Abstract
Large prosomal scent glands constitute a major synapomorphic character of the arachnid order Opiliones. These glands produce a variety of chemicals very specific to opilionid taxa of different taxonomic levels, and thus represent a model system to investigate the evolutionary traits in exocrine secretion chemistry across a phylogenetically old group of animals. The chemically best-studied opilionid group is certainly Laniatores, and currently available chemical data allow first hypotheses linking the phylogeny of this group to the evolution of major chemical classes of secretion chemistry. Such hypotheses are essential to decide upon a best-fitting explanation of the distribution of scent-gland secretion compounds across extant laniatorean taxa, and hence represent a key toward a well-founded opilionid chemosystematics.
Introduction
A recently published chemosystematic approach by Caetano and Machado (2013) to explain the taxonomic distribution of chemical classes of secretion compounds in the large laniatorean family Gonyleptidae considers benzoquinones ancestral in this family, vinyl-ketones synapomorphic for one gonyleptid subgroup and alkylphenols as derived from benzoquinones, having evolved several times independently. Here, we provide an alternative view arising from a more adequate ancestral character-state reconstruction method for secretion chemistry, and we furnish evidence for a common ancestry of alkylphenols and the derived status of benzoquinones in grassatorean secretions.
Opilionid scent-gland chemistry
Chemical characters from exocrine secretions of homologous glands offer the opportunity of chemosystematic studies based on the principles of cladistics, hence introducing an independent set of characters for phylogeny reconstruction in addition to traditional sets of morphological and molecular data. So far, only a few studies have focused on secretion-based chemosystematics along with chemical character evolution in the Arthropoda, and only a few arthropod taxa provide a promising basis for successful chemosystematic studies at all. For instance, basic chemosystematic studies have been conducted for insect groups rich in exocrine glands such as beetles (e.g. Dettner, 1987), termites (e.g. Prestwich, 1983) and diverse hymenopterans (e.g. Cane, 1983), as well as arachnids such as glandulate oribatid mites (Sakata and Norton, 2001; Heethoff et al., 2011; Raspotnig et al., 2011a) and millipedes (e.g. Bodner and Raspotnig, 2012). However, another group particularly well suited for chemosystematics is certainly the Opiliones, the third largest order of arachnids, comprising about 6500 described species.
All opilionids possess large prosomal scent glands, and the chemistry of scent-gland secretions is stunningly diverse in extant taxa. As opilionids are an ancient group of arachnids with fossils known from the Lower Devonian (Dunlop et al., 2004), scent glands represent a well-suited model for studying the evolution of exocrine secretion chemistry in a homologous gland system across hundreds of millions of years. So far, the secretion chemistry in all four opilionid suborders has been exemplarily investigated, revealing sets of naphthoquinones and methyl ketones in the Cyphophthalmi (Raspotnig et al., 2005, 2012), naphthoquinones in phalangiid Eupnoi (Wiemer et al., 1978) and in Dyspnoi (Raspotnig et al., 2010), various acycles, including ethyl ketones in sclerosomatid Eupnoi (Ekpa et al., 1985), and volatile ketones in several Dyspnoi (Raspotnig et al., 2014). The chemically best-studied group, however, is certainly the Laniatores, from which at least four major chemical classes of secretion components have been reported: (1) nitrogen-containing compounds including various tobacco alkaloids from travunioid Insidiatores (Raspotnig et al., 2011b), and an array of (2) alkylphenols, (3) benzoquinones and (4) vinyl-ketones from the Grassatores (Roach et al., 1980; Duffield et al., 1981; Eisner et al., 2004; Hara et al., 2005; Wouters et al., 2013). Several authors have hypothesized about the evolutionary history of these compounds, including Hara et al. (2005), Raspotnig et al. (2011b), Raspotnig (2012) and, recently, Caetano and Machado (2013).
Evolutionary history of laniatorean secretion chemistry: current opinions
In their recent paper, Caetano and Machado (2013) reconstructed the phylogeny of the large laniatorean family Gonyleptidae by combining ecological, behavioural and chemical characters of 28 species, prior to mapping the main classes of scent-gland-derived compounds—benzoquinones, alkylphenols and vinyl ketones—onto their phylogenetic tree, by means of ancestral character-state reconstruction (ASR) under a parsimony regime of unordered, equal weighted characters. Their results implied benzoquinones to be the ancestral state in gonyleptid secretions, vinyl ketones to be a synapomorphy of gonyleptids of clade “K92,” and alkylphenols to have evolved five times independently in the Gonyleptidae. Several authors have acted on the proposal of a multiple independent evolution of alkylphenols from the ancestral state of benzoquinones and cited this particular situation as given (Rocha et al., 2013a,b; Wouters et al., 2013). We consider this proposed evolutionary scenario as rather problematic, and show that a few (in our opinion necessary) changes in the original data set of Caetano and Machado as well as in the evaluation method lead to completely different results.
Alternative approaches to explain the evolution of laniatorean secretion chemistry
We propose to use a more representative selection of gonyleptid outgroups, including also those from which alkylphenols have already been reported (“outgroup rooting”): in our opinion, outgroup-taxa selection by Caetano and Machado (2013) was extremely biased, choosing the only hitherto known non-alkylphenol producing manaosbiid as an outgroup. In fact, all chemically investigated grassatorean species outside the Gonyleptoidea as well as most basal gonyleptoids exclusively and characteristically produce alkylphenols, as outlined below.
Ancestral character-state reconstruction (ASR) is a frequently used method to test hypotheses regarding character evolution, mainly by mapping characters on given phylogenies, with approaches relying on maximum parsimony methods still being most common. As ASR by means of parsimony appears to suffer from several limitations (Cunningham, 1999; Ekman et al., 2008; Skinner, 2010), many authors have emphasized the importance of adapting ASR methods to specific demands as well as the advantages of comparing different ASR approaches for obtaining more differentiated pictures about the evolutionary traits under consideration (e.g. Royer-Carenzi et al., 2013). For instance, the inclusion of likelihood methods may add alternative views of character evolution (e.g. Pagel, 1999), but requires branch-length information for reliable ASR and is thus not applicable to analyses that rely on cladograms. Regarding maximum parsimony-based ASR, the default approach is often the assignment of equal costs to character state changes. In many cases, however, such an assumption does not reflect the real situation. How to deal with asymmetric transformation probabilities, that is, non-equal probabilities of gains and losses in binary characters, has been addressed in many studies and may be met by introducing step matrices, making certain transformations less likely than others (e.g. Cunningham et al., 1998; Ree and Donoghue, 1998; Cunningham, 1999; Omland, 1999). Especially when dealing with chemical compounds in exocrine secretions, it is clear that the gain of a compound (its biosynthesis) is much more difficult, and thus more unlikely, than its loss. The particular situation of chemosystematics and ASR has been emphasized by, for example, Barkman (2001). In brief, the evolution of a chemical compound requires an elaborate biosynthetic machinery, which typically comprises a cascade of different enzymes; whereas the breakdown of only one of these components (e.g. one particular enzyme) may already lead to the loss of the product. In order to accommodate this particular situation for ASR, knowledge of the biosynthetic pathways to specific compounds or classes of compounds—that is, the number of biosynthetic steps (n) required to finish the biosynthetic process—is important. From this information, a step matrix that most adequately reflects the costs of gains and losses (e.g. gain is n times less likely than loss) can be deduced. Thus, a parsimony reconstruction of chemical characters under equal weights for gains and losses, as done for alkylphenols in gonyleptid scent glands by Caetano and Machado (2013), may easily result in a heavily biased evolutionary scenario, clearly overestimating the number of independent gains of a class of compounds.
Moreover, the biosynthesis of phenols and benzoquinones in laniatorean harvestmen has recently been the focus of an in-depth study, showing that the benzoquinones arise from phenolic precursors (Rocha et al., 2013a), clearly suggesting a derived status of benzoquinones from ancestrally present phenols. With respect to ASR, we have to point out that alkylphenols and benzoquinones, sharing a common biosynthetic route in gonyleptids, cannot be treated as independent characters. We do though acknowledge that the convergent evolution of chemical compounds in exocrine secretions is not a rare phenomenon in the Arthropoda in general (“semiochemical parsimony” sensu Blum 1996) but it is rare within a specific taxon (e.g. Gonyleptoidea). In these terms, we consider it unlikely that, for example, alkylphenols evolved multiple times in a taxon mainly relying on alkylphenolic–benzoquinonic chemistry.
Chemistry database: outgroup and ingroup information
Alkylphenols in non-gonyleptoid grassatoreans
In grassatoreans outside gonyleptoids, exclusively phenolic secretions have been detected: alkylphenols were reported from two species of Phalangodidae, Bishopella laciniosa and Texella bifurcata (Shear et al., 2010a), and one species of Stygnommatidae, Stygnomma spinifera (Duffield et al., 1981). Moreover, according to our preliminary data on a number of American and European phalangodids, several Epedanoidea from Asia, and assamiids, exclusively phenolic secretions are present in these lower grassatorean families (Raspotnig, unpublished).
Alkylphenols in the Gonyleptoidea
Chemical data are available for a few representatives of four (of the seven) gonyleptoid families, namely Gonyleptidae, Cosmetidae, Manaosbiidae and Stygnopsidae. From stygnopsids, according to Sharma and Giribet (2011) the basal gonyleptoid family, exclusively alkylphenolic secretions have been reported. However, chemical information on stygnopsids so far relies on the secretions of two species only: Chinquipellobunus madlae and Hoplobunus mexicanus (Pomini et al., 2010; Shear et al., 2010b). For cosmetids and gonyleptids, a few examples for the production of exclusively alkylphenolic secretions also exist: from seven cosmetids investigated, two produced alkylphenols, Cynorta astora and Eucynortula albipunctata (e.g. Eisner et al., 1977; Roach et al., 1980; Rocha et al., 2013a); from about 50 gonyleptids studied, at least seven produce exclusively alkylphenols, namely Daguerra inermis (Pachylinae), Camarana flavipalpi, Pseudopachylus longipes, Cryptogeobius crassipes (all Tricommatinae), Progonyleptoidellus striatus, Mitopernoides variabilis (both Progonyleptoidellinae) and Metarthrodes longipes (Caelopyginae) (Hara et al., 2005; Gnaspini and Hara, 2007; Caetano and Machado, 2013; Rocha et al., 2013a). From two chemically investigated manaosbiids, Zygopachylus albomarginis secretes alkylphenols together with benzoquinones, and similar mixtures are known from several gonyleptids, such as Discocyrtus oliveroi, Pachyloidellus goliath (both Pachylinae), Chavesincola inexpectabilis (Heteropachylinae) and Mischonyx cuspidatus (Gonyleptinae) (Eisner et al., 1977; Roach et al., 1980; Acosta et al., 1993; Caetano and Machado, 2013). However, the majority of gonlyleptoids above Stygnopsidae seem to produce exclusively benzoquinonic secretions (e.g. Hara et al., 2005; Gnaspini and Hara, 2007; Rocha et al., 2013a) with clade “K92” (= vinyl-ketone producers) being an exception (Wouters et al., 2013). However, there are large gaps in the knowledge of gonyleptoid secretion chemistry. Data for the families Agoristenidae, Stygnidae and Cranaidae are conspicuously missing.
Taxon sampling and phylogenetic trees
We are aware of the influence of taxon sampling on ACRs. Although the probability of a correct reconstruction increases with the number of representative taxa investigated, the true reconstruction, at least theoretically, can be achieved only by including all taxa of the group in consideration and by subsequently mapping the characters onto an unambiguous species tree. This is de facto impossible, and thus a certain degree of tentativeness will always remain. The Gonyleptidae comprises more than 800 species in 16 subfamilies (Kury, 2003), and the phylogenetic tree of Caetano and Machado is based on 28 selected species, although representing most of the subfamilies. It does not, however, contain all the so far chemically analysed species. As several subfamilies have been shown to be paraphyletic (Caetano and Machado, 2013; Pinto-da-Rocha et al., 2014), it is not possible to place additional, previously chemically analysed species correctly into this particular tree. To our knowledge, only two further studies provide alternative phylogenetic hypotheses on gonyleptids. Pinto-da-Rocha (2002) used morphological characters. The so far most comprehensive phylogenetic study, based on a molecular multilocus approach, is from Pinto-da-Rocha et al. (2014), who included 101 species from all gonyleptid subfamilies. It is superfluous to say that all three hypotheses are different. Thus, in the absence of chemical data for most of the species included by Pinto-da-Rocha (2002) and Pinto-da-Rocha et al. (2014), a hypothesis on chemical character evolution in gonyleptoid secretions is currently only possible based on the phylogeny by Caetano and Machado (2013), although we acknowledge that statistical support in their tree is weak for several nodes.
Alkylphenols as ancestral grassatorean scent-gland constituents?
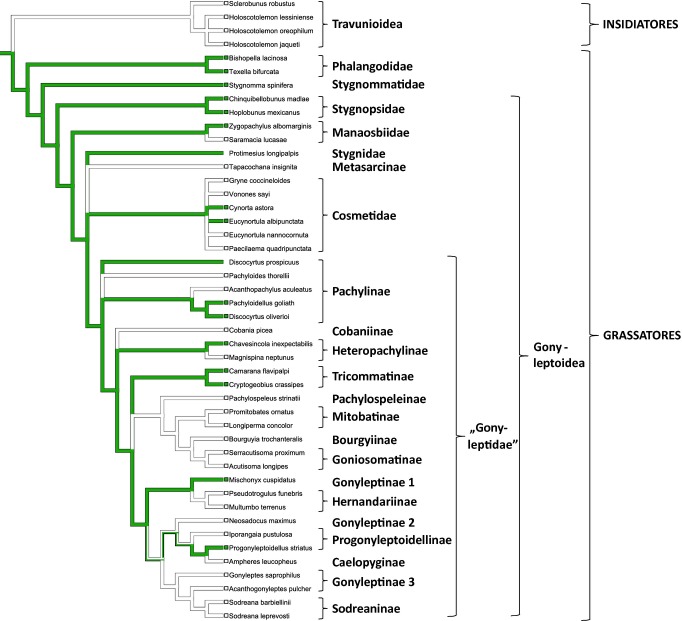
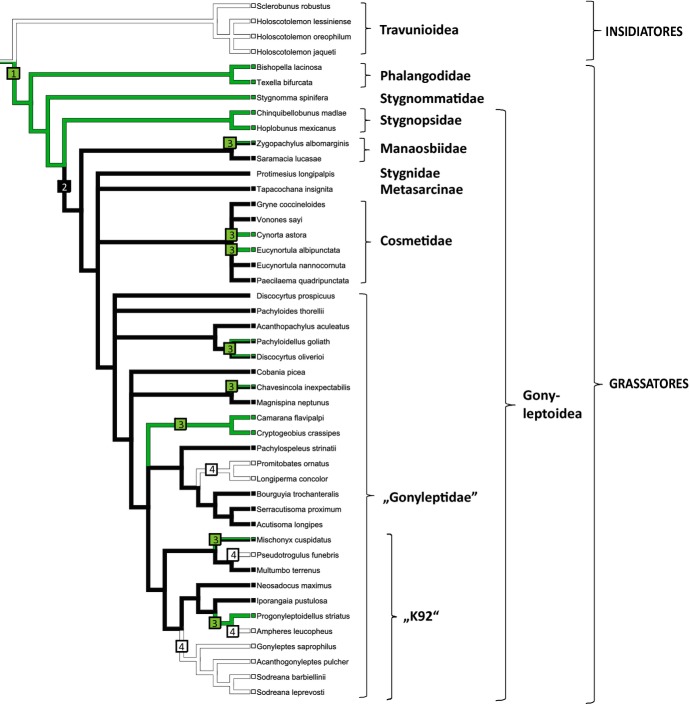
For our ASR approach, we thus used the tree proposed by Caetano and Machado, supplemented by outgroups on which chemical information has previously been published. In particular, we added non-grassatoreans such as travunioids, non-gonyleptoid grassatoreans such as phalangodids, stygnommatids and gonyleptoids such as stygnopsids, as well as manaosbiids and cosmetids (Figs1 and 2). These additional taxa, in particular the lower grassatorean families, were placed as proposed in the laniatorean phylogeny of Sharma and Giribet (2011). For cosmetids, we chose to show a polytomy for all chemically analysed species, because their phylogenetic relationships are still unresolved. All other aspects in the tree of Caetano and Machado were left unchanged. We subsequently performed ACRs under different assumptions: (I) under an unordered parsimony regime of equal-weighted gains and losses (as done by Caetano and Machado, 2013); (II) under a parsimony regime using step matrices, making gains less likely than losses; and (III) under an ordered parsimony regime, accounting for information on the dependency of alkylphenol and benzoquinone production in gonyleptids and the reversible conversion of alkylphenols to benzoquinones (Rocha et al., 2013a). All reconstructions were conducted in Mesquite Version 2.75 (Maddison and Maddison, 2011). The ASR was based on a single-tree input, thus it was not possible to account for topological uncertainty in the analysis. The reconstructions obtained (I–III) show diverging results. In (I) the ASR using the parsimony approach under equal weights for gains and losses still indicates that alkylphenols arose several times independently in the Gonyleptidae even though they are indicated to represent old grassatorean equipment as evidenced from outgroups, basically being consistent with the findings of Caetano and Machado (2013; not shown). As this kind of reconstruction ignores the above-mentioned differences in probabilities of gaining or losing a particular trait as well as information on a common route of alkylphenol and benzoquinone biosynthesis, we believe that this reconstruction does not adequately reflect alkylphenol evolution. By contrast, reconstruction (II), an ASR approach using weighted parsimony, strongly suggests a common alkylphenol ancestry (Fig.1). Considering a multistep biosynthesis of alkylphenols from acetate and propionate units, as recently suggested by Rocha et al. (2013a), a step-matrix making gains several times less likely than losses might be proposed. For Magnispina neptunus (Heteropachylinae), a known benzoquinone producer, the route to its main secretion component 2-ethyl-1,4-benzoquinone might include at least six steps (scheme 1 in Rocha et al., 2013a), starting with the repeated condensation of acetate units to form a polyketide chain, followed by cyclization, enolysation and decarboxylation, then resulting in an ethylphenol. Phenol p-oxidation leads to the corresponding ethyl-1,4-hydroquinone, and further oxidation to the ethyl-1,4-benzoquinone. According to Rocha et al. (2013a), small amounts of the intermediate hydroquinones can generally be detected in benzoquinone-producing Gonyleptidae. An ACR on phenols and benzoquinones must take such a multistep biosynthesis into account. Indeed, even when introducing a rather low weighting factor of three (for gains) into a step matrix, a scenario as pictured in Fig.1 arises, indicating that alkylphenols are ancestral in grassatorean secretions, having evolved only once in early grassatoreans. In (III), using an ordered parsimony approach as strongly implied by alkylphenol and benzoquinone biosynthesis, the scenario of Fig.2 arises, and should be interpreted as follows: a common biosynthetic pathway to alkylphenols and benzoquinones evolved only once. Benzoquinones are derived from ancestral alkylphenols by p-oxidation and benzoquinones replaced alkylphenols in the Gonyleptoidea higher than Stygnopsidae. The final biosynthetic step toward benzoquinones is indicated to have been lost several times independently (seven times when using the approach herein, see Fig.2). Such a scenario requires only the loss of p-oxidation, being much more likely than the multiple independent development of the whole biosynthetic machinery toward alkylphenols. The picture obtained from this ordered parsimony approach superficially resembles the reconstruction from Caetano and Machado (2013), but it shows a completely different pattern of alkylphenol and benzoquinone evolution in gonyleptoid harvestmen.
Fig 1.
Ancestral character-state reconstruction (ASR) of the scent-gland associated character “alkylphenols” in the Laniatores, basically using the phylogenetic hypothesis of Caetano and Machado (2013), but slightly modified by adding outgroups (insidiatorean Travunioidea, Phalangodidae, Stygnopsidae: placement according to Sharma and Giribet, 2011) and in addition previously chemically analysed species of Manaosbiidae and Cosmetidae. The ASR is based on maximum parsimony and a step matrix, making gains less likely than losses by a factor of three. Alkylphenols were coded as “0” (not present, white) or “1” (present, green). Alkylphenols appear to have evolved only once, at the base of Grassatoreans, but are reduced or replaced, respectively, in a number of taxa. Please note that the family “Gonyleptidae” is handled under the exclusion of Metasarcinae, as in Caetano and Machado (2013). One representative of Stygnidae, Protimesius longipalpis, has been included in the ASR of scent-gland-derived chemical classes by Caetano and Machado, although the secretion of this species (as the secretions for all Stygnidae) is unknown. We retained the species in our reconstruction (secretion chemistry coded as “?”). Pachyloidellus goliath (Pachylinae, Gonyleptidae) was designated as a non-alkylphenol-producing species in the reconstruction by Caetano and Machado (2013), despite being known to produce a mixture of alkylphenols and benzoquinones (Acosta et al., 1993; Rocha et al., 2013a). We coded alkylphenols in this species as “1.”
Fig 2.
Ancestral character-state reconstruction of the scent-gland associated characters “alkylphenols” and “benzoquinones” with an indication of its interpretion, again using the tree of Fig.1. As indicated by their biosynthesis, benzoquinones are considered derived from the ancestral state of alkylphenols by p-oxidation (Rocha et al., 2013a), thus a maximum parsimony reconstruction, relying on ordered characters, is shown (character state “green”: alkylphenols; character state “black”: benzoquinones). (1) The common biosynthethic pathway to alkylphenols and benzoquinones is ancestral and evolved in basal grassatoreans. Benzoquinones are derived from the ancestral state of alkylphenols, mainly relying on phenol p-oxidation. (2) The extension of the pathway from alkylphenols to benzoquinones may have happened in gonyleptoids after the split-off of the Stygnopsidae. (3) In a number of gonyleptoid taxa, however, this final step to benzoquinone synthesis has been lost independently, again leading to the ancestral state of alkylphenols. (4) In other taxa, both alkylphenols and benzoquinones have been lost completely. Note the travunioid Insidiatores (outgroup) that primarily produce neither alkylphenols nor benzoquinones but rely on nitrogen-compound-rich secretions (Raspotnig et al., 2011b). Note the gonyleptid clade “K92” in which alkylphenol and/or benzoquinones are frequently reduced; the whole clade is characterized by vinyl-ketone-rich secretions (not indicated, see Caetano and Machado, 2013).
We can also imagine that for some of these seven incidences, p-oxidation has not been lost but possibly never evolved at all, especially when considering the alternative phylogenetic hypothesis on Gonyleptoidea by Pinto-da-Rocha et al. (2014). In their tree, the Tricommatinae in particular is placed outside the Gonyleptidae sensu stricto, possibly having split-off before addition of an oxidation step to form benzoquinones and thus possibly exclusively relying on alkylphenols. Tricommatines have generally been regarded as a small gonyleptid subfamily, currently comprising 53 species (Kury, 2000), and thus far insufficient chemical data on these are available. However, two species (from the genera Camarana and Cryptogeobius), both of which exclusively rely on alkylphenolic secretions, are included in the tree shown in Figs1 and 2. Consistently, a third species analysed, Pseudopachylus longipes (not included in Figs1 and 2), also exhibits an exclusively alkylphenolic secretion (Hara et al., 2005). Following our arguments presented above, we consider alkylphenolic–benzoquinone mixtures, as found in some gonyleptoids, to represent secretions partially subjected to loss of p-oxidation. Such partial reductions, that is, only affecting a few or one compound in the secretion of a particular species, might indeed have happened several times independently, according to Fig.2 (and the tree of Caetano and Machado, 2013) at least once in the Manaosbiidae and three times in the Gonyleptidae. We, however, point out that this scenario may change by the inclusion of Manaosbiidae within Gonyleptidae, as proposed by Pinto-da-Rocha et al. (2014). Moreover, our analysis indicates multiple further and more drastic regression events leading to the loss of both alkylphenols and benzquinones in gonyleptid secretions, mainly within the clade “K92” of vinyl-ketone producers (Fig.2); whereas Mitobatinae (as represented by Promitobates ornatus and Longiperma concolor) may not produce secretions at all (Caetano and Machado, 2013).
Conclusion
When considering all information on laniatorean phenols and benzoquinones in an ACR, a hypothesis of multiple independent development of alkylphenols during gonyleptid evolution must be rejected, or at least, a much more differentiated view on the evolutionary history of alkylphenols and benzoquinones is obtained. Alkylphenols are indicated to be ancestral compounds in the scent-gland secretions of grassatoreans, most likely having evolved in the ancestors of Phalangodidae and the remaining Grassatores. According to Sharma and Giribet (2011), Phalangodidae is the basal group of Grassatores, and the split-off of the Phalangodidae had happened by about 300 Ma in the Late Carboniferous. On the other hand, benzoquinones are considered to be derived from alkylphenols by p-oxidation, and are proposed to have arisen only once within the Gonyleptoidea, possibly sometime in the Triassic, probably after the split-off of the Stygnopsidae.
Acknowledgments
This work was supported by a grant from the Austrian Science Fund (FWF), project number P21819-B16.
References
- Acosta LE, Poretti TI, Mascarelli PE. The defensive secretion of Pachyloidellus goliath (Opiliones, Laniatores, Gonyleptidae) Bonn. Zool. Beitr. 1993;44:19–31. [Google Scholar]
- Barkman TJ. Character coding of secondary chemical variation for use in phylogenetic analyses. Biochem. Syst. Ecol. 2001;29:1–20. doi: 10.1016/s0305-1978(00)00031-4. [DOI] [PubMed] [Google Scholar]
- Blum MS. Semiochemical parsimony in the Arthropoda. Annu. Rev. Entomol. 1996;41:353–374. doi: 10.1146/annurev.en.41.010196.002033. [DOI] [PubMed] [Google Scholar]
- Bodner M, Raspotnig G. Millipedes that smell like bugs: (E)-alkenals in the defensive secretion of the julid diplopod Allajulus dicentrus. J. Chem. Ecol. 2012;38:547–556. doi: 10.1007/s10886-012-0127-5. [DOI] [PubMed] [Google Scholar]
- Caetano D, Machado G. The ecological tale of Gonyleptidae (Arachnida, Opiliones) evolution: phylogeny of a Neotropical lineage of armoured harvestmen using ecological, behavioural and chemical characters. Cladistics. 2013;29:589–609. doi: 10.1111/cla.12009. [DOI] [PubMed] [Google Scholar]
- Cane JT. Chemical evolution and chemosystematics of the Dufour's gland secretions of the lactone-producing bees (Hymenoptera: Colletidae, Halictidae, and Oxaeidae) Evolution. 1983;37:657–674. doi: 10.1111/j.1558-5646.1983.tb05588.x. [DOI] [PubMed] [Google Scholar]
- Cunningham CW. Some limitations of ancestral character-state reconstruction when testing evolutionary hypotheses. Syst. Biol. 1999;48:665–674. [Google Scholar]
- Cunningham CW, Omland KE, Oakley TH. Reconstructing ancestral states: a critical reappraisal. Tree. 1998;13:361–366. doi: 10.1016/s0169-5347(98)01382-2. [DOI] [PubMed] [Google Scholar]
- Dettner K. Chemosystematics and evolution of beetle chemical defenses. Annu. Rev. Entomol. 1987;32:17–48. [Google Scholar]
- Duffield RM, Olubajo O, Wheeler JW, Shear WA. Alkylphenols in the defensive secretion of the neartic opilionid, Stygnomma spinifera. J. Chem. Ecol. 1981;7:445–452. doi: 10.1007/BF00995767. [DOI] [PubMed] [Google Scholar]
- Dunlop JA, Anderson LI, Kerp H, Hass H. A harvestman (Arachnida: Opiliones) from the Early Devonian Rhynie cherts, Aberdeenshire, Scotland. Trans. Roy. Soc. Edinburgh Earth Sci. 2004;94:341–354. [Google Scholar]
- Eisner T, Jones TH, Hicks K, Silberglied RE, Meinwald J. Quinones and phenols in the defensive secretions of neotropical opilionids. J. Chem. Ecol. 1977;3:321–329. [Google Scholar]
- Eisner T, Rossini C, Eisner M. Chemical defense of an opilionid (Acanthopachylus aculeatus. J. Exp. Biol. 2004;207:1313–1321. doi: 10.1242/jeb.00849. [DOI] [PubMed] [Google Scholar]
- Ekman S, Andersen HL, Wedin M. The limitations of ancestral state reconstruction and the evolution of the ascus in the Lecanorales (lichenized Ascomycota) Syst. Biol. 2008;57:141–156. doi: 10.1080/10635150801910451. [DOI] [PubMed] [Google Scholar]
- Ekpa O, Wheeler JW, Cokendolpher JC, Duffield RM. Ketones and alcohols in the defensive secretion of Leiobunum townsendi Weed and a review of the known exocrine secretions of Palpatores (Arachnida: Opiliones) Comp. Biochem. Physiol. 1985;81B:555–557. [Google Scholar]
- Gnaspini P, Hara MR. Defense mechanisms. In: Pinto-da-Rocha R, Machado G, Giribet G, editors. Harvestmen: The Biology of Opiliones. Cambridge, MA: Harvard University Press; 2007. pp. 374–399. [Google Scholar]
- Hara MR, Cavalheiro AJ, Gnaspini P, Santos DYAC. A comparative analysis of the chemical nature of defensive secretions of Gonyleptidae (Arachnida: Opiliones: Laniatores) Biochem. Syst. Ecol. 2005;33:1210–1225. [Google Scholar]
- Heethoff M, Laumann M, Weigmann G, Raspotnig G. Integrative taxonomy: combining morphological, molecular, and chemical data for species delineation in the parthenogenetic Trhypochthonius tectorum complex (Acari, Oribatida, Trhypochthoniidae) Front. Zool. 2011;8:2. doi: 10.1186/1742-9994-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kury AB. 2000. onwards. Classification of Opiliones. Museu Nacional/UFRJ website. http://www.museunacional.ufrj.br/mndi/Aracnologia/opiliones.html.
- Kury AB. Annotated catalogue of the Laniatores of the New World (Arachnida, Opiliones) Revista Iberica de Aracnología, Zaragoza. 2003;1:1–325. Special Monograph. [Google Scholar]
- Maddison WP, Maddison DR. 2011. Mesquite: a modular system for evolutionary analysis, Version 2.75. http://mesquiteproject.org.
- Omland KE. The assumptions and challenges of ancestral state reconstructions. Syst. Biol. 1999;48:604–611. [Google Scholar]
- Pagel M. The maximum likelihood approach to reconstructing ancestral character states of discrete characters on phylogenies. Syst. Biol. 1999;48:612–622. [Google Scholar]
- Pinto-da-Rocha R. Systematic review and cladistic analysis of the Brazilian subfamily Caelopyginae (Opiliones: Gonyleptidae) Arquiv. Zool. 2002;36:357–464. [Google Scholar]
- Pinto-da-Rocha R, Bragagnolo C, Marques FPL, Antunes M., Jr Phylogeny of harvestmen family Gonyleptidae inferred from a multilocus approach (Arachnida: Opiliones) Cladistics. 2014 doi: 10.1111/cla.12065. doi: 10.1111/cla.12065. [DOI] [PubMed] [Google Scholar]
- Pomini AM, Machado G, Pinto-da-Rocha R, Macías-Ordóñez R, Marsaioli AJ. Lines of defense in the harvestmen Hoplobunus mexicanus (Arachnida: Opiliones): aposematism, stridulation, thanatosis, and irritant chemicals. Biochem. Syst. Ecol. 2010;38:300–308. [Google Scholar]
- Prestwich GD. Chemical systematic of termite exocrine secretions. Annu. Rev. Ecol. Syst. 1983;14:287–311. [Google Scholar]
- Raspotnig G. Scent gland chemistry and chemosystematics in harvestmen. Biol. Serbica. 2012;34:5–18. [Google Scholar]
- Raspotnig G, Fauler G, Leis M, Leis HJ. Chemical profiles of scent gland secretions in the cyphophthalmid opilionid harvestmen, Siro duricorius and S. exilis. J. Chem. Ecol. 2005;31:1353–1368. doi: 10.1007/s10886-005-5291-4. [DOI] [PubMed] [Google Scholar]
- Raspotnig G, Leutgeb V, Schaider M, Komposch C. Naphthoquinones and anthraquinones from scent glands of a dyspnoid harvestman, Paranemastoma quadripunctatum. J. Chem. Ecol. 2010;36:158–162. doi: 10.1007/s10886-010-9745-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raspotnig G, Leutgeb V, Krisper G, Leis HJ. Discrimination of Oribotritia species by oil gland chemistry (Acari, Oribatida) Exp. Appl. Acarol. 2011a;54:211–224. doi: 10.1007/s10493-011-9434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raspotnig G, Schaider M, Föttinger P, Komposch C, Karaman I. Nitrogen-containing compounds in the scent gland secretions of European cladonychiid harvestmen (Opiliones, Laniatores, Travunioidea) J. Chem. Ecol. 2011b;37:912–921. doi: 10.1007/s10886-011-9996-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raspotnig G, Schwab J, Karaman I. High conservatism in the composition of scent gland secretions in the Cyphophthalmi: evidence from Pettalidae (Arachnida, Opiliones) J. Chem. Ecol. 2012;38:437–440. doi: 10.1007/s10886-012-0108-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raspotnig G, Schaider M, Stabentheiner E, Leis HJ, Karaman I. On the enigmatic scent glands of dyspnoan harvestmen (Arachnida, Opiliones): first evidence for the production of volatile secretions. Chemoecology. 2014;24:43–55. doi: 10.1007/s00049-014-0146-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ree RH, Donoghue MJ. Step matrices and the interpretation of homoplasy. Syst. Biol. 1998;47:582–588. doi: 10.1080/106351598260590. [DOI] [PubMed] [Google Scholar]
- Roach B, Eisner T, Meinwald J. Defensive substances in opilionids. J. Chem. Ecol. 1980;6:511–516. [Google Scholar]
- Rocha DFO, Wouters FC, Zampieri DS, Brocksom TJ, Machado G, Marsaioli AJ. Harvestmen phenols and benzoquinones: characterisation and biosynthetic pathway. Molecules. 2013a;18:11429–11451. doi: 10.3390/molecules180911429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha DFO, Wouters FC, Machado G, Marsaioli AJ. First biosynthetic pathway of 1-hepten-3-one in Iporangia pustulosa (Opiliones) Sci. Rep. 2013b;3:3156. doi: 10.1038/srep03156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer-Carenzi M, Pontarotti P, Didier G. Choosing the best ancestral character state reconstruction method. Math. Biosci. 2013;242:95–109. doi: 10.1016/j.mbs.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Sakata T, Norton RA. Opisthonotal gland chemistry of early-derivative oribatid mites (Acari) and its relevance to systematic relationships of Astigmata. Int. J. Acarol. 2001;27:281–292. [Google Scholar]
- Sharma PP, Giribet G. The evolutionary and biogeographic history of the armoured harvestmen – Laniatores phylogeny based on ten molecular markers, with the description of two new families of Opiliones (Arachnida) Invert. Syst. 2011;25:106–142. [Google Scholar]
- Shear WA, Jones TH, Snyder AJ. Chemical defense of phalangodid harvestmen: Bishopella laciniosa (Croby & Bishop) and Texella bifurcata (Briggs) produce 2-methyl-5-ethylphenol (Opiliones: Grassatores: Phalangodidae) Bull. Brit. Arachnol. Soc. 2010a;15:27–28. [Google Scholar]
- Shear WA, Snyder AJ, Jones TH, Garaffo HM, Andriamaharavo NR. The chemical defense of the Texas cave harvestman Chinquipellobunus madlae: first report on the family Stygnopsidae and on a North American troglobiont harvestman (Opiliones: Gonyleptoidea) J. Arachnol. 2010b;38:126–127. [Google Scholar]
- Skinner A. Rate heterogeneity, ancestral character state reconstruction, and the evolution of limb morphology in Lerista (Scincidae, Squamata) Syst. Biol. 2010;59:723–740. doi: 10.1093/sysbio/syq055. [DOI] [PubMed] [Google Scholar]
- Wiemer DF, Hicks K, Meinwald J, Eisner T. Naphthoquinones in the defensive secretion of an opilionid. Experientia. 1978;34:969–970. [Google Scholar]
- Wouters FC, Rocha DFO, Conçalves CCS, Machado G, Marsaioli AJ. Additional vinyl ketones and their pyranyl ketones in gonyleptid harvestmen (Arachnida: Opiliones) suggest these metabolites are widespread in his family. J. Nat. Prod. 2013;76:1559–1564. doi: 10.1021/np4001569. [DOI] [PubMed] [Google Scholar]