Abstract
We investigated the association between maternal zinc level during pregnancy and the risks of low birth weight (LBW) and small for gestational age (SGA) infants in a large population-based birth cohort study. In this study, 3187 pregnant women were recruited. For serum zinc level, 2940 pregnant women were sufficient (≥56 μg/dL) and 247 deficient (<56 μg/dL). Of interest, 7.3% newborns were with LBW among subjects with low zinc level (RR: 3.48; 95% CI: 2.03, 5.96; P < 0.001). Adjusted RR for LBW was 3.41 (95% CI: 1.97, 5.91; P < 0.001) among subjects with low zinc level. Moreover, 15.0% newborns were with SGA among subjects with low zinc level (RR: 1.98; 95% CI: 1.36, 2.88; P < 0.001). Adjusted RR for SGA was 1.93 (95% CI: 1.32, 2.82; P < 0.001) among subjects with low zinc level. A nested case-control study within above cohort showed that maternal serum zinc level was lower in SGA cases as compared with controls. By contrast, maternal serum C-reactive protein, TNF-α and IL-8 levels were significantly higher in SGA cases than that of controls. Moreover, nuclear NF-κB p65 was significantly up-regulated in placentas of SGA cases as compared with controls. Taken together, maternal zinc deficiency during pregnancy elevates the risks of LBW and SGA infants.
Fetal growth restriction (FGR), which manifests as low birth weight (LBW) or small for gestational age (SGA), increases infant mortality and morbidity1,2. Almost 25 years ago, Barker and coworkers described FGR as highly correlated with increased risk for the development of cardiovascular diseases during adulthood3,4. Since then, numerous epidemiologic studies have demonstrated an association between FGR and an increased risk for adult onset of metabolic as well as non-metabolic diseases5,6. Thus, the etiology and underlying mechanism for FGR is of great concern.
Zinc (Zn) is a structural constituent that is essential for cell growth, development and differentiation7. Several earlier reports demonstrate that maternal zinc deficiency during pregnancy is linked with adverse pregnant outcomes including abortion, preterm delivery, stillbirth and fetal neural tube defects8,9,10,11. A double-blind and randomized controlled study shows that zinc supplementation during pregnancy improves birth length after adjusting for maternal height, pre-pregnancy weight and parity12. According to a recent systematic review, prenatal zinc supplementation leads to a statistically significant lower incidence of preterm birth13,14,15. The link between maternal zinc status during pregnancy and birth weight has been investigated in several small epidemiological studies16,17. Nevertheless, the association between maternal zinc status during pregnancy and the incidences of LBW and SGA infants needs to be determined in a large longitudinal investigation.
The objective of the present study was to assess maternal serum zinc level during pregnancy in a large population-based birth cohort study. We were to further analyze the association between maternal serum zinc level at different gestational stages and the risks of LBW and SGA infants. We also sought to explore the link among maternal serum zinc level during pregnancy, placental inflammation and the incidence of LBW and SGA infants.
Results
In the present study, 3187 pregnant women were recruited (Fig. 1). The average serum zinc concentration was 91.0 μg/dL among 3187 pregnant women. For serum zinc level, 2940 pregnant women were sufficient (≥56 μg/dL) and 247 deficient (<56 μg/dL). No subject was drinking alcohol or smoking cigarette during pregnancy (data not shown). The demographic characteristics of pregnant women and their newborns were compared between subjects with normal zinc level and low zinc level. No significant difference on mother’s age, BMI before pregnancy and monthly income per person was observed between two groups (Table 1). As expected, 96.2% of subjects with normal zinc level and 92.3% of subjects with low zinc level were primiparous. The average gestational age at birth was 39.1 weeks among subjects with normal zinc level and 39.1 weeks among subjects with low zinc level, respectively. There was no significant difference on gestational age at birth between two groups (Table 1). The average birth weight was 3401 g among subjects with normal zinc level and 3357 g among subjects with low zinc level, respectively. There was a downward trend on the average birth weight in subjects with low zinc level as compared with that of subjects with normal zinc level (P = 0.210). Further analysis showed that several maternal characteristics, including maternal age, monthly income, parity and gravidity, did not influence serum zinc level during pregnancy (Table 2). Of interest, the average serum zinc levels were significantly lower among subjects with BMI < 18.5 kg/m2 than those of subjects with normal BMI (18.5–24.9 kg/m2) (Table 2).
Figure 1. Flow diagram of recruitment and follow-up in this birth cohort study.
