Abstract
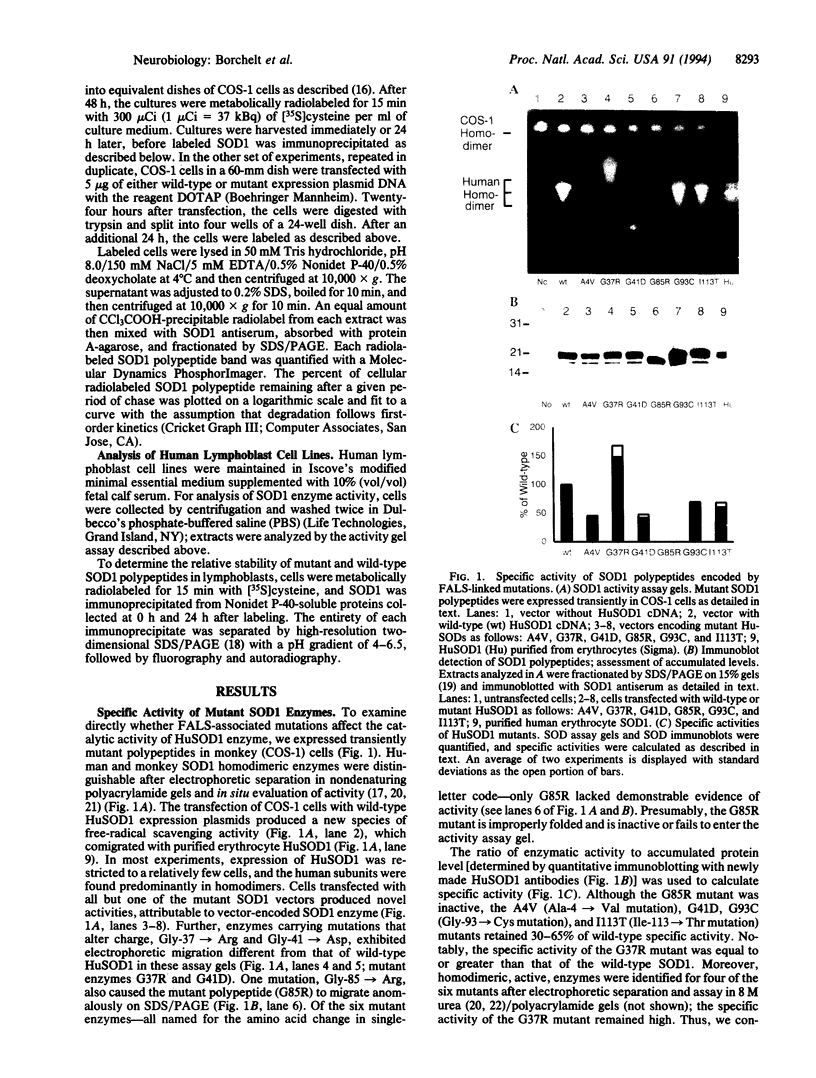
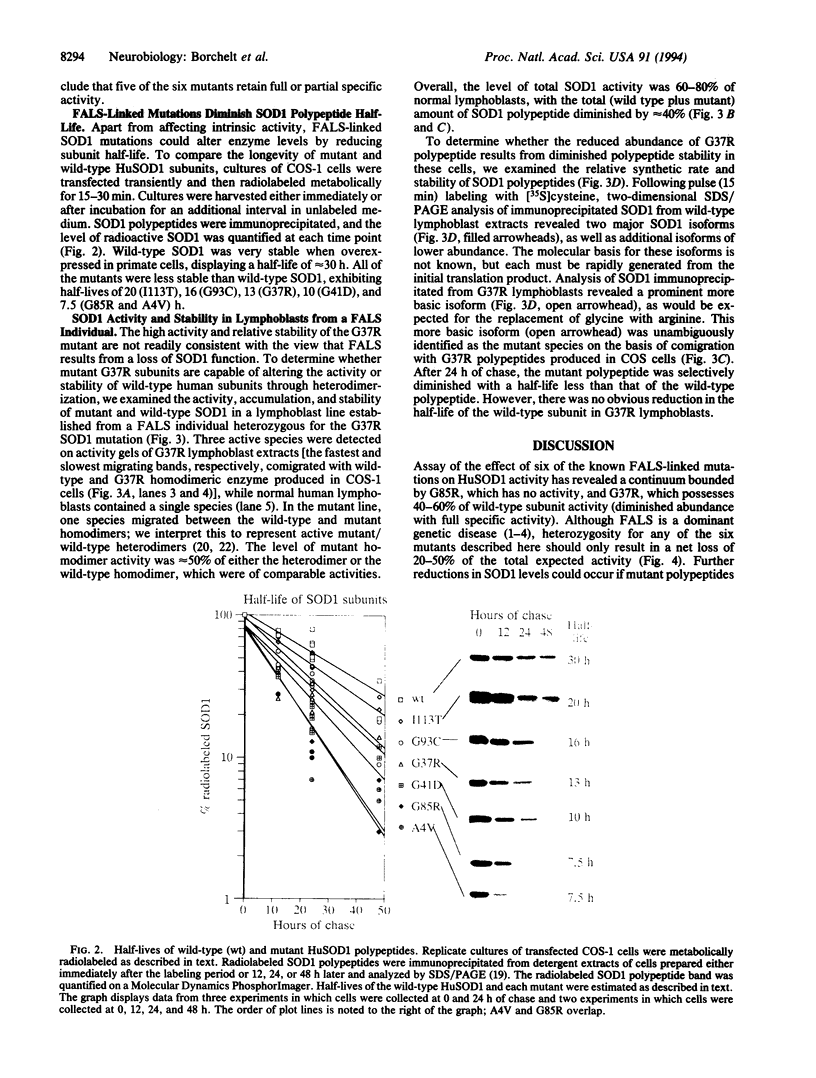
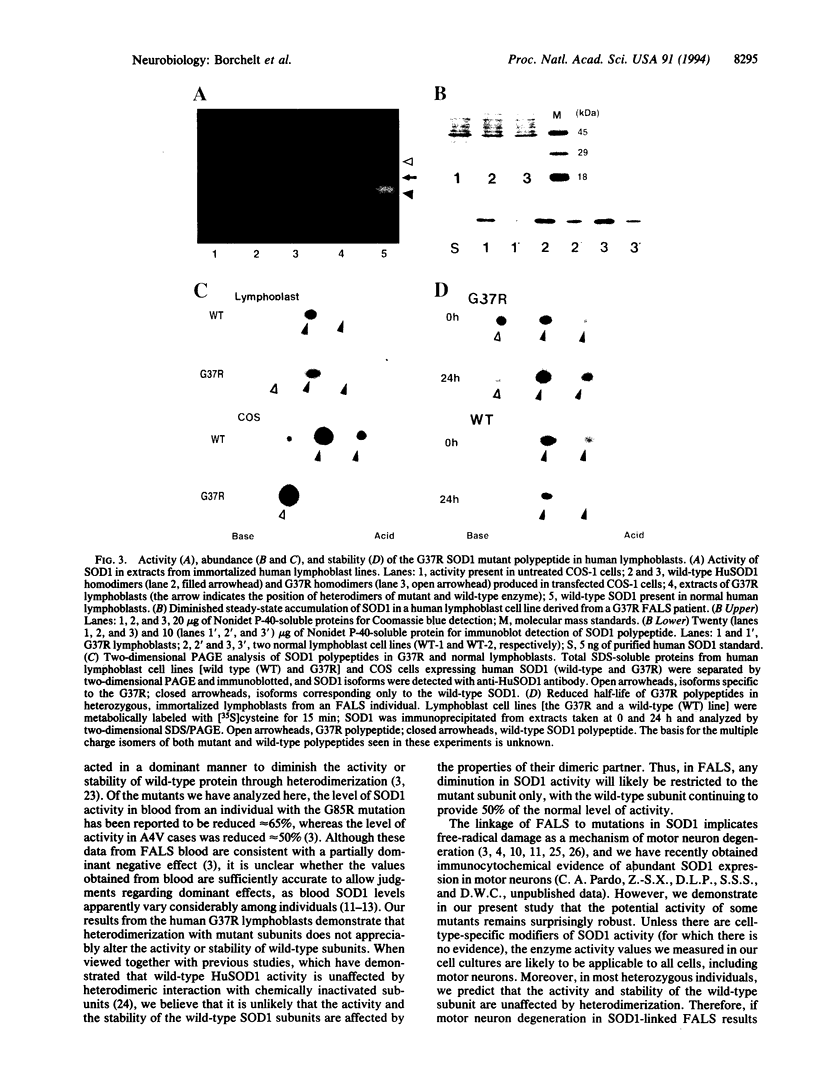
Familial amyotrophic lateral sclerosis (FALS) has been linked to mutations in the homodimeric enzyme Cu/Zn superoxide dismutase 1 (SOD1). Assay by transient expression in primate cells of six FALS mutant enzymes revealed a continuum of enzymatic activity bounded by the enzyme carrying the mutation Gly-85-->Arg, which was inactive, and mutant enzyme G37R carrying the Gly-37-->Arg change, which retained full specific activity but displayed a 2-fold reduction in polypeptide stability. The G37R mutant displayed similar properties in transformed lymphocytes from an individual heterozygous for the G37R and wild-type SOD1 genes; heterodimeric enzymes composed of mutant and wild-type subunits were detected, but there was no measurable diminution in the stability and activity of the wild-type subunits. Thus, for mutants such as G37R, either surprisingly modest losses in activity (involving only the mutant subunit) can yield motor neuron death, or alternatively, mutant SOD1 may acquire properties that injure motor neurons by one or more mechanisms unrelated to the metabolism of oxygen radicals.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki M., Ogasawara M., Matsubara Y., Narisawa K., Nakamura S., Itoyama Y., Abe K. Mild ALS in Japan associated with novel SOD mutation. Nat Genet. 1993 Dec;5(4):323–324. doi: 10.1038/ng1293-323. [DOI] [PubMed] [Google Scholar]
- Avraham K. B., Schickler M., Sapoznikov D., Yarom R., Groner Y. Down's syndrome: abnormal neuromuscular junction in tongue of transgenic mice with elevated levels of human Cu/Zn-superoxide dismutase. Cell. 1988 Sep 9;54(6):823–829. doi: 10.1016/s0092-8674(88)91153-1. [DOI] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Beckman J. S., Carson M., Smith C. D., Koppenol W. H. ALS, SOD and peroxynitrite. Nature. 1993 Aug 12;364(6438):584–584. doi: 10.1038/364584a0. [DOI] [PubMed] [Google Scholar]
- Bowling A. C., Schulz J. B., Brown R. H., Jr, Beal M. F. Superoxide dismutase activity, oxidative damage, and mitochondrial energy metabolism in familial and sporadic amyotrophic lateral sclerosis. J Neurochem. 1993 Dec;61(6):2322–2325. doi: 10.1111/j.1471-4159.1993.tb07478.x. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H. X., Hentati A., Tainer J. A., Iqbal Z., Cayabyab A., Hung W. Y., Getzoff E. D., Hu P., Herzfeldt B., Roos R. P. Amyotrophic lateral sclerosis and structural defects in Cu,Zn superoxide dismutase. Science. 1993 Aug 20;261(5124):1047–1051. doi: 10.1126/science.8351519. [DOI] [PubMed] [Google Scholar]
- Elroy-Stein O., Bernstein Y., Groner Y. Overproduction of human Cu/Zn-superoxide dismutase in transfected cells: extenuation of paraquat-mediated cytotoxicity and enhancement of lipid peroxidation. EMBO J. 1986 Mar;5(3):615–622. doi: 10.1002/j.1460-2075.1986.tb04255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshafey A., Lanyon W. G., Connor J. M. Identification of a new missense point mutation in exon 4 of the Cu/Zn superoxide dismutase (SOD-1) gene in a family with amyotrophic lateral sclerosis. Hum Mol Genet. 1994 Feb;3(2):363–364. doi: 10.1093/hmg/3.2.363. [DOI] [PubMed] [Google Scholar]
- Epstein C. J., Avraham K. B., Lovett M., Smith S., Elroy-Stein O., Rotman G., Bry C., Groner Y. Transgenic mice with increased Cu/Zn-superoxide dismutase activity: animal model of dosage effects in Down syndrome. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8044–8048. doi: 10.1073/pnas.84.22.8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):35–97. doi: 10.1002/9780470122860.ch2. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol. 1986;58:61–97. doi: 10.1002/9780470123041.ch2. [DOI] [PubMed] [Google Scholar]
- Guemouri L., Artur Y., Herbeth B., Jeandel C., Cuny G., Siest G. Biological variability of superoxide dismutase, glutathione peroxidase, and catalase in blood. Clin Chem. 1991 Nov;37(11):1932–1937. [PubMed] [Google Scholar]
- Gurney M. E., Pu H., Chiu A. Y., Dal Canto M. C., Polchow C. Y., Alexander D. D., Caliendo J., Hentati A., Kwon Y. W., Deng H. X. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994 Jun 17;264(5166):1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee M. K., Rebhun L. I., Frankfurter A. Posttranslational modification of class III beta-tubulin. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7195–7199. doi: 10.1073/pnas.87.18.7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinowski D. P., Fridovich I. Bovine erythrocyte superoxide dismutase: diazo coupling, subunit interactions, and electrophoretic variants. Biochemistry. 1979 Jan 9;18(1):237–244. doi: 10.1021/bi00568a037. [DOI] [PubMed] [Google Scholar]
- McNamara J. O., Fridovich I. Human genetics. Did radicals strike Lou Gehrig? Nature. 1993 Mar 4;362(6415):20–21. doi: 10.1038/362020a0. [DOI] [PubMed] [Google Scholar]
- Mizushima S., Nagata S. pEF-BOS, a powerful mammalian expression vector. Nucleic Acids Res. 1990 Sep 11;18(17):5322–5322. doi: 10.1093/nar/18.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder D. W., Kurland L. T., Offord K. P., Beard C. M. Familial adult motor neuron disease: amyotrophic lateral sclerosis. Neurology. 1986 Apr;36(4):511–517. doi: 10.1212/wnl.36.4.511. [DOI] [PubMed] [Google Scholar]
- Park E. C., Horvitz H. R. Mutations with dominant effects on the behavior and morphology of the nematode Caenorhabditis elegans. Genetics. 1986 Aug;113(4):821–852. doi: 10.1093/genetics/113.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robberecht W., Sapp P., Viaene M. K., Rosen D., McKenna-Yasek D., Haines J., Horvitz R., Theys P., Brown R., Jr Cu/Zn superoxide dismutase activity in familial and sporadic amyotrophic lateral sclerosis. J Neurochem. 1994 Jan;62(1):384–387. doi: 10.1046/j.1471-4159.1994.62010384.x. [DOI] [PubMed] [Google Scholar]
- Rosen D. R., Siddique T., Patterson D., Figlewicz D. A., Sapp P., Hentati A., Donaldson D., Goto J., O'Regan J. P., Deng H. X. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993 Mar 4;362(6415):59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Sherman L., Dafni N., Lieman-Hurwitz J., Groner Y. Nucleotide sequence and expression of human chromosome 21-encoded superoxide dismutase mRNA. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5465–5469. doi: 10.1073/pnas.80.18.5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddique T., Pericak-Vance M. A., Brooks B. R., Roos R. P., Hung W. Y., Antel J. P., Munsat T. L., Phillips K., Warner K., Speer M. Linkage analysis in familial amyotrophic lateral sclerosis. Neurology. 1989 Jul;39(7):919–925. doi: 10.1212/wnl.39.7.919. [DOI] [PubMed] [Google Scholar]
- de Lustig E. S., Serra J. A., Kohan S., Canziani G. A., Famulari A. L., Dominguez R. O. Copper-zinc superoxide dismutase activity in red blood cells and serum in demented patients and in aging. J Neurol Sci. 1993 Mar;115(1):18–25. doi: 10.1016/0022-510x(93)90062-4. [DOI] [PubMed] [Google Scholar]