-minute for QRS duration. There was a statistically significant within-group difference for the control group for PR interval, F(2,48) = 3.620, P = 0.047, for unclear reasons. Bonferroni post hoc testing demonstrated a significant difference between baseline testing and post 1-minute for the PR interval. In all cases of within-group repeated measures, sphericity was not violated. Overall, the cardiovascular changes, although statistically significant, were transient and minimal in clinical effect. This would suggest that supine diversified anterior upper thoracic SMT resulted in no appreciable clinical changes in cardiovascular function in hypertensive participants compared to the control group. Tables 2–4 demonstrate the relationships between all of the cardiovascular dependent variables measured.
Table 2. Blood pressure and related blood flow variable recordings at baseline, post 1-minute, and post 10-minute.
| Baseline | Post 1-minute | Post 10-minute | P-value | |
| R. systolic blood pressure (mmHg) | ||||
| Control | 141.0±16.3 | 143.0±17.7 | 141.2±17.5 | 0.412 |
| Thoracic SMT | 149.0±21.7 | 147.6±24.9 | 146.3±20.5 | 0.464 |
| Baseline P | 0.145 | |||
| R. diastolic blood pressure (mmHg) | ||||
| Control | 83.5±8.6 | 83.4±8.6 | 81.7±10.0 | 0.235 |
| Thoracic SMT | 87.2±15.1 | 85.1±15.7 | 85.6±14.6 | 0.102 |
| Baseline P | 0.292 | |||
| L. systolic blood pressure (mmHg) | ||||
| Control | 142.4±17.9 | 139.2±16.3 | 144.4±18.4 | 0.107 |
| Thoracic SMT | 146.4±21.1 | 145.0±20.4 | 144.4±23.6 | 0.599 |
| Baseline P | 0.478 | |||
| L. diastolic blood pressure (mmHg) | ||||
| Control | 82.5±9.5 | 81.6±9.4 | 83.3±9.1 | 0.260 |
| Thoracic SMT | 85.4±16.8 | 85.1±16.9 | 85.2±15.2 | 0.971 |
| Baseline P | 0.454 | |||
| Pulse pressure (mmHg) | ||||
| Control | 57.5±12.0 | 59.5±13.1 | 59.5±12.1 | 0.359 |
| Thoracic SMT | 61.8±12.8 | 62.5±16.6 | 60.7±14.0 | 0.663 |
| Baseline P | 0.224 | |||
| Mean arterial pressure (mmHg) | ||||
| Control | 102.6±10.3 | 103.3±10.8 | 101.5±11.7 | 0.287 |
| Thoracic SMT | 107.8±16.5 | 106.0±17.6 | 105.8±15.5 | 0.152 |
| Baseline P | 0.193 | |||
| Heart rate (bpm) | ||||
| Control | 70.9±10.6 | 71.7±8.9 | 71.0±10.4 | 0.682 |
| Thoracic SMT | 71.8±12.5 | 71.5±12.3 | 70.9±12.1 | 0.498 |
| Baseline P | 0.789 | |||
| Rate pressure product (mmHg bpm) | ||||
| Control | 9987±1838 | 10 224±1680 | 9978±1652 | 0.316 |
| Thoracic SMT | 10 772±2713 | 10 645±2925 | 10 438±2560 | 0.288 |
| Baseline P | 0.238 | |||
Data listed as mean±standard deviation (SD).
Within-group data were analyzed with repeated measures ANOVA.
Between-group baseline data were compared with an independent samples t-test.
SMT: spinal manipulative therapy.
Table 3. Change in pulse oximetry readings from baseline to post 1-minute and post 10-minutes.
| Baseline | Post 1-minute | Post 10-minutes | P-value | |
| Pulse oximetry – right index finger (%) | ||||
| Control | 97.6±1.5 | 97.8±0.9 | 97.8±1.0 | 0.514 |
| Thoracic SMT | 97.4±1.4 | 97.7±1.1 | 97.8±0.7 | 0.370 |
| Baseline P | 0.700 | |||
| Pulse oximetry – left index finger (%) | ||||
| Control | 98.0±1.1 | 97.5±1.0 | 97.7±1.2 | 0.290 |
| Thoracic SMT | 96.9±1.9 | 97.4±1.2 | 97.2±1.2 | 0.307 |
| Baseline P | 0.020* | |||
Data listed as mean±standard deviation (SD).
*Statistically significant data.
Within-group data were analyzed with repeated measures ANOVA.
Between-group baseline data were compared with an independent samples t-test.
SMT: spinal manipulative therapy.
Table 4. Change in ECG readings from baseline to post 1-minute and post 10-minute.
| Baseline | Post 1-minute | Post 10-minute | P-value | |
| PR interval (ms) | ||||
| Control | 141.0±28.4 | 144.6±27.8 | 143.9±26.5 | 0.047* |
| Thoracic SMT | 156.2±21.2 | 154.9±23.4 | 158.7±12.1 | 0.046* |
| Baseline P | 0.037* | |||
| QRS duration (ms) | ||||
| Control | 93.0±18.2 | 94.9±18.8 | 93.8±18.3 | 0.150 |
| Thoracic SMT | 91.8±8.5 | 94.1±9.1 | 89.9±8.3 | 0.025* |
| Baseline P | 0.767 | |||
| QT (ms) | ||||
| Control | 396.7±26.9 | 397.1±27.7 | 400.0±28.1 | 0.078 |
| Thoracic SMT | 392.8±30.4 | 386.2±65.7 | 396.0±28.2 | 0.483 |
| Baseline P | 0.631 | |||
| QTc (ms) | ||||
| Control | 428.0±21.7 | 430.0±19.1 | 432.2±18.2 | 0.286 |
| Thoracic SMT | 425.3±29.4 | 415.2±69.1 | 426.2±28.6 | 0.419 |
| Baseline P | 0.715 | |||
Data listed as mean±standard deviation (SD).
*Statistically significant data.
Within-group data were analyzed with repeated measures ANOVA.
Between-group baseline data were compared with an independent samples t-test
SMT: spinal manipulative therapy.
Discussion
The purpose of this study was to determine if supine diversified anterior upper thoracic SMT resulted in a significant unsafe drop in blood pressure or in some other way significantly impacted variables of cardiovascular function amongst hypertensive individuals. This study’s original hypothesis was that upper thoracic SMT would transiently lower blood pressure similar to the results of Yates et al.11 with hypertensive patients, yet have minimal impact on other cardiovascular parameters. Contextually, minimal clinically important difference (MCID) represents the smallest amount of change for an outcome that would be important to a patient or clinician.26 For hypertensive patients this would be a drop in systolic blood pressure of 10 mmHg and a drop in diastolic blood pressure of 5 mmHg.27,28 Studies have demonstrated prevalence rates of various cardiovascular pathologies (coronary death, myocardial infarction, or stroke) to significantly increase with hypertension.27–32 Ultimately, the MCID was not achieved for BP changes in this study.
This experiment differs from the work of other researchers,6,9,10,14,33 as it involved hypertensive participants who were taking blood pressure medication. Most of the preliminary studies on thoracic spine manipulation and blood pressure have used normotensive participants10,14 or excluded participants on medications.6 Holt et al.6 did use some participants with stage-1 hypertension in their research study, but it is unclear how many participants had hypertension in their treatment group. Their overall findings (−0.3 mmHg systolic blood pressure and −0.6 mmHg diastolic blood pressure), which were similar to the present study, suggest that there is minimal change in blood pressure in response to thoracic spine SMT.6
The findings of this present study strongly contrast with those of Yates et al.11 (−14.7 mmHg systolic blood pressure and −13.0 mmHg diastolic blood pressure) and Torns5 (−20.2 mmHg systolic blood pressure and −6.8 mmHg diastolic blood pressure).
It is unclear why Torns5 and Yates et al.11 both found significant decreases in blood pressure in response to SMT when the present study did not. The results of Yates et al.11 might be explained by the low participant number in their treatment group, which consisted of only seven participants (three of which were on blood pressure medication). The likelihood of statistical error with such a small sample size is significant. The difference between the present study’s findings and those of Torns5 is much harder to rationalize. Torns5 had 18 participants who were classified as pre-hypertensive to stage-2 hypertensive. Perhaps the difference in the findings of Torns5 and the present study is due to the region of the spine that was manipulated. Some researchers have suggested that cervical spine SMT results in greater lowering of blood pressure than thoracic spine SMT.6 The findings of Torns5 were so drastically different in relation to the findings of the present study and other researchers that they warrant further corroboration.
Meanwhile, the recent systematic review published by Mangum et al.34 further corroborates the findings of this study. Those authors found that there is currently a lack of low bias evidence to support the use of SMT to positively impact hypertension. The researchers classified half of the studies that they reviewed as having a high risk of bias according to the Cochrane Collaboration High Risk of Bias standards.35 They also found that, when SMT was compared to effleurage, no statistically significant drop in blood pressure occurred in clinical trials that were categorized as having low bias.
Study limitations
In the present study’s design, the researchers did not attempt to exclusively manipulate fixated segments of the upper thoracic spine or to manipulate participants specifically complaining of thoracic pain. It may be argued that, when a patient with a painful or fixated region of the thoracic spine is manipulated, the results of this study may be different. Future researchers should explore if there is any link between thoracic back pain and cardiovascular response markers.
Following a post hoc power analysis using G*Power version 3.1.3 (Universität Kiel, Germany),36,37 researchers determined study power was 0.6697. This analysis was in accordance with a desired effect size of 0.5, α of 0.05, and 25 participants per group. In consideration of this analysis, the current study was underpowered and the possibility of type II error exists. Ideally, in order to have a power of 0.90, researchers would need 44 participants per group.
Another limitation of the study is that the researchers did not collect data on how many years the patients had had hypertension. However, the researchers have no reason to believe that this would have impacted the results of this study.
Lastly, the researchers did not include, in the exclusionary criteria, severe cardiovascular conditions or specific non-cardiovascular medications that could have impacted the cardiovascular system due to their side effects. It is plausible that consideration of these factors may have made the study’s participants less homogenous.
Conclusions
The results from this study suggest that supine diversified anterior upper thoracic SMT appears to have minimal impact on any of the cardiovascular physiology variables tested. There were statistically significant changes noted in PR interval and QRS duration, but they did not reach a clinically significant level. The broader implications of this research in this field support that spinal manipulation to this region appears to minimally impact cardiovascular physiology in normotensive or hypertensive individuals and it appears to be safe.
Disclaimer Statements
Contributors John Ward is the lead author, and managed the study, analyzed data, and reviewed the manuscript. Ken Tyer provided all study interventions and reviewed the manuscript. Jesse Coats was involved in data collection and reviewed the manuscript. Gabbrielle Williams was involved in data collection and reviewed the manuscript. Kristina Kulcak was involved in data collection and reviewed the manuscript.
Funding This study was supported with an internal grant from TCC.
Conflicts of interest No conflicts of interest were reported for this study.
Ethics approval Texas Chiropractic College IRB.
References
- 1.Qureshi AI, Suri MF, Kirmani JF, Divani AA. Prevalence and trends of prehypertension and hypertension in United States: National Health and Nutrition Examination Surveys 1976 to 2000. Med Sci Monit. 2005;11:403–9. [PubMed] [Google Scholar]
- 2.World Health Organization. A global brief on hypertension: silent killer, global public health crisis. Geneva, Switzerland: World Health Organization; 2013, Available from http://www.who.int/cardiovascular_diseases/publications/global_brief_hypertension/en/ [Google Scholar]
- 3.Causes of Death. [online database]. Geneva: World Health Organization; 2008, Available from http://www.who.int/healthinfo/global_burden_disease/cod_2008_sources_methods.pdf. [Google Scholar]
- 4.Bakris G, Dickholtz M, Meyer P, Kravitz G, Avery E, Miller M, et al. Atlas vertebra realignment and achievement of arterial pressure goal in hypertensive patients: a pilot study. J Hum Hypertens. 2007;21:347–52. doi: 10.1038/sj.jhh.1002133. [DOI] [PubMed] [Google Scholar]
- 5.Torns S. Atlas vertebra realignment and arterial blood pressure regulation in 42 subjects. J Upper Cervical Chiropr Res. 2012;2:40–5. [Google Scholar]
- 6.Holt K, Beck R, Sexton S, Haavik T. Reflex effects of a spinal adjustment on blood pressure. Chiropr J Aust. 2010;40:95–9. [Google Scholar]
- 7.Knutson G. Significant changes in systolic blood pressure post vectored upper cervical adjustment vs resting control groups: a possible effect of the cervicosympathetic and/or pressor reflex. J Manipulative Physiol Ther. 2001;24:101–9. doi: 10.1067/mmt.2001.112564. [DOI] [PubMed] [Google Scholar]
- 8.McKnight M, DeBoer K. Preliminary study of blood pressure changes in normotensive subjects undergoing chiropractic care. J Manipulative Physiol Ther. 1988;11:261–6. [PubMed] [Google Scholar]
- 9.Watanabe N, Polus B. A single mechanical impulse to the neck: does it influence autonomic regulation of cardiovascular function? Chiropr J Aust. 2007;37:42–8. [Google Scholar]
- 10.Welch A, Boone R. Sympathetic and parasympathetic response to specific diversified adjustments to chiropractic vertebral subluxations of the cervical and thoracic spine. J Chiropr Med. 2008;7:86–93. doi: 10.1016/j.jcm.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yates G, Lamping D, Abram N, Wright C. Effects of chiropractic treatment on blood pressure and anxiety: a randomized, controlled trial. J Manipulative Physiol Ther. 1988;11:484–8. [PubMed] [Google Scholar]
- 12.Tran T, Kirby J. The effect of upper thoracic adjustment upon the normal physiology of the heart. J Am Chiropr Assoc. 1977;6:58–62. [Google Scholar]
- 13.Plaugher G, Bachman T. Chiropractic management of a hypertensive patient. J Manipulative Physiol Ther. 1993;16:544–9. [PubMed] [Google Scholar]
- 14.Ward J, Coats J, Tyer K, Weigand S, Williams G. Immediate effects of anterior upper thoracic spine manipulation on cardiovascular response. J Manipulative Physiol Ther. 2013;36:101–10. doi: 10.1016/j.jmpt.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Drake R, Vogl A, Mitchel A. Gray’s Anatomy for students, 2nd edn. Philadelphia: Churchill Livingstone; 2010. p. 203. [Google Scholar]
- 16.Hall J. Guyton and hall textbook of medical physiology, 12th edn. Philadelphia: Saunders-Elsevier; 2011. p. 111. [Google Scholar]
- 17.Klabunde R. Cardiovascular physiology concepts, 2nd edn. Philadelphia: Lippincott Williams & Wilkins; 2012. pp. 6, 62, 127–30. [Google Scholar]
- 18.Mohram D, Heller L. Cardiovascular physiology, 7th edn. New York: McGraw Hill; 2010. pp. 12–3, 193–6. [Google Scholar]
- 19.Crawford J, Hickson G, Wiles M. The management of hypertensive disease: a review of spinal manipulation and the efficacy of conservative therapeusis. J Manipulative Physiol Ther. 1986;9:27–32. [PubMed] [Google Scholar]
- 20.Harris W, Wagnon R. The effects of chiropractic adjustments on distal skin temperature. J Manipulative Physiol Ther. 1987;10:57–60. [PubMed] [Google Scholar]
- 21.Sterling M, Jull G, Wright A. Cervical mobilisation: concurrent effects on pain, sympathetic nervous system activity and motor activity. Man Ther. 2001;6:72–81. doi: 10.1054/math.2000.0378. [DOI] [PubMed] [Google Scholar]
- 22.McGee D. Hypertension: a case study. Chiropractic. 1992;7:98–9. [Google Scholar]
- 23.Bergmann T, Peterson D. Chiropractic technique: principles and procedures, 3rd edn. St. Louis: Elsevier-Mosby; 2011. pp. 188–232. [Google Scholar]
- 24.Vera-Cala L, Orostequi M, Valencia-Angel L, López N, Bautista L. Accuracy of the Omron HEM-705 CP for blood pressure measurement in large epidemiologic studies. Arq Bras Cardiol. 2011;96:393–8. doi: 10.1590/s0066-782x2011005000038. [DOI] [PubMed] [Google Scholar]
- 25.Field A. Discovering statistics using SPSS, 2nd edn. Thousand Oaks: Sage; 2005. pp. 346–8. [Google Scholar]
- 26.McAlister F, O’Connor A, Wells G, Grover S, Laupcis A. When should hypertension be treated? The different perspectives of Canadian family physicians and patients. Can Med Assoc J. 2000;163:403–8. [PMC free article] [PubMed] [Google Scholar]
- 27.Grover S, Paquet S, Levinton C, Coupal L, Zowall H. Estimating the benefits of modifying cardiovascular risk factors: a comparison of primary versus secondary prevention. Arch Intern Med. 1998;158:655–62. doi: 10.1001/archinte.158.6.655. [DOI] [PubMed] [Google Scholar]
- 28.MacDonald S, Joffres M, Stachenko S, Horlick L, Fodor G. Multiple cardiovascular disease risk factors in Canadian adults. Can Med Assoc J. 1992;146:2021–9. [PMC free article] [PubMed] [Google Scholar]
- 29.Retchin S, Brown R, Yeh S, Chu D, Moreno L. Outcomes of stroke patients in Medicare fee for service and managed care. JAMA. 1997;278:119–24. [PubMed] [Google Scholar]
- 30.Dennis M, Burn J, Sandercock P, Bamford J, Wade D, Warlow C. Long-term survival after first-ever stroke: the Oxfordshire Community Stroke Project. Stroke. 1993;24:796–800. doi: 10.1161/01.str.24.6.796. [DOI] [PubMed] [Google Scholar]
- 31.Sonke G, Beagelhole R, Stewart A, Jackson R, Stewart F. Sex differences in case fatality before and after admission to hospital for acute cardiac events: analysis of community based coronary heart disease register. BMJ. 1996;313:853–5. doi: 10.1136/bmj.313.7061.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicod P, Gilpin E, Dittrich H, Polikar R, Hjalmarson A, Blacky A. Short- and long-term clinical outcome after Q wave and non-Q wave myocardial infarction in a large patient population. Circulation. 1989;79:528–36. doi: 10.1161/01.cir.79.3.528. [DOI] [PubMed] [Google Scholar]
- 33.Ward J, Tyer K, Coats J, Williams G, Weigand S, Cockburn D. Immediate effects of atlas manipulation on cardiovascular physiology. Clin Chiropr. 2012;15:147–57. [Google Scholar]
- 34.Mangum K, Partna L, Vavrek D. Spinal manipulation for the treatment of hypertension: a systematic qualitative literature review. J Manipulative Physiol Ther. 2012;35:235–43. doi: 10.1016/j.jmpt.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Higgins J, Green S editors. Cochrane handbook for systematic reviews of interventions 5.0.2. The Cochrane Collaboration; 2009, Available from www.cochrane-handbook.org. [Google Scholar]
- 36.Buchner A, Erdfelder E, Faul F. How to Use G*Power [WWW document]; 1997. Available from http://www.psycho.uni-duesseldorf.de/aap/projects/gpower/how_to_use_gpower.html. [Google Scholar]
- 37.Erdfelder E, Faul F, Buchner A. GPOWER: a general power analysis program. Behav Res Methods Instrum Comput. 1996;28:1–11. [Google Scholar]