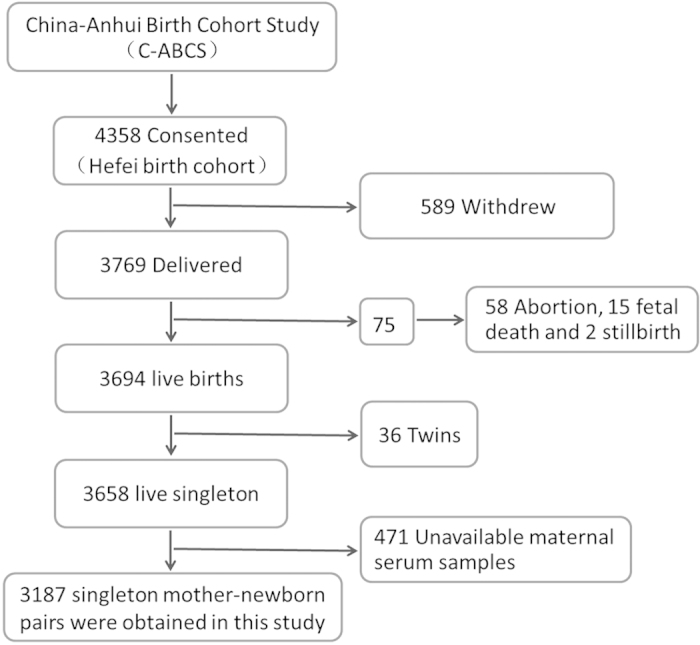
Table 1. Characteristics of 3187 mothers and their newborns.
| Parameters |
Maternal serum zinc levela |
P | |
|---|---|---|---|
| Deficiency (n = 247) | Sufficiency (n = 2940) | ||
| Maternal characteristics | |||
| Age (y, means ± SD) | 27.1 ± 3.3 | 27.5 ± 3.2 | 0.092 |
| ≤24 [n(%)] | 53 (21.5) | 454 (15.4) | |
| 25–29 [n(%)] | 150 (60.7) | 1850 (62.9) | 0.032 |
| ≥30 [n(%)] | 44 (17.8) | 636 (21.6) | |
| BMIb (kg/m2, means ± SD) | 20.2 ± 2.3 | 20.2 ± 2.2 | 0.995 |
| <18.5 kg/m2 [n(%)] | 59 (23.9) | 623 (21.2) | |
| 18.5–24.9 kg/m2 [n(%)] | 171 (69.2) | 2143 (72.9) | 0.461 |
| >25 kg/m2 [n(%)] | 17 (6.9) | 174 (5.9) | |
| Parity [n(%)] | |||
| 1 | 228 (92.3) | 2828 (96.2) | 0.003 |
| ≥2 | 19 (7.7) | 112 (3.8) | |
| Monthly income [n(%)] | |||
| Low incomec | 119 (48.2) | 1345 (45.7) | 0.749 |
| Middle incomec | 123 (49.8) | 1538 (52.3) | |
| High incomec | 5 (2.0) | 57 (1.9) | |
| Newborn characteristics | |||
| Gestational age (wk, means ± SD) | 39.1 ± 1.2 | 39.1 ± 1.4 | 0.954 |
| Birth weight (g, means ± SD) | 3357 ± 539 | 3401 ± 458 | 0.210 |
| Time for collecting serum [n(%)] | |||
| First trimester | 48 (19.4) | 1027 (34.9) | 0.000 |
| Second trimester | 186 (75.3) | 1833 (62.3) | |
| Third trimester | 13 (5.3) | 80 (2.7) | |
aDeficiency for serum zinc<56 μg/dL, and sufficiency for serum zinc ≥ 56 μg/dL.
bBMI before pregnancy.
cLow income (1) for <2000 RMB per month; middle income (2) for ≥2000 RMB per month; high income (3) for ≥4000 RMB per month.
Table 2. Influence of maternal characteristics on serum zinc level during pregnancy.
| Characteristics | n | Maternal serum zinc (μg/dL, means ± SD) | P |
|---|---|---|---|
| Maternal age (y) | |||
| ≤24 | 507 | 92.8 ± 37.8 | 0.141 |
| 25–29 | 2000 | 90.2 ± 27.3 | |
| ≥30 | 680 | 92.0 ± 31.4 | |
| Monthly incomea | |||
| Low | 1464 | 90.5 ± 29.0 | 0.431 |
| Middle | 1661 | 91.3 ± 31.0 | |
| High | 62 | 94.9 ± 29.6 | |
| BMIb (kg/m2) | |||
| <18.5 | 682 | 88.2 ± 28.6* | 0.019 |
| 18.5–24.9 | 2314 | 91.7 ± 30.0 | |
| ≥25 | 191 | 92.8 ± 35.7 | |
| Parity | |||
| 1 | 3056 | 91.1 ± 29.9 | 0.251 |
| ≥2 | 131 | 88.0 ± 35.4 | |
| Gravidity | |||
| 1 | 1661 | 91.1 ± 30.1 | 0.768 |
| ≥2 | 1526 | 90.8 ± 30.1 | |
aLow income for <2000 RMB per month; middle income for ≥2000 RMB per month; high income for ≥4000 RMB per month.
bPre-pregnancy BMI.
*P < 0.01 as compared with normal BMI (18.5–24.9 kg/m2).
The association between serum zinc level during pregnancy and the risk of LBW infants was analyzed. As shown in Table 3, 2.2% newborns were with LBW among subjects with normal zinc level. Moreover, 7.3% newborns were with LBW among subjects with low zinc level (RR: 3.48; 95% CI: 2.03, 5.96; P < 0.001). Adjusted RR for LBW was 3.41 (95% CI: 1.97, 5.91; P < 0.001) among subjects with low zinc level using multiple logistic regression model (Table 3). The association between maternal zinc level during early gestational stage and the risk of LBW infants was further analyzed. As shown in Table 4, 2.6% newborns were with LBW among subjects with normal zinc level during the first trimester. Of interest, 6.5% newborns were with LBW among subjects with low zinc level during the first trimester (RR: 2.63; 95% CI: 0.58, 12.00; P = 0.211). Adjusted RR for LBW was 2.64 (95% CI: 0.58, 12.05; P = 0.211) among subjects with low zinc level during the first trimester (Table 4). The association between maternal zinc level during later gestational stages and the risk of LBW infants was analyzed. As shown in Table 5, 2.1% newborns were with LBW among subjects with normal zinc level during the second and third trimesters. Of interest, 7.4% newborns were with LBW among subjects with low zinc level during the second and third trimesters (RR: 3.70; 95% CI: 2.06, 6.62; P < 0.001). Adjusted RR for LBW was 3.81 (95% CI: 2.12, 6.85; P < 0.001) among subjects with low zinc level during the second and third trimesters (Table 5).
Table 3. The incidence and relative risk ( RR ) for LBW and SGA infants based on maternal serum Zn level.
|
Maternal serum Zn levela |
P | ||
|---|---|---|---|
| Deficiency (n = 247) | Sufficiency (n = 2940) | ||
| LBW | |||
| Number of LBW | 18 | 65 | |
| Incidence (%) | 7.3 | 2.2 | <0.001 |
| Univariate RR (95%CI) | 3.48 (2.03, 5.96) | 1.00 | <0.001 |
| Adjusted RR (95%CI)b | 3.41 (1.97, 5.91) | 1.00 | <0.001 |
| SGA | |||
| Number of SGA | 37 | 240 | |
| Incidence (%) | 15.0 | 8.2 | <0.001 |
| Univariate RR (95%CI) | 1.98 (1.36, 2.88) | 1.00 | <0.001 |
| Adjusted RR (95%CI)b | 1.93 (1.32, 2.82) | 1.00 | <0.001 |
aDeficiency for serum zinc < 56 μg/dL, and sufficiency for serum zinc ≥ 56 μg/dL.
bAdjusted for pre-pregnancy BMI, maternal age, gestational week for collecting serum and monthly income per person.
Table 4. The incidence and relative risk ( RR ) for LBW and SGA infants based on maternal serum Zn level in the first trimester.
|
Maternal serum Zn levela |
P | ||
|---|---|---|---|
| Deficiency (n = 31) | Sufficiency (n = 627) | ||
| LBW | |||
| Number of LBW | 2 | 16 | |
| Incidence (%) | 6.5 | 2.6 | 0.462 |
| Univariate RR (95%CI) | 2.63 (0.58, 12.00) | 1.00 | 0.211 |
| Adjusted RR (95%CI)b | 2.64 (0.58, 12.05) | 1.00 | 0.211 |
| SGA | |||
| Number of SGA | 7 | 52 | |
| Incidence (%) | 22.6 | 8.3 | 0.007 |
| Univariate RR (95%CI) | 3.23 (1.33, 7.84) | 1.00 | 0.009 |
| Adjusted RR (95%CI)b | 3.12 (1.27, 7.66) | 1.00 | 0.013 |
aDeficiency for serum zinc < 56 μg/dL, and sufficiency for serum zinc ≥ 56 μg/dL.
bAdjusted for pre-pregnancy BMI, maternal age, and monthly income per person.
Table 5. The incidence and relative risk ( RR ) for LBW and SGA infants based on maternal serum Zn level in the second and third trimesters.
|
Maternal serum Zn levela |
P | ||
|---|---|---|---|
| Deficiency (n = 216) | Sufficiency (n = 2313) | ||
| LBW | |||
| Number of LBW | 16 | 49 | |
| Incidence (%) | 7.4 | 2.1 | <0.001 |
| Univariate RR (95%CI) | 3.70 (2.06, 6.62) | 1.00 | <0.001 |
| Adjusted RR (95%CI)b | 3.81 (2.12, 6.85) | 1.00 | <0.001 |
| SGA | |||
| Number of SGA | 30 | 188 | |
| Incidence (%) | 13.9 | 8.1 | 0.004 |
| Univariate RR (95%CI) | 1.82 (1.21, 2.76) | 1.00 | 0.004 |
| Adjusted RR (95%CI)b | 1.82 (1.20, 2.75) | 1.00 | 0.005 |
aDeficiency for serum zinc < 56 μg/dL, and sufficiency for serum zinc ≥ 56 μg/dL.
bAdjusted for pre-pregnancy BMI, maternal age, and monthly income per person.
The association between zinc level during pregnancy and the risk of SGA infants was then analyzed. As shown in Table 3, 8.2% newborns were with SGA among subjects with normal zinc level. Of interest, 15.0% newborns were with SGA among subjects with low zinc level (RR: 1.98; 95% CI: 1.36, 2.88; P < 0.001). Adjusted RR for SGA was 1.93 (95% CI: 1.32, 2.82; P < 0.001) among subjects with low zinc level (Table 3). The association between zinc level during early gestational stage and the risk of SGA infants was then analyzed. As shown in Table 4, 8.3% newborns were with SGA among subjects with normal zinc level during the first trimester. Of interest, 22.6% newborns were with SGA among subjects with low zinc level during the first trimester (RR: 3.23; 95% CI: 1.33, 7.84; P = 0.009). Adjusted RR for SGA was 3.12 (95% CI: 1.27, 7.66; P = 0.013) among subjects with low zinc level during the first trimester (Table 4). The association between zinc level during later gestational stages and the risk of SGA infants was then analyzed. As shown in Table 5, 8.1% newborns were with SGA among subjects with normal zinc level during the second and third trimesters. Of interest, 13.9% newborns were with SGA among subjects with low zinc level during the second and third trimesters (RR: 1.82; 95% CI: 1.21, 2.76; P = 0.004). Adjusted RR for SGA was 1.82 (95% CI: 1.20, 2.75; P = 0.005) among subjects with low zinc level during the second and third trimesters (Table 5).
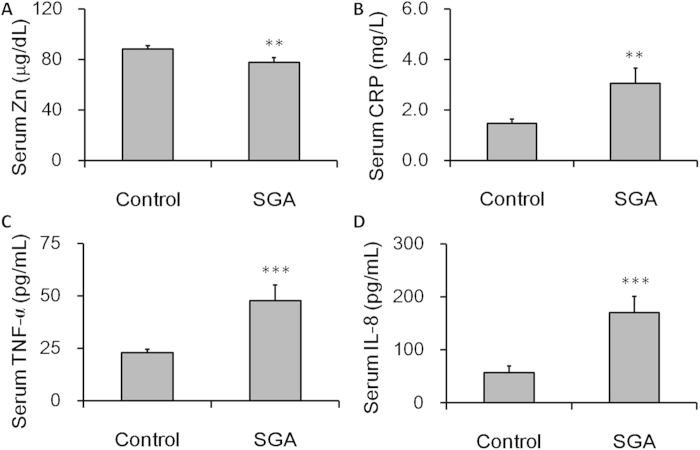
We compared serum zinc level from SGA cases and controls. As expected, the level of serum zinc during pregnancy was lower in SGA cases as compared with controls (Fig. 2A). We then compared the levels of CRP, TNF-α and IL-8 in maternal sera from SGA cases and controls. As shown in Fig. 2B, the level of CRP in maternal sera was significantly higher in SGA cases than that of controls. In addition, the level of TNF-α and IL-8 in maternal sera was also higher in SGA cases than that of controls (Fig. 2C,D).
Figure 2. Serum zinc level during early gestational stage and inflammatory cytokines in maternal sera from SGA cases and controls.
Serum zinc level and inflammatory cytokines during the first trimester were measured in 50 SGA cases and 100 controls. (A) Zinc. (B) CRP. (C) TNF-α. (D) IL-8. All data were expressed as means ± SEM. **P < 0.01, ***P < 0.001 as compared with controls.
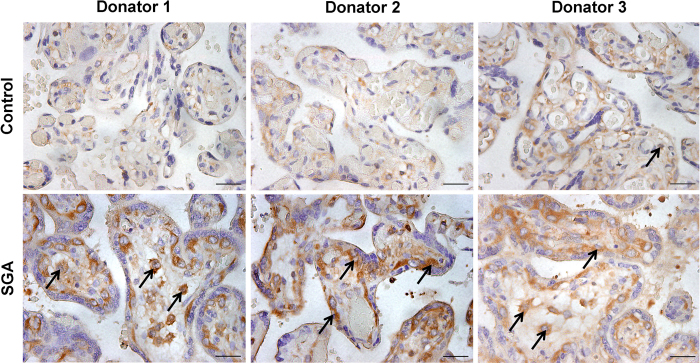
We compared zinc level in umbilical sera from 30 SGA cases and 30 controls. Unexpectedly, there was no significant difference on the level of zinc in umbilical sera between SGA cases and controls (Fig. 3). We then compared the expression of placental NF-κB p65 between SGA cases and controls. As expected, nuclear NF-κB p65 was significantly up-regulated in placentas of SGA cases as compared with controls. Furthermore, nuclear translocation of NF-kB p65 was mainly observed in trophoblasts of SGA placenta (Fig. 4).
Figure 3. Zinc level in umbilical sera from SGA cases and controls.
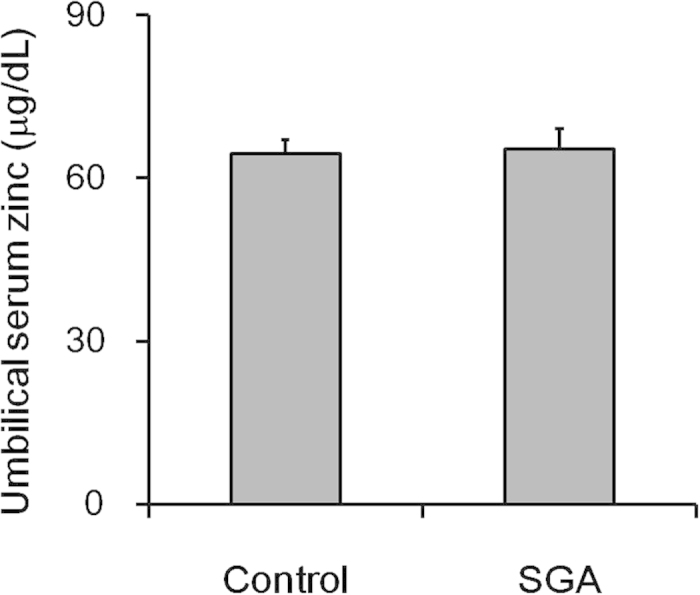
Zinc level in umbilical sera was measured in 30 SGA cases and 30 controls. All data were expressed as means ± SEM.
Figure 4. NF-κB p65 in placentas from SGA cases and controls.
Placental NF-κB p65 was measured in controls and SGA cases using immunohistochemistry. Arrows indicate NF-кB p65-positive cells. Scale bar: 50 μm.
Discussions
The present study assessed serum zinc concentration among 3187 pregnant women. Our results showed that the average serum zinc concentration during pregnancy for all subjects was 91.0 μg/dL. According to the suggested lower cutoffs of serum zinc concentrations by NHANES II18, 2940 pregnant women (92.2%) were sufficient (≥56 μg/dL) and 247 (7.8%) deficient ( <56 μg/dL). These results are similar to the results from NHANES in 1976–198018. Several reports demonstrate that socio-demographic status and economic income are associated with zinc deficiency19,20. In addition, age and gender are important determinants of serum zinc concentrations21. The present study investigated the effects of maternal age, monthly income, parity and gravidity on serum zinc concentration during pregnancy. Surprisingly, these factors had little effect on serum zinc concentration during pregnancy. According to several earlier reports, maternal serum or plasm zinc concentrations declined as pregnancy progressed18,22,23. Of interest, the present study showed that the average zinc levels were significantly lower among subjects with BMI < 18.5 than those of subjects with BMI18.5–24.9. The inconsistency of the present results with past findings may be related to following reasons: first, different suggested lower cutoffs of serum zinc concentrations were chosen; sencond, pregnant woman population with different age, BMI, average monthly income, and gestational ages was investigated; third, past results came most frequently from small samples. To our knowledge, the present study is the first to assess serum zinc concentration during pregnancy in a large population-based birth cohort study.
It remains contradictory whether maternal zinc deficiency during pregnancy elevates the risks of LBW and SGA infants. An earlier study demonstrated that there was a threshold below which the prevalence of LBW infants was increased17. By contrast, two recent reports found that maternal zinc level during pregnancy was not significantly associated with the incidence of LBW and SGA infants24,25. In the present study, we showed that maternal zinc deficiency during pregnancy elevated the risks of LBW and SGA births in a large population-based birth cohort study. This result will provide a scientific basis for zinc to prevent or control LBW and SGA especially in high-risk pregnant women with zinc deficiency. The next step is to determine whether maternal zinc supplementation during pregnancy reduces the risks of LBW and SGA infants.
Until now, no report analyzed the effects of zinc deficiency at different gestational stages on fetal growth. The present study compared the effects of zinc deficiency at different gestational stages on the incidence of LBW and SGA infants. Results suggest that maternal zinc deficiency at different gestational stages produces differential effects on fetal growth. Maternal zinc deficiency during early gestational stage elevates the risk of SGA infants, while zinc deficiency during later gestational stages results in the incidence of LBW and SGA infants. In addition, no significant difference in the level of zinc in umbilical sera is observed between SGA cases and controls. Although the confirmation for this result requires a larger sample size, the current result suggests that FGR, caused by maternal zinc deficiency during pregnancy, may not be attributed to zinc deficiency in the fetus.
Increasing evidence has demonstrated that zinc has an anti-inflammatory effect26. An earlier study found that injection with zinc during pregnancy ameliorated inflammation-associated teratogenicity in mice27. According to a recent report from our laboratory, oral supplementation with zinc protected mice from LPS-induced IUGR through its anti-inflammatory activity28. The present study showed that maternal serum zinc concentration was significantly lower in SGA cases than that of controls. In contrast with low zinc level, maternal serum CRP, TNF-α and IL-8, three important inflammatory cytokines, were markedly higher in SGA cases than those of controls. Correspondingly, NF-κB, a regulator of inflammatory cytokines, was activated in placentas from SGA cases. Thus, it is reasonable to speculate that activation of placental NF-κB signaling and elevation of serum inflammatory cytokines are attributed to low zinc level in SGA infants. Indeed, several recent studies showed that inflammatory cytokines were elevated in the cord blood from SGA infants29,30. Taken together, these results suggest that there is an association between placental inflammation and the incidence of SGA infants among subjects with low zinc level during pregnancy.
In the present study, we laid emphasis on the association of maternal zinc deficiency during pregnancy and the risks of LBW and SGA infants. The present study has some limitations. First, the present study only analyzed the effects of maternal zinc deficiency during pregnancy on fetal growth. Indeed, several reports demonstrated that maternal deficiency of other micronutrients, such as folate and vitamin B-12, elevated the risks of LBW and SGA infants31,32. Supplementation with either folic acid or multivitamins reduced the risk of SGA infant33,34. Second, the present study did not clarify the mechanism why maternal zinc deficiency during pregnancy elevated the risks of LBW and SGA infants. Additional work is required to determine whether maternal zinc supplementation during pregnancy reduces the risks of LBW and SGA infants and other adverse pregnancy outcomes. In addition, the mechanism by which maternal zinc deficiency during pregnancy induces FGR needs to be explored in animal experiments.
In summary, the present study investigated the association between maternal zinc level during pregnancy and the risks of LBW and SGA infants in a large population-based birth cohort study. The present results allow us to reach the following conclusions. First, maternal zinc deficiency during pregnancy elevates the risks of LBW and SGA infants; second, maternal zinc deficiency during early gestational stage elevates the risk of SGA infant, while zinc deficiency during later gestational stages results in the incidence of LBW infant; third, there is an association among maternal serum zinc level, placental inflammation and the incidence of SGA infants.
Methods
Study design
To avoid repeated freeze-thaw cycles for all serum samples and to ensure sufficient sample sizes, the present study analyzed a subsample of the China-Anhui Birth Cohort Study (C-ABCS) cohort35 that recruited 4358 pregnant women from Hefei city of Anhui province from January 1 to December 31 in 2009. For this study, eligible participants were mother-and-singleton -offspring pairs in which serum samples from mothers were available for analysis of serum zinc level and offspring had a detailed birth records. Thirty-six pregnant women giving birth to twins, 15 fetal deaths, 2 stillbirths, 58 abortions and 589 withdrew were excluded from this study. In addition, 471 cases were also excluded from this study due to maternal sera exhausted for other experiments (Fig. 1). Total 3187 mother-and-singleton-offspring pairs were eligible for this study. All neonates were weighed at birth. The present study was approved by the ethics committee of Anhui Medical University. The methods were carried out in accordance with the approved guidelines. Oral and written consents were obtained from all pregnant women.
Definition of SGA and LBW
In this study, SGA births were live-born infants that were <10th percentile of birth weight according to nomograms based on gender and gestational age from a reference population of 13,454 infants delivered at C-ABCS36. LBW births were live-born infants that were less than 2500 g for birth weight.
Nested case-control study
To analyze the association among serum zinc status, inflammatory cytokines and FGR, a nested case-control study within above cohort were designed. In the case-control study, 50 SGA cases and 100 controls were randomly chosen. For control subjects, a common control series (case: control = 1:2) was selected from the non–case subjects in this cohort. The controls were matched with these cases with regard to pre-pregnancy BMI, maternal age, time for collecting serum and average monthly income. The present study obtained ethics approval from the ethics committee of Anhui Medical University. Oral and written consents were obtained from all pregnant women.
Collection of placentas and umbilical sera
To compare the expression of placental NF-κB p65 in SGA cases and controls, a small case-control study was designed. Thirty SGA placentas and 30 control placentas were collected by the Maternal and Child Care Service Centre of Maanshan city (Maanshan, Anhui province, China). To analyze the association between zinc level in umbilical sera and IUGR, umbilical sera were also collected from 30 SGA cases and 30 controls by the Maternal and Child Care Service Centre of Maanshan city. The controls were matched with these cases with regard to pre-pregnancy BMI, maternal age, socio-economic status, parity and gestational week of collecting serum. The present study obtained ethics approval from the ethics committee of Anhui Medical University. Oral and written consents were obtained from all pregnant women.
Measurement of serum zinc
Maternal fasting blood during pregnancy was collected in the morning. The blood samples kept under refrigeration were allowed to clot for 30 mins. Then maternal serum was obtained after centrifuging for 15 mins at 3000 g. After discarding hemolytic specimens, available sera were stored at –80 ºC until analysis. To avoid contaminaion of exogenous zinc, all centrifuge tubes, storage vials and transfer pipettes were soaked for 24 hrs in ultrapure 10% HNO3 at room temperature. Serum zinc concentration was determined by flame atomic absorption spectroscopy (FAAS) as previously described37. Serum samples were diluted with 1% HNO3 according to 1:35 (v/v). The diluted solution was then detected using FAAS. Each sample was analyzed in triplicate. Precision of the method was measured by coefficients of variation. Mean CV for measurement of serum zinc was 4.9% for within-day determinations and 4.3% for day-to-day determinations. The detection limit of this method was 0.2 μg/dL.
Enzyme-linked immunosorbent assay (ELISA)
Commercial human TNF-α and IL-8 ELISA kits (R&D Systems, Abingdon, Oxon, UK) and human C-reactive protein (CRP) ELISA kits (TSZ ELISA, Waltham, MA, USA) were used to measure TNF-α, IL-8 and CRP in maternal serum according to the manufacturer’s protocols.
Immunohistochemistry
Human placenta tissues from SGA cases and controls were fixed in 4% paraformaldehyde and embedded in paraffin according to the standard procedure. Paraffin embedded tissues were cut 5 μm thick and stained with hematoxylin and eosin (H & E) for morphological analysis. For immunohistochemistry, paraffin-embedded placental sections were deparaffinized and rehydrated in a graded ethanol series. After antigen retrieval and quenching of endogenous peroxidase, sections were overnight incubated with NF-кB p65 monoclonal antibodies (1:200 dilution) at 4 °C. The color reaction was developed with HRP-linked polymer detection system and counterstaining with hematoxylin.
Statistical analysis
Maternal serum zinc level during pregnancy was divided into two groups according to suggested criteria18: serum zinc concentration less than 56 μg/dL for zinc deficiency and serum zinc concentration more than 56 μg/dL for zinc sufficiency. Incidence and relative risk (RR) for LBW and SGA were calculated between two groups. For adjustment of pre-pregnancy BMI, maternal age, time for collecting serum and average monthly income, logistic regression model was used to estimate RR with 95% confidence intervals (95% CI) with respect to LBW and SGA incidence. All quantified data were expressed as means ± SEM. All statistical tests were two-sided using an alpha level of 0.05. ANOVA and the Student-Newmann-Keuls post hoc test were used to determine differences among different groups. Student t test was used to determine differences between two groups.
Additional Information
How to cite this article: Wang, H. et al. Maternal zinc deficiency during pregnancy elevates the risks of fetal growth restriction: a population-based birth cohort study. Sci. Rep. 5, 11262; doi: 10.1038/srep11262 (2015).
Acknowledgments
We thank the Maternal and Child Care Service Centre of Hefei and Maanshan city, and all participants in the study. This study was partially supported by National Natural Science Foundation of China (81172711, 81473016, 81471467) and National Key Technology R & D Program (2006BAI05A03 to C-ABCS).
Footnotes
Author Contributions The authors’ responsibilities were as follows–D.X.X., F.B.T. and H.W.: designed the research; Y.F.H., H.W., Y.H.C., Y.W., Z.Y. and L.F.: conducted the research; J.H.H., P.Y.S., Y.Y.X. and C.Z.: provided essential materials and subjects; D.X.X., F.B.T. and H.W.: wrote the manuscript; D.X.X., F.B.T., H.W. and Y.F.H.: had primary responsibility for the final content of the manuscript; H.W., Y.F.H., J.H.H., Y.H.C., P.Y.S., Y.W., Z.Y., L.F., Y.Y.X., C.Z., F.B.T. and D.X.X.: analyzed data and read and approved the final manuscript.
References
- Saenger P., Czernichow P., Hughes I. & Reiter E. O. Small for gestational age: short stature and beyond. Endocr Rev. 28, 219–251 (2007). [DOI] [PubMed] [Google Scholar]
- Joss-Moore L. A. & Lane R. H. The developmental origins of adult disease. Curr Opin Pediatr. 21, 230–234 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D. J., Osmond C., Golding J., Kuh D. & Wadsworth M. E. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 298, 564–567 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D. J. et al. Fetal nutrition and cardiovascular disease in adult life. Lancet. 341, 938–941 (1993). [DOI] [PubMed] [Google Scholar]
- Brufani C. et al. Obese children with low birth weight demonstrate impaired beta-cell function during oral glucose tolerance test. J Clin Endocrinol Metab. 94, 4448–4452 (2009). [DOI] [PubMed] [Google Scholar]
- Liao C. H. et al. Schizophrenia patients at higher risk of diabetes, hypertension and hyperlipidemia: a population-based study. Schizophr Res. 126, 110–116 (2011). [DOI] [PubMed] [Google Scholar]
- Hirano T. et al. Roles of zinc and zinc signaling in immunity: zinc as an intracellular signaling molecule. Adv Immunol. 97, 149–176 (2008). [DOI] [PubMed] [Google Scholar]
- Buamah P. K., Russell M., Bates G., Ward A. M. & Skillen A. W. Maternal zinc status: a determination of central nervous system malformation. Br J Obstet Gynaecol. 91, 788–790 (1984). [DOI] [PubMed] [Google Scholar]
- Lehti K. K. Stillbirth rates and folic acid and zinc status in low-socioeconomic pregnant women of Brazilian Amazon. Nutrition. 9, 156–158 (1993). [PubMed] [Google Scholar]
- Scholl T. O., Hediger M. L., Schall J. I., Fischer R. L. & Khoo C. S. Low zinc intake during pregnancy: its association with preterm and very preterm delivery. Am J Epidemiol. 137, 1115–1124 (1993). [DOI] [PubMed] [Google Scholar]
- Graham T. W. et al. Serum zinc and copper concentrations in relation to spontaneous abortion in cows: implications for human fetal loss. J Reprod Fertil. 102, 253–262 (1994). [DOI] [PubMed] [Google Scholar]
- Prawirohartono E. P., Nystrom L., Nurdiati D. S., Hakimi M. & Lind T. The impact of prenatal vitamin A and zinc supplementation on birth size and neonatal survival - a double-blind, randomized controlled trial in a rural area of Indonesia. Int J Vitam Nutr Res. 83, 14–25 (2013). [DOI] [PubMed] [Google Scholar]
- Piso B., Zechmeister-Koss I. & Winkler R. Antenatal interventions to reduce preterm birth: an overview of Cochrane Systematic Reviews. BMC Res Notes. 7, 265 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori R. et al. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst Rev. 7, CD000230 (2012). [DOI] [PubMed] [Google Scholar]
- Ota E. et al. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst Rev. 2, CD000230 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmer K. & Thompson R. P. Maternal zinc and intrauterine growth retardation. Clin Sci (Lond). 68, 395–399 (1985). [DOI] [PubMed] [Google Scholar]
- Neggers Y. H. et al. A positive association between maternal serum zinc concentration and birth weight. Am J Clin Nutr. 51, 678–684 (1990). [DOI] [PubMed] [Google Scholar]
- Hotz C., Peerson J. M. & Brown K. H. Suggested lower cutoffs of serum zinc concentrations for assessing zinc status: reanalysis of the second National Health and Nutrition Examination Survey data (1976-1980). Am J Clin Nutr. 78, 756–764 (2003). [DOI] [PubMed] [Google Scholar]
- Cole C. R. et al. Zinc and iron deficiency and their interrelations in low-income African American and Hispanic children in Atlanta. Am J Clin Nutr. 91, 1027–1034 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebremedhin S., Enquselassie F. & Umeta M. Prevalence of prenatal zinc deficiency and its association with socio-demographic, dietary and health care related factors in rural Sidama, Southern Ethiopia: a cross-sectional study. BMC Public Health. 11, 898 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud J. et al. Determinants of serum zinc concentrations in a population of French middle-age subjects (SU.VI.MAX cohort). Eur J Clin Nutr. 64, 1057–1064 (2010). [DOI] [PubMed] [Google Scholar]
- Tamura T., Goldenberg R. L., Johnston K. E. & DuBard M. Maternal plasma zinc concentrations and pregnancy outcome. Am J Clin Nutr. 71, 109–113 (2000). [DOI] [PubMed] [Google Scholar]
- Gibson R. S. & Huddle J. M. Suboptimal zinc status in pregnant Malawian women: its association with low intakes of poorly available zinc, frequent reproductive cycling, and malaria. Am J Clin Nutr. 67, 702–709 (1998). [DOI] [PubMed] [Google Scholar]
- Gebremedhin S., Enquselassie F. & Umeta M. Independent and joint effects of prenatal Zinc and Vitamin A Deficiencies on birthweight in rural Sidama, Southern Ethiopia: prospective cohort study. PLoS One. 7, e50213 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry H. D. et al. Maternal selenium, copper and zinc concentrations in pregnancy associated with small-for-gestational-age infants. Matern Child Nutr. 10, 327–334 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase H. & Rink L. Functional significance of zinc-related signaling pathways in immune cells. Annu Rev Nutr. 29, 133–152 (2009). [DOI] [PubMed] [Google Scholar]
- Carey L. C., Berbee P. L., Coyle P., Philcox J. C. & Rofe A. M. Zinc treatment prevents lipopolysaccharide-induced teratogenicity in mice. Birth Defects Res A Clin Mol Teratol. 67, 240–245 (2003). [DOI] [PubMed] [Google Scholar]
- Chen Y. H. et al. Zinc supplementation during pregnancy protects against lipopolysaccharide-induced fetal growth restriction and demise through its anti-inflammatory effect. J Immunol. 189, 454–463 (2012). [DOI] [PubMed] [Google Scholar]
- Amarilyo G. et al. Increased cord serum inflammatory markers in small-for-gestational-age neonates. J Perinatol. 31, 30–32 (2011). [DOI] [PubMed] [Google Scholar]
- Lausten-Thomsen U., Olsen M., Greisen G. & Schmiegelow K. Inflammatory markers in umbilical cord blood from small-for-gestational-age newborns. Fetal Pediatr Pathol. 33, 114–118 (2014). [DOI] [PubMed] [Google Scholar]
- van Eijsden M., Smits L. J., van der Wal M. F. & Bonsel G. J. Association between short interpregnancy intervals and term birth weight: the role of folate depletion. Am J Clin Nutr. 88, 147–153 (2008). [DOI] [PubMed] [Google Scholar]
- Dwarkanath P. et al. High folate and low vitamin B-12 intakes during pregnancy are associated with small-for-gestational age infants in South Indian women: a prospective observational cohort study. Am J Clin Nutr. 98, 1450–1458 (2013). [DOI] [PubMed] [Google Scholar]
- Catov J. M., Bodnar L. M., Ness R. B., Markovic N. & Roberts J. M. Association of periconceptional multivitamin use and risk of preterm or small-for-gestational-age births. Am J Epidemiol. 166, 296–303 (2007). [DOI] [PubMed] [Google Scholar]
- Timmermans S., Jaddoe V. W., Hofman A., Steegers-Theunissen R. P. & Steegers E. A. Periconception folic acid supplementation, fetal growth and the risks of low birth weight and preterm birth: the Generation R Study. Br J Nutr. 102, 777–785 (2009). [DOI] [PubMed] [Google Scholar]
- Tao F. B. et al. Cohort Profile: the China-Anhui Birth Cohort Study. Int J Epidemiol. 42, 709–721 (2013). [DOI] [PubMed] [Google Scholar]
- Marchant T. et al. Neonatal mortality risk associated with preterm birth in East Africa, adjusted by weight for gestational age: individual participant level meta-analysis. PLoS Med. 9, e1001292 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahlavska A., Slansky V. & Benediktova B. The use of spectrum analytical methods in drug analysis. 3. Determination of iron using atomic absorption spectrophotometry. Pharmazie. 28, 241–242 (1973). [PubMed] [Google Scholar